A Clinical Overview of DPP-4 Inhibitors for Type 2 Diabetes
RELEASE DATE:
October 1, 2018
EXPIRATION DATE:
October 31, 2020
FACULTY:
Michelle Jeon, PharmD
Assistant Professor of Pharmacy Practice
St. Louis College of Pharmacy
Shared Faculty/Clinical Pharmacist
Walgreens Pharmacy
Roxane L. Took, PharmD, BCACP
Assistant Professor of Pharmacy Practice
St. Louis College of Pharmacy
Ambulatory Shared Faculty Pharmacist
Schnuck Markets, Inc.
Golden L. Peters, PharmD, BCPS
Associate Professor of Pharmacy Practice
St. Louis College of Pharmacy
Clinical Pharmacy Specialist, Primary Care
Veterans Affairs St. Louis Health Care System,
John Cochran Division
St. Louis, Missouri
FACULTY DISCLOSURE STATEMENTS:
Drs. Jeon, Took, and Peters have no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT:
Pharmacy
 Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 430-0000-18-049-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE:
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER:
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients' conditions and possible contraindications or dangers in use, review of any applicable manufacturer's product information, and comparison with recommendations of other authorities.
GOAL:
To give the pharmacist a clinical and pharmacotherapeutic overview of dipeptidyl peptidase-4 (DPP-4) inhibitors available in the United States for treatment of type 2 diabetes.
OBJECTIVES:
After completing this activity, the participant should be able to:
- Describe DPP-4 inhibitors' mechanism of action in lowering blood glucose concentrations in patients with type 2 diabetes.
- Identify class and individual glycemic effects of DPP-4 inhibitors in the treatment of type 2 diabetes.
- Recall significant clinical benefits of DPP-4 inhibitors and recognize potential adverse effects that may occur with initiation of sitagliptin, saxagliptin, linagliptin, or alogliptin.
- Discuss DPP-4 inhibitors' role as add-on therapy in patients with type 2 diabetes.
ABSTRACT: Since the approval of the first dipeptidyl peptidase-4 (DPP-4) inhibitor in the United States in 2006, the use of these agents as second-line oral antihyperglycemic therapy in type 2 diabetes management has been established. Several clinical trials and meta-analyses have been conducted to identify potential additional benefits and risks associated with each DPP-4 inhibitor. In general, DPP-4 inhibitors are effective at promoting moderate lowering of A1C and are associated with few adverse events. Although there is evidence that DPP-4 inhibitors confer cardiovascular safety and renoprotection, the potential risks of hospitalization for heart failure and acute pancreatitis are disadvantages to their use as add-on therapy.
Dipeptidyl peptidase-4 (DPP-4) inhibitors were first investigated in 2002 for the management of hyperglycemia in patients with type 2 diabetes.1 There are four FDA-approved single-entity DPP-4 inhibitors: sitagliptin (the first agent; approved in 2006), saxagliptin, linagliptin, and alogliptin. (Another DPP-4 inhibitor, vildagliptin, is widely prescribed in Europe but is not available in the U.S.) These agents are also available in combination with other diabetes medications (i.e., metformin, empagliflozin, pioglitazone, dapagliflozin, ertugliflozin).2 DPP-4 inhibitors are one of the two incretin-based medication classes; the other class, glucagon-like peptide-1 (GLP-1) receptor agonists, will be discussed only briefly in this article.
