Managing HIV Coinfections and Complications
RELEASE DATE
April 1, 2024
EXPIRATION DATE
April 30, 2026
FACULTY
Kristen Mathew, PharmD Candidate 2024
St. John’s University
College of Pharmacy and Health Sciences
Queens, New York
Yamilex Tomala, PharmD Candidate 2024
St. John’s University
College of Pharmacy and Health Sciences
Queens, New York
Tina Caliendo, PharmD, BCGP, BCACP
Associate Professor
Department of Pharmacy Practice
St. John’s University
College of Pharmacy and Health Sciences
Queens, New York
FACULTY DISCLOSURE STATEMENTS
Ms. Mathew, Ms. Tomala, and Dr. Caliendo have no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT
 Pharmacy
Pharmacy
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-24-037-H02-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients' conditions and possible contraindications or dangers in use, review of any applicable manufacturer's product information, and comparison with recommendations of other authorities.
GOAL
To expand pharmacists' comprehension of HIV coinfections and complications to further contribute to positive patient health outcomes.
OBJECTIVES
After completing this activity, the participant should be able to:
- Identify common coinfections in HIV-positive patients and the pharmacologic approaches for their management.
- Recognize the various opportunistic infections associated with HIV and recommendations for their prevention and treatment.
- Explain the cardiovascular and renal complications associated with HIV, including the role of pharmacists in monitoring and managing these conditions.
- Describe the pharmacist's role in enhancing medication adherence and patient education in HIV care through motivational interviewing and collaborative practice agreements.
ABSTRACT: Human immunodeficiency virus (HIV) is a significant global public health issue that requires lifelong treatment with antiretroviral therapy (ART) and close patient monitoring. Besides early initiation of appropriate ART, other comorbidities and risk factors must be considered when providing comprehensive and holistic care. Tuberculosis, hepatitis, sexually transmitted infections, and other opportunistic infections should be prevented and treated with appropriate, patient-specific agents to reduce the risk of morbidity and mortality in patients living with HIV. Cardiovascular and renal complications are also common risks in HIV, requiring extra care and monitoring from pharmacists. Pharmacists play an essential role in collaborative practice and patient education in HIV care.
Human immunodeficiency virus (HIV) is a retrovirus that targets and attacks the CD4+ T cells of the immune system, eventually leading to their depletion.1 Primarily, CD4+ cells are found in the body to fight against bacteria, viruses, and other organisms. The progressive loss in CD4+ cells causes immunodeficiency, increasing susceptibility to infections and illnesses. CD4+ T-lymphocyte cell-count monitoring assesses immune function at baseline diagnosis and throughout therapy. To ensure that counts remain at normal levels of about 500 to 1,500 cells/mm3 , CD4+ monitoring is indicated every 3 to 6 months. Without treatment, the body may decline to a CD4+ value of less than 200 cells/mm3. This is classified as acquired immune deficiency syndrome (AIDS), the last stage of HIV infection.2 AIDS may also be diagnosed in the presence of certain opportunistic infections, which increase mortality.1,2
In 2022, the World Health Organization (WHO) reported that 39 million people are living with HIV worldwide. Of those, 630,000 people died of HIV-related illnesses around the globe in 2022. Although HIV-related deaths have decreased by 50% since 2010, HIV is still a significant global public health issue.3 To decrease the risk of HIV-related deaths secondary to coinfections and complications, it is essential to initiate antiretroviral therapy (ART) early and increase the CD4+ count. Pharmacists can make a significant impact by having adequate knowledge of common coinfections and complications in HIV to optimize patient care and population health.4
COMMON COINFECTIONS
Tuberculosis
Tuberculosis (TB) is a bacterial infection caused by Mycobacterium tuberculosis, affecting the lungs. WHO estimates that people living with HIV are 16 to 27 times more likely to develop TB disease than people without HIV.5 One form of TB infection, called latent TB, can become active in those who have a weakened immune system due to poorly controlled HIV.5 Living with an HIV and TB coinfection provides advantages to both pathogens and leads to acceleration of both diseases, which may lead to death.6 For this reason, all persons with HIV should be evaluated for active or latent TB infection at the time of HIV diagnosis, regardless of their environmental risk for TB infection.
For patients with latent TB infections, one preferred therapy includes the 3HP regimen: rifapentine (weight-based dosing) by mouth once weekly, isoniazid 15 mg/kg by mouth once weekly (900 mg maximum), and pyridoxine 50 mg by mouth once weekly for 12 weeks.7 This regimen is recommended only for virally suppressed patients who are receiving efavirenz, raltegravir, or once-daily dolutegravir–based ART.7 Another preferred therapy is called the 3HR regimen, which consists of isoniazid 300 mg by mouth daily, rifampin 600 mg by mouth daily, and pyridoxine 25 mg to 50 mg by mouth daily for 3 months. Some of these medications require dosing adjustments when used in combination with antiretroviral (ARV) drugs (see TABLE 1).7
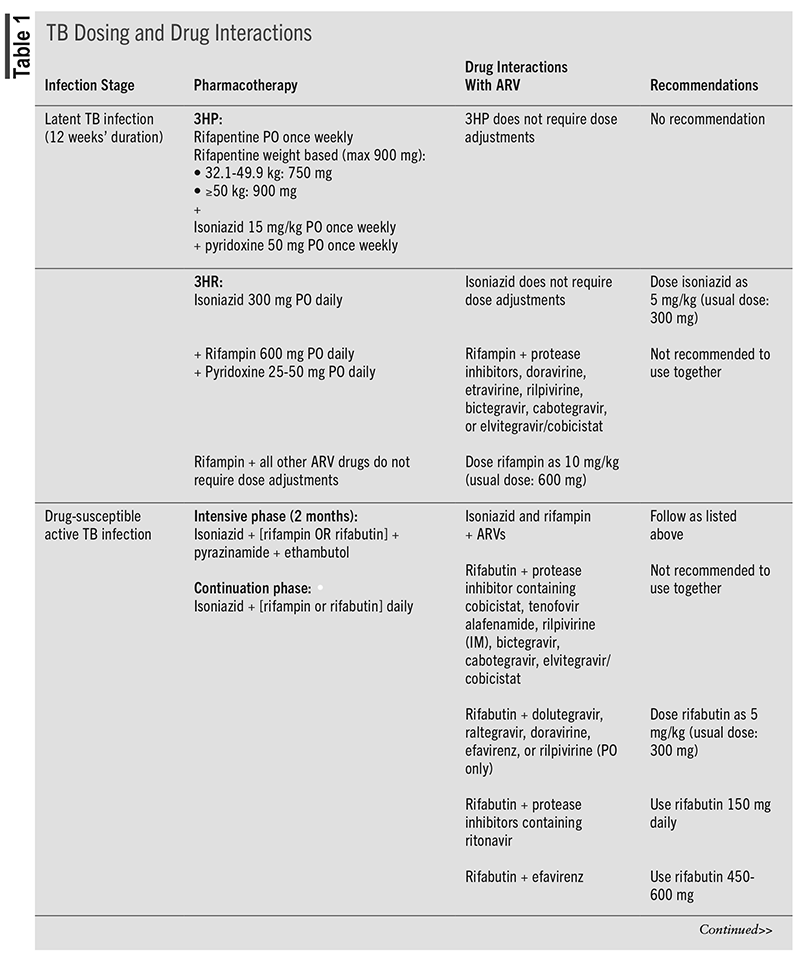
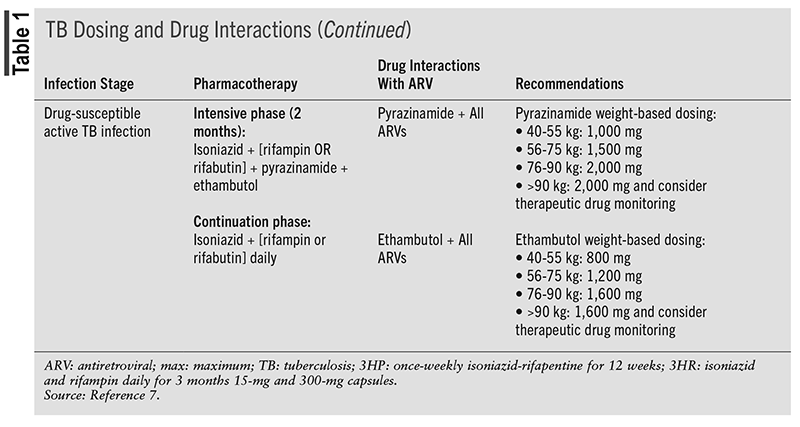
Standard therapy for the treatment of active TB for patients living with HIV and drug-susceptible TB consist of 2 months of isoniazid, rifampin or rifabutin, pyrazinamide, and ethambutol in the intensive phase, followed by 4 months of isoniazid and rifampin in the continuation phase. If a drug-susceptibility report reveals that there is sensitivity to isoniazid and rifampin, then ethambutol can be discontinued. Similarly, pharmacists must note some challenges that may arise with TB treatment, such as drug interactions and drug resistance.7
Hepatitis B Virus
Another common coinfection includes hepatitis B virus (HBV), which should be tested for in patients living with HIV. It is estimated that 8% to 10% of persons living with HIV worldwide have chronic HBV infection. Compared with individuals with HBV infection alone, people with HIV/HBV coinfection can experience an accelerated progression of liver disease, including increased risks for hepatocellular carcinoma, liver-related mortality, and all-cause mortality. Before ART was common, the mortality rate was eight times higher for those with HIV/ HBV coinfection compared with those with HIV alone and 18 times higher compared with those with HBV infection alone. Although this has improved with ART, end-stage liver disease is still a main concern following HBV infection.8,9
HBV is commonly transmitted through percutaneous or mucosal exposure to infectious blood or bodily fluids, including sexual contact and sharing needles, syringes, and other drug-injection equipment during pregnancy or delivery.9 To help prevent infection, patients living with HIV should receive the hepatitis B vaccination series. In any situation where patients feel they may have been exposed to the virus, they should seek medical evaluation to see if postexposure prophylaxis with hepatitis B vaccination and/or immune globulin is recommended. Before starting ART, it is recommended to screen patients for HBV and hepatitis C virus (HCV) infections. If necessary, periodic screenings should also be conducted after the initiation of ART. This is important because managing these coinfections can impact the selection of ART and the potential for drug-induced liver toxicity.10