Mechanism of Action
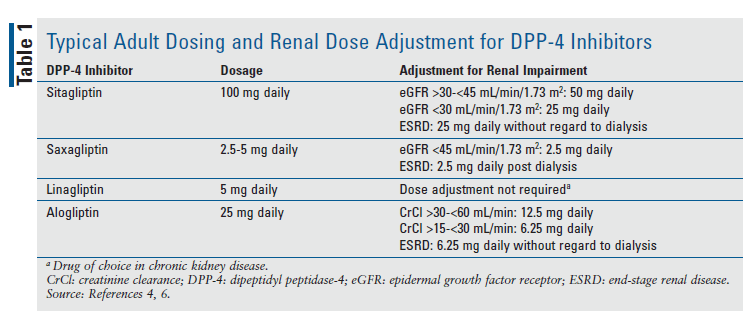
In a healthy person, incretins such as GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) are released by the intestines after food is ingested. When blood glucose (BG) concentrations are normal or high, the intestines secrete GLP-1 and GIP to act on pancreatic beta cells through intracellular signaling pathways that involve cyclic adenosine monophosphate (cAMP). The action of GLP-1 and GIP on the pancreas causes a reduction in glucagon secretion that results in diminished hepatic glucose production.3,4 These incretins are rapidly inactivated by DPP-4, an enzyme expressed throughout the vascular endothelial cells, venous capillary beds, gut, liver, lungs, and kidneys.5 Medications that inhibit DPP-4 work by slowing the degradation of incretins, subsequently sustaining GLP-1 and GIP activity. Because DPP-4 inhibitors work in a glucose-dependent manner, they do not usually cause hypoglycemia. DPP-4 inhibitors have a half-life of 12.4 to 24 hours and are dosed once daily.4 TABLE 1 summarizes typical dosing for adults and adjustments for renal disease.4,6
Efficacy
A1C-Lowering Effects: Compared with other oral antihyperglycemic agents, DPP-4 inhibitors are considered to have intermediate glycemic efficacy. In phase III trials, these agents have demonstrated an average A1C decrease of about 0.5% when administered as monotherapy. Sitagliptin and alogliptin may have slightly greater A1C-lowering effects than saxagliptin and linagliptin; studies have shown a 0.6% A1C decrease after approximately 6 months of sitagliptin or alogliptin therapy, compared with 0.5% for saxagliptin and 0.4% for linagliptin.7-10 It should be noted that these studies involved patients with an average baseline A1C of 8%, so results may be variable in clinical practice.
DPP-4 inhibitors appear to have additive A1C-lowering effects in combination with other oral agents used to treat diabetes. Efficacy studies have demonstrated this additive effect with metformin, sulfonylureas, and thiazolidinediones given in combination with saxagliptin and linagliptin.11,12
Glucose-Lowering Effects: DPP-4 inhibitors effectively reduce both fasting and postprandial glucose levels. However, because of their glucose-dependent mechanism of action, these agents tend to have a more significant effect on postprandial glucose than on fasting BG (–40 mg/dL vs. –11 mg/dL, respectively).13 As a result, initiation of therapy may be more beneficial for patients who require greater glycemic control throughout the day than during the night.
Beta-Cell Function: Pancreatic beta-cell function and diabetes progression have an inverse relationship. Because beta-cell function is essential for proper intrinsic insulin response to increased BG levels, therapies that have the potential to improve cell function may help slow the progression of insulin resistance.14 A 2017 meta-analysis of 52 randomized, controlled trials that evaluated DPP-4 inhibitors' effects on beta-cell function found that, in monotherapy studies, all agents except linagliptin resulted in improvements in cell function. Collective data on the class effect of DPP-4 inhibitor therapy on beta-cell function revealed that monotherapy and combination therapy improved beta-cell function. This analysis also indicated that sitagliptin was the only DPP-4 inhibitor that improved insulin resistance when used as monotherapy.15
Potential Additional Benefits
Several studies have investigated potential additional benefits of DPP-4 inhibitors, including microvascular and macrovascular effects, effects on blood pressure (BP) and weight, and risk of hypoglycemia.
Cardiovascular Safety: In 2008, the U.S. Department of Health and Human Services and the FDA published guidance for pharmaceutical companies that develop and produce antidiabetic medications.16 This statement recommended that sponsors demonstrate the safety of their products for patients with cardiovascular (CV) disease, as most phase II and III trials excluded such patients. As a result, several studies of alogliptin, sitagliptin, and saxagliptin were performed to determine CV safety in high-risk patients.17-19 The CAROLINA and CARMELINA studies of linagliptin demonstrated CV safety, but results have not yet been made available (Clinicaltrials.gov identifiers: NCT01243424 and NCT01897532).