A combination of tenofovir alafenamide (TAF) plus emtricitabine (FTC) or tenofovir disoproxil fumarate (TDF) plus lamivudine (3TC) or FTC should be used as the nucleoside reverse transcriptase inhibitor backbone of an ARV regimen and for the treatment of both HIV and HBV infection. TAF and TDF selection should be based on consideration of patient-specific factors, such as nephrotoxicity, weight loss, and bone loss. As an alternative, entecavir can be used in addition to a fully suppressive ARV regimen; however, entecavir should not be considered as part of the ARV regimen. Other HBV regimens, such as telbivudine or adefovir, are not recommended.10
Hepatitis C Virus
Like HBV, HCV is another concern in patients living with HIV that should be screened for. The rate of coinfection differ among various risk groups, including people who inject drugs, men who have sex with men (MSM), and high-risk heterosexual individuals. Although transmission via injection- drug use remains the most common mode of HCV acquisition in the United States (62%-80%), sexual transmission is another high transmission route among MSM with HIV who also have risk factors.11 Concomitant infection of HIV with HCV has shown a faster progression of liver injury compared with individuals without both.12
As there are no available recommended vaccines or postexposure prophylaxis to prevent HCV infection, patients should be counseled on prevention and safe practices, such as avoiding the reuse or sharing of needles. Appropriate ART in the acute, or early, phase of contracting HCV prevents further hepatocellular injury and inflammation commonly seen in the chronic, or long-term, phase of HCV. Preventing and controlling HCV infection with treatment improve patient outcomes and decrease the likelihood of ARV-associated, drug-induced liver injury.
Patients with any HCV genotype who have never been infected or treated for HCV and who do not have cirrhosis may be treated with three glecaprevir 100-mg/pibrentasvir 40-mg tablets daily for 8 weeks or one sofosbuvir 400-mg/velpatasvir 100-mg tablet daily for 12 weeks.13 However, if patients living with HIV contract HCV, preferred antiviral regimens are one elbasvir 50-mg/grazoprevir 100-mg tablet daily for 12 weeks, three glecaprevir 100-mg/pibrentasvir 40-mg tablets daily for 8 weeks, or one sofosbuvir 400-mg/velpatasvir 100-mg tablet daily for 12 weeks.14 Like TB drugs, pharmacists should also be mindful of drug interactions and dosage recommendations with coexistent medications.12
Sexually Transmitted Infections
Persons living with HIV have an elevated risk of contracting sexually transmitted infections (STIs). For this reason, these patients must be screened regularly and be counseled on prevention.15 Some common STIs include syphilis, gonorrhea, and chlamydia. Treatment for these STIs is similar whether or not a patient has HIV (see TABLE 2).

OPPORTUNISTIC INFECTIONS
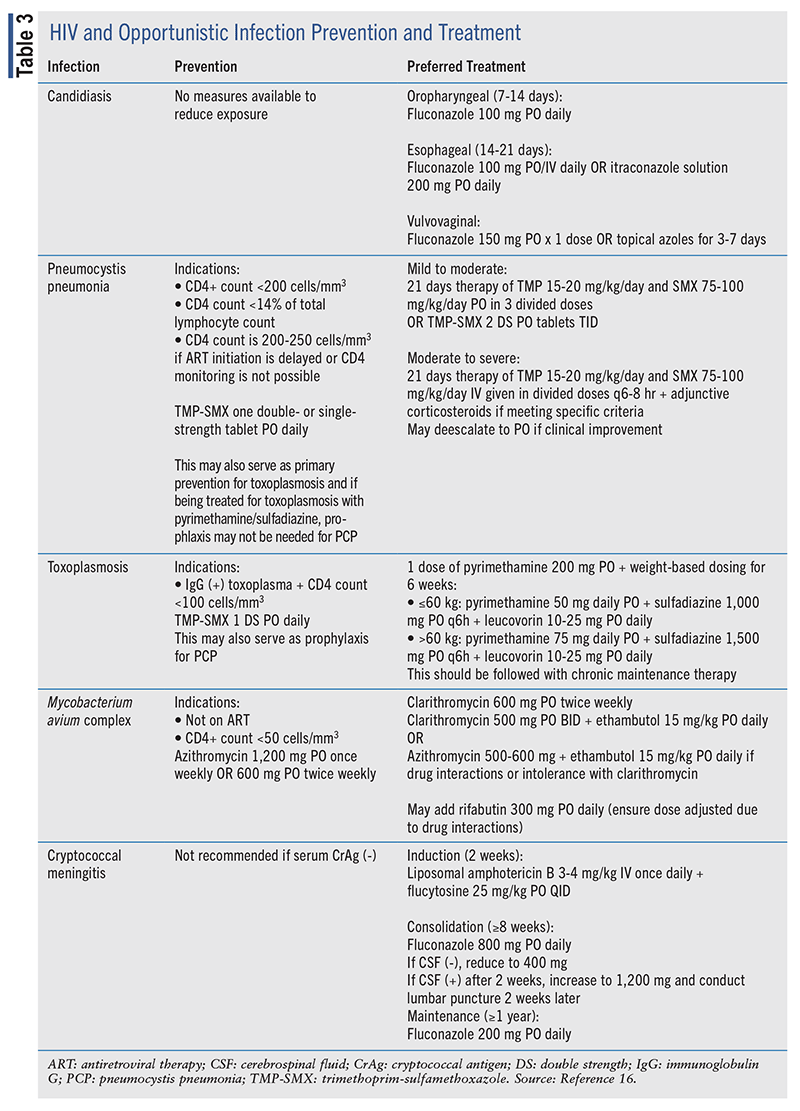
Opportunistic infections (OIs) are defined as infections that become more frequent or severe due to the weakened immune system of an individual with an HIV infection. Selected opportunistic infections include candidiasis, pneumocystis pneumonia (PCP), toxoplasmosis, Mycobacterium avium complex (MAC), and cryptococcal meningitis. To help prevent morbidity and mortality of OIs, clinicians should work with individuals with HIV to achieve and maintain viral suppression. For patients who are unable to sustain immunity and viral suppression, it is important to provide sufficient prevention and treatment options to enhance outcomes (see TABLE 3).16
Candidiasis
There are various locations in the body where a person living with HIV may contract candidiasis, including the oropharyngeal, esophageal, and vulvovaginal areas.
To treat oropharyngeal candidiasis, oral fluconazole is the preferred therapy due to its efficacy, tolerability, and minimal drug interactions. If not, miconazole 50-mg mucoadhesive buccal tablets once daily, clotrimazole 10-mg troches by mouth five times daily, nystatin suspension, itraconazole 200-mg solution by mouth, or posaconazole 400-mg suspension twice daily on Day 1 and then daily thereafter may be used as alternative treatment options.
If the infection is in the esophageal area, oral/intravenous fluconazole and oral itraconazole solution are preferred treatment options due to their efficacy. Alternative treatment options include oral isavuconazole with varying loading and maintenance doses for 2 weeks, oral/intravenous voriconazole 200 mg twice daily, or intravenous amphotericin B (deoxycholate or lipid formulations).