BP-Lowering Effect: The American Diabetes Association (ADA) recommends tight glycemic control and BP reduction to help prevent chronic kidney disease (CKD) in patients with diabetes.20 Sitagliptin is the only DPP-4 inhibitor to show significant reduction of BP. In a prospective cohort study of 36 patients with albuminuria who were initiated on sitagliptin 50 mg daily, patients experienced a significant change in both systolic and diastolic BP that was apparent after 6 months (baseline systolic BP 140 +/– 18 mmHg dropped to 129 +/– 17 mmHg and baseline diastolic BP 77 +/– 10 mmHg fell to 73 +/– 7 mmHg; P <.017).21
All other DPP-4 inhibitors available in the U.S. have neutral effects on BP.21-25 Vildagliptin has neutral-to-positive effects on BP, with results less noteworthy than those for sitagliptin (lowering of 2.7 mmHg for systolic BP and 1.64 mmHg for diastolic BP).26,27
Effect on Weight: Unlike some medications used to treat diabetes, DPP-4 inhibitors are neutral in their effect on weight.23,28,29 In a retrospective observational study of 303 patients who received alogliptin treatment for 1 year or longer, the mean BMI was 25.04 kg/m2 (range: 16.3-41.5 kg/m2) at baseline, and at the conclusion of the study it was 25 kg/m2, indicating no change in weight after 1 year.23 This lack of effect on weight constitutes an advantage of DPP-4 inhibitors over older oral anti-hyperglycemic medications that typically cause a net weight gain, such as sulfonylureas and thiazolidinediones. However, since the approval of DPP-4 inhibitors, other agents for treatment of type 2 diabetes have been developed that have weight-loss properties, including GLP-1 agonists and sodium-glucose cotranporter 2 (SGLT2) inhibitors.
Low Hypoglycemia Risk: A major benefit of DPP-4 inhibitors is their low risk of hypoglycemia. As monotherapy, alogliptin, linagliptin, saxagliptin, and sitagliptin have a 0.6% to 6.6% incidence of hypoglycemia. In clinical studies, linagliptin monotherapy had a 6.6% risk of hypoglycemia versus a 0.6% risk for sitagliptin. The risk of hypoglycemia is highest in patients taking an additional BG-lowering agent, such as insulin or a sulfonylurea; with combination therapy, the incidence increases to about 25%.3,11,12,30
Renoprotective Effects: Patients with diabetes are at increased risk for microvascular complications such as nephropathy, neuropathy, and retinopathy. ACE inhibitors and angiotensin II receptor blockers (ARBs) are currently recommended as first-line treatment in diabetes patients with hypertension to delay the onset and progression of CKD.20 A cross-over study evaluated 12 patients with type 2 diabetes treated with an ARB who were initiated on either alogliptin or sitagliptin. The urine albumin/creatinine ratio (ACR) was lowered by DPP-4 inhibitor therapy, with the most significant change occurring in patients taking alogliptin versus sitagliptin. The effect is thought to be related to renal production of cAMP—a secondary messenger on the GLP-1 receptor signal pathway—and changes in plasma levels of stromal cell–derived factor 1-alpha (SDF-1-alpha), a substrate of DPP-4. Because alogliptin is a more selective DPP-4 inhibitor than sitagliptin, it offers renoprotective benefits by enhancing systemic and renal oxidative advantages independent of lowering A1C.31 Similar ACR-lowering effects have been found in patients with varying levels of albuminuria that had no correlation with race or lowering of A1C and did not affect epidermal growth factor receptor. These renal benefits were more significant in patients with an ACR >300 mg/g.31-33