Patients with vulvovaginal candidiasis may be treated with the preferred oral fluconazole or topical azoles, such as clotrimazole and miconazole, for 3 to 7 days. Alternative therapy includes oral itraconazole solution 200 mg for 3 to 7 days or vaginal boric acid 600-mg suppository daily for 14 days. Severe recurrent vaginitis should be treated with 100- to 200-mg oral fluconazole or topical antifungals for 7 days or longer.16
Pneumocystis Pneumonia
PCP is caused by Pneumocystis jirovecii, a ubiquitous fungus, and is spread by the airborne route. PCP has declined since the increased use of prophylaxis and ART. However, it remains a considerable risk for people who are unaware of an HIV infection, patients who are not receiving ongoing HIV care, or those with advanced immunosuppression with a CD4+ less than 100 cells/mm3.16
Primary prevention should be started for adults and adolescents living with HIV with a CD4+ count less than 200 cells/mm3 , a CD4+ cell percentage <14% of total lymphocyte count, or a CD4+ count between 200 and 250 cells/mm3 if ART initiation is delayed or CD4+ monitoring is not possible. The preferred primary prophylaxis is trimethoprim/sulfamethoxazole (TMP-SMX), as outlined in TABLE 3. Alternative therapy may include TMP-SMX double-strength tablets by mouth three times weekly or pentamidine, atovaquone, and dapsone as monotherapy or in combination with pyrimethamine and/or leucovorin. Primary prevention may be discontinued if the CD4+ count increases to greater than 200 cells/mm3 for 3 or more months in response to ART or when the CD4+ count is between 100 and 200 cells/mm3 in addition to an HIV viral count remaining below detection for 3 to 6 months.16
If an individual is infected with PCP, the preferred therapy for a moderate-to-severe infection is a 21-day course of intravenous TMP-SMX with adjunctive corticosteroids if indicated. The intravenous formulation may be switched to oral formulation if there is clinical improvement. Alternatively, patients may be treated with intravenous pentamidine 4 mg/kg once daily. In the event of toxicities, the dose may be reduced to pentamidine 3 mg/kg IV once daily. Alternatively, dapsone, primaquine, and atovaquone may also be used for mild-to-moderate infections.16 As a reminder to all clinicians, a patient should be assessed for a glucose-6-phosphate dehydrogenase (G6PD) deficiency before initiating dapsone or primaquine.
Toxoplasmosis
The protozoan Toxoplasma gondii causes toxoplasmic encephalitis (TE) infection. Primary infection can occur after consuming undercooked meat containing tissue cysts or ingesting oocysts exposed to cat feces. Patients must be counseled to avoid raw or uncooked meat and shellfish and wash their hands after contact with raw meat and cat litter or gardening. It is also advisable for patients to thoroughly rinse fruits and vegetables before consuming them in their raw state.
Following an initial diagnosis of HIV, individuals should be tested for immunoglobulin G antibodies for toxoplasma. Seropositive patients with a CD4+ count of less than 100 cells/μL should receive prophylaxis against TE. Patients vulnerable to toxoplasmosis are also at a heightened risk of developing PCP due to a low CD4+ count and should be prescribed PCP prophylaxis as well (see TABLE 3). Prophylaxis may be discontinued if the CD4+ count increases above 200 cells/mm3 for over 3 months in response to ART or if the CD4 count is between 100 and 200 and the viral load is below detectable limits for 3 to 6 months.
If a patient is infected with TE, the preferred treatment regimen includes pyrimethamine, sulfadiazine, and leucovorin. After the acute infection is treated, chronic maintenance therapy should be continued with pyrimethamine 25 mg to 50 mg by mouth daily, sulfadiazine 2,000 mg to 4,000 mg by mouth daily (in 2 to 4 divided doses), and leucovorin 10 to 25 mg by mouth daily. The discontinuation of chronic maintenance therapy is considered once the patient shows no symptoms and the CD4+ count stays above 200 cells/mm3 for more than 6 months in response to ART.16
Mycobacterium Avium Complex
MAC infection rates are more prevalent in persons with HIV who have a CD4+ cell count of less than 50 cells/mm3, RNA viral levels greater than 1,000 copies/mL, or previous or concurrent OIs. It is believed that contraction occurs through inhaling, ingesting, or exposure to MAC bacteria in the respiratory or gastrointestinal tract.
To help prevent MAC disease, individuals with a CD4+ count below 50 cells/mm3 who are not on ART or who continue to have detectable viral levels on ART without access to fully suppressive regimens should undergo primary prophylaxis. The preferred primary prophylaxis is azithromycin or clarithromycin.
If MAC is contracted, the initial treatment should include two or more antimycobacterial drugs to prevent or delay resistance. These regimens include clarithromycin or azithromycin plus ethambutol (see TABLE 3). In some cases, due to prescriber preference, increased mortality risk, drug resistance, high mycobacterial loads, or ineffective ARV therapy, a third agent like rifabutin is added. If patients are not on ART, regimens should be started as soon as possible at the same time as initiation of antimycobacterial therapy to improve outcomes.16
Cryptococcal Meningitis
Cryptococcal infections are more likely to occur in persons with HIV who have a CD4+ cell count of less than 100 cells/mm3. In persons living with HIV, a cryptococcal infection presents as subacute meningitis or meningoencephalitis. Patients who are newly diagnosed with HIV who have no signs of meningitis should be screened with serum cryptococcal antigen testing if CD4+ counts are less than 100 cells/mm3. If this test is positive, the patient should undergo further cerebrospinal fluid (CSF) evaluation.