Prevention of Retinopathy: One of the least-studied potential benefits of DPP-4 inhibitors is the ability to decrease blood flow and increase vasodilation, thereby preventing retinopathy in diabetic patients. A randomized, placebo-controlled, double-blind, crossover trial was conducted in 42 patients with type 2 diabetes.24 Patients had a mean age of 60 years, mean diabetes duration of 4 years, mean A1C of 6.99%, and BP 132/79 mmHg. Retinal capillary flow, which is used to measure the vasodilatory capacity of retinal capillaries in order to identify early vascular remodeling, was assessed at baseline and after 6 weeks of saxagliptin or placebo administration. Baseline retinal capillary flow was lowered significantly (288 +/– 13.2 vs. 314 +/– 14.1; P = .033), indicating improvements in retinal health and reversal of vascular remodeling. There was also a twofold increase in vasodilatory capacity in patients treated with saxagliptin versus placebo, signifying an improvement in the eye vasculature, although the increase was nonsignificant.24 A more recent double-blind, randomized, controlled trial that investigated the microvascular effects of linagliptin in nondiabetic patients with arterial hypertension resulted in a significant improvement in retinal capillary perfusion and retinal arterial blood flow.34 Other significant findings indicative of improvements in diabetic retinopathy have occurred with vildagliptin.35 Clinical data are limited regarding the use of DPP-4 inhibitors to prevent retinopathy; more studies are needed.
Diabetic Wound Healing: Prolonged or poor wound healing can be a serious complication of uncontrolled diabetes. Two studies have been performed to determine DPP-4 inhibitors' effect on wound healing.27,36 In a controlled study, 63 patients with type 2 diabetes and a lower-extremity ulcer were randomized to either saxagliptin 5 mg or placebo daily.36 Patients had a baseline Wagner classification of one of the following: grade 2—ulcer extension to ligament, tendon, joint capsule, or deep fascia without abscess or osteomyelitis; grade 3—deep ulcer with abscess, osteomyelitis, or joint sepsis; or grade 4—gangrene localized to part of forefoot or heel.37 There was a significant improvement in the healed-ulcer rate and time for complete wound closure in the saxagliptin arm independent of glycemic control. It is believed that the DPP-4 enzyme is involved in tissue repair, thereby increasing SDF-1-alpha; this subsequently causes an increase in collagen synthesis, which results in reduced scarring, repair, and regeneration.36,38 A nonsignificant decrease in recurrent ulcers and amputations also was noted.36
The second study was conducted in 106 patients with type 2 diabetes and chronic nonhealing foot ulcers (>3 months) who were treated with vildagliptin.27 This randomized, placebo-controlled trial found a significant decrease in wound area after 3 months of treatment and a significant difference in wound-closure rate at week 12 (31% vs. 15%). After 12 weeks of treatment, a significant increase in granulation tissue was also observed. This outcome is thought to be an effect of all medications that increase GLP-1, because of both an increase in SDF-1-alpha and capillary density due to increased hypoxia-inducible factor 1-alpha and vascular endothelial growth factor.27
Potential Negative Effects
DPP-4 inhibitors are generally well tolerated, with adverse events occurring in fewer than 10% of patients. The most common side effects for all agents in this drug class are nasopharyngitis, headache, and upper respiratory tract infection. Saxagliptin may also cause urinary tract infections and peripheral edema (3.6% and 6.8%, respectively).11 Postmarketing case studies have reported a rare but serious autoimmune reaction—bullous pemphigoid—after initiation of DPP-4 inhibitor therapy.39,40 This skin condition, which is characterized by erythematous fluid-filled blisters around flexural areas of the body, should result in medical treatment and immediate discontinuation of the drug.
Heart Failure: In 2013, the SAVOR-TIMI trial reported a significantly increased risk of hospitalization for heart failure (HF) in patients taking saxagliptin. This study of more than 16,400 subjects found a 27% increased rate of hospitalization for acute HF after approximately 2 years of saxagliptin therapy (P = .007).17
Following SAVOR-TIMI, several studies were performed to determine whether increased