If a patient is diagnosed with cryptococcal meningitis, he or she must undergo treatment in three phases: induction, consolidation, and maintenance. The induction phase consists of 2 weeks of therapy with liposomal amphotericin with flucytosine. Flucytosine is renally dosed, and serum concentrations should be monitored 2 hours after 3 to 5 doses have been administered. After successful induction treatment, the patient should move on to consolidation therapy, which involves taking oral fluconazole at a dose of 800 mg daily for at least 8 weeks. If the patient remains clinically stable and has negative CSF cultures after 2 weeks of induction, the dosage may be decreased to 400 mg once daily. However, if there is a positive CSF culture, the dose may be increased to 1,200 mg and lumbar puncture must be conducted 2 weeks later. This phase should last 8 weeks from the time of a negative CSF culture. After the consolidation therapy, maintenance therapy includes fluconazole for 1 year from initiation of antifungal treatment. Patients can be considered to have completed therapy if they remain asymptomatic from a cryptococcal infection and have a CD4+ count greater than 100 cells/mm3 with suppressed RNA viral load in response to ART.16
CARDIOVASCULAR AND RENAL COMPLICATIONS OF HIV
Individuals who live with HIV, even those treated with ART, possess a higher risk of cardiovascular complications when compared with the general population. Cardiovascular complications can present themselves through a multifaceted mechanism. Typically, HIV leads to a dysregulated immune system and chronic inflammation, causing activated leukocytes, such as monocytes, and cytokines, such as interleukin 6 (IL-6), to be actively expressed in the body.17 In the case of HIV, a chronic, lifelong condition, this chronic inflammatory state can precipitate endothelial cell injury, leading to atherosclerosis and plaque buildup. It is important to note that active monocytes express tissue factor, a protein linked to thrombin formation and, eventually, clot formation. This procoagulant state can increase the risk of complications such as venous thromboembolism. Studies have shown that elevated levels of inflammatory biomarkers such as IL-6, C-reactive protein (CRP), and D-dimer are typically higher in those affected with HIV.17 Those treated with current ART also exhibit elevated levels of these biomarkers when compared with those not living with HIV; however, these levels decrease when initiated on appropriate therapy. Although current guidelines do not recommend anticoagulation treatment for these individuals for the prevention of myocardial infarction or stroke, it is necessary to routinely monitor patient-specific risk factors in those who live with HIV.
Renal complications are common in those with HIV, with a prevalence ranging from 2.4% to 17%.18 The mechanism by which HIV affects renal cells is unclear; however, a proposed mechanism includes cell-to-cell interaction with helper T cells. Those who have untreated HIV present symptoms of azotemia and proteinuria. One of the common renal complications patients present with is HIV-associated immune-mediated glomerulonephritis, wherein the virus affects tubular and glomerular epithelial cells.18 Microscopic kidney examinations show electron-dense deposits similar to findings in individuals with lupus-induced renal disease or immunoglobulin A (IgA) nephropathy.18 Another common renal complication in those affected with HIV is HIV-associated thrombotic microangiopathy.18 Patients present with symptoms of proteinuria, hematuria, hemolytic anemia, hemolytic thrombocytopenia, and rapid organ damage.18 It is typically diagnosed with the confirmation of schistocytes or erythrocyte fragments on a blood smear.18 Long-term health complications rise with untreated HIV, and thus initiation of appropriate ART provides better quality of life and survival outcomes.
THE ROLE OF THE PHARMACIST
Pharmacologic Considerations for Patients
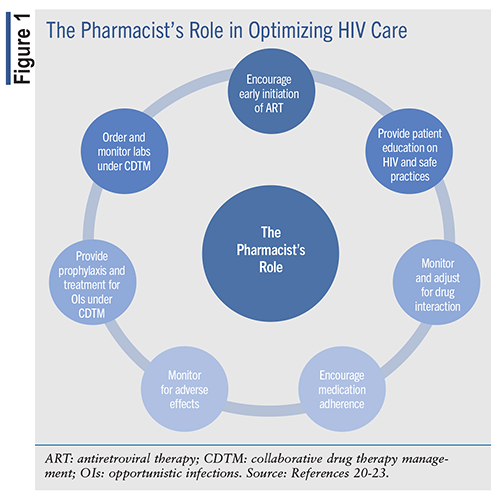
Patients presenting to visits for HIV care must be assessed for a complete medical history, physical examination, and laboratory evaluation and should be counseled regarding the implications of HIV infection.19 Pharmacists must ensure that early initiation of ART is prioritized to reduce morbidity and mortality and to prevent the transmission of HIV and other comorbidities to others. Pharmacists should encourage patients to initiate therapy on the day of diagnosis since there may be a delay in care or follow-up in future visits. Once initiated, pharmacists must assess significant drug interactions with other concomitant medications the patient may be taking (see FIGURE 1). Along with monitoring for medication side effects, obtaining proper care is essential. These same recommendations should be used in the treatment of coinfections and complications. However, the prevention of these complications must be practiced first.
Medication Adherence and Education
Informing and educating patients about their medications are key elements to providing the best care and managing HIV and its coinfections and complications effectively. First, pharmacists should inform patients about the significance of adhering to their medication regimens to maintain therapeutic drug concentrations and prevent coinfections. Poor adherence increases the risk of subtherapeutic drug concentrations that may lead to HIV resistance and a decrease in the CD4+ count.
A study conducted with Kaiser Permanente in Caifornia compared different clinic team structures to determine which group of healthcare team members would increase adherence. The highest adherence increases in a year were attributed to a team comprised of a clinical pharmacist, a social worker/benefits coordinator, and a non–HIV-specialized primary care provider. Another study conducted by the same institution evaluated refill adherence at 24 months, which was significantly higher in patients seen by HIV clinical pharmacists.20 Multiple studies show that screening barriers to adherence, motivational interviewing, OI prophylaxis, pharmaceutical care (management of side effects and drug interactions, medication reconciliation), and providing handouts to patients significantly improved patient adherence.21 Pharmacist intervention in care teams has positively impacted patient care and health outcomes.
Enhancing adherence requires pharmacists to discuss adherence strategies before initiating and making changes in ARV therapy. Medication adherence should be a consistent topic during each follow-up visit, where ongoing exploration of adherence strategies is identified. Pharmacists may implement strategies such as switching to once-daily regimens when possible, utilizing telehealth and technological advancements, and providing financial assistance programs for ARV medications.20,21 Another strategy includes motivational interviewing, where pharmacists can channel the patient's own motivation to direct behavioral changes toward long-term treatment and nonpharmacologic implementations.22 Once patients discover their motivation to improve medication adherence, the medication can effectively suppress the viral load and reduce the risk of immunodeficiency and associated coinfections.
In addition to adherence counseling, pharmacists can provide personalized education on HIV as a chronic condition, treatment, prophylaxis for OIs, and treatment of coinfections and complications.20 Additionally, patients need guidance on safe practices to prevent transmission and prevent coinfections, empowering them to take control of their health.
Finally, some pharmacists are certified in HIV care and may work under collaborative drug therapy management (CDTM) agreements. CDTM agreements allow pharmacists to practice under collaborative practice and become more involved in direct patient-care management. Establishing CDTM protocols with physicians empowers pharmacists to choose and initiate ART or OI prophylaxis. They can also take charge of ordering and interpreting relevant laboratories to monitor the efficacy or toxicity of a regimen, simplify regimens using fixed-dose combination tablets, and manage common ART-related side effects like nausea and diarrhea.20 These pharmacists should ensure appropriate supportive treatment and switch to alternative ART regimens if indicated.20
Pharmacists are the most accessible healthcare professionals. Individuals in urban areas live within 2 miles of a pharmacy and individuals in rural areas live within 15 miles of a pharmacy in the U.S.24 Due to this improved patient accessibility, pharmacists have an obligation to stay updated on the latest guidelines and research to ensure that the care provided remains current and aligned with the various evolving aspects of HIV management. The pharmacist's role in promoting a comprehensive, patient-centered care approach is vital to the prevention and treatment of coinfections and complications in HIV care. Obtaining patient consent to monitor laboratory values such as HIV viral load and drug resistance test results, CD4+ counts, and failed drug regimens can be beneficial for individualized and comprehensive care under these agreements.24 In addition, pharmacists can provide supportive care by offering immunizations and engaging in collaborative models locally or statewide for HIV testing, reminder tools, and adherence packaging.24
CONCLUSION
HIV is a significant cause of morbidity and mortality, especially in the setting of coinfections and complications. Initiating ART early is essential to proactively prevent these coinfections and complications. In cases of severe immunosuppression where ART initiation is delayed or ineffective, pharmacists must use their expertise in pharmacotherapy to provide patient-centered therapeutic options to manage HIV and its coinfections and complications. Prevention and treatment of tuberculosis, hepatitis, STIs, and OIs must be emphasized to provide holistic, comprehensive care to each patient. Cardiovascular and renal complications are common in patients with HIV and should be adequately monitored by pharmacists. Furthermore, pharmacists can provide patient education, help patients find personalized medication adherence strategies, and monitor adverse effects, laboratory values, and drug interactions to optimize treatment regimens.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
- NIH. HIV and AIDS: the basics. 2021. https://hivinfo.nih.gov/understanding-hiv/fact-sheets/hiv-and-aids-basics. Accessed January 15, 2024.
- Battistini Garcia SA, Guzman N. Acquired immune deficiency syndrome CD4+ count. [Updated 2023 Aug 14]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
- World Health Organization. HIV data and statistics. 2023. www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/strategic-information/hiv-data-and-statistics. Accessed January 15, 2024.
- CDC. AIDS and opportunistic infections. 2019. www.cdc.gov/hiv/basics/livingwithhiv/opportunisticinfections.html. Accessed January 15, 2024.
- Camuto A. HIV and co-infections. amfAR, The Foundation for AIDS Research. March 29, 2022. www.amfar.org/treat-asia/hiv-and-co-infections/#:~:text=In%20addition%20to%20causing%20serious. Accessed January 15, 2024.
- Yang Q, Han J, Shen J, et al. Diagnosis and treatment of tuberculosis in adults with HIV. Medicine (Baltimore). 2022;101(35):e30405.
- NIH. Mycobacterium tuberculosis infection and disease. https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/mycobacterium-0. Accessed January 15, 2024.
- Corcorran MA, Kim N. Chronic hepatitis B and HIV coinfection. Top Antivir Med. 2023;31(1):14-22.
- CDC. Hepatitis B. March 9, 2023. www.cdc.gov/hepatitis/hbv/index.htm#:~:text=Hepatitis%20B%20Information&text=This%20can%20happen%20through%20sexual. Accessed January 15, 2024.
- NIH. Hepatitis B virus/HIV coinfection. clinicalinfo.hiv.gov. September 21, 2022. https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/hepatitis-b-virus-hiv-coinfection?view=full. Accessed January 15, 2024.
- CDC. Epidemiology and prevention of HIV and viral hepatitis co-infections. 2020. www.cdc.gov/hepatitis/populations/hiv.htm.
- NIH. Hepatitis C virus/HIV coinfection. clinicalinfo.hiv.gov. March 23, 2023. https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/coinfections-hepatitis-c-virus-hcv?view=full.
- Simplified HCV treatment for treatment-naive patients without cirrhosis. www.hcvguidelines.org.www.hcvguidelines.org/treatment-naive/simplified-treatment. Accessed January 15, 2024.
- HCV Guidance. Patients with HIV/HCV coinfection. www.hcvguidelines.org. Accessed January 15, 2024.
- Walensky R, Jernigan D, Bunnell R, et al. Sexually Transmitted Infections Treatment Guidelines, 2021. Morb Mort Wkly Rep. www.cdc.gov/std/treatment-guidelines/STI-Guidelines-2021.pdf. Accessed January 15, 2024.
- NIH, CDC, HIV Medicine Association, Infectious Diseases Society of America. Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-opportunistic-infection. Accessed January 15, 2024.
- Perkins MV, Joseph SB, Dittmer DP, Mackman N. Cardiovascular disease and thrombosis in HIV infection. Arterioscler Thromb Vasc Biol. 2023;43(2):175-191.
- Yussuf FM, Barbarawi A, Nor MA, et al. A systematic review exploring the range of renal complications of human immunodeficiency virus. Cureus. 2023;15(3):e36755.
- NIH. Baseline evaluation. https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/baseline-evaluation?view=full. Accessed January 15, 2024.
- Cocohoba, J. The pharmacist’s role in HIV care. In W. David Hardy, ed. Fundamentals of HIV Medicine. September 1, 2023.
- Adherence to the Continuum of Care. Clinicalinfo.HIV.gov.clinicalinfo.hiv.gov. March 23, 2023. https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/adherence-continuum-care?view=full. Accessed January 15, 2024.
- Hogan A, Catley D, Goggin K, et al. Mechanisms of motivational interviewing for antiretroviral medication adherence in people with HIV. AIDS Behav. 2020;24:2956-2965.
- McCree DH, Byrd KK, Johnston M, et al. Roles for pharmacists in the "Ending the HIV Epidemic: A Plan for America" initiative. Public Health Reports. 2020;135(5):547-554.
- Myers JE, Farhat D, Guzman A, Arya V. Pharmacists in HIV prevention: an untapped potential. Am J Public Health. 2019;109:859-861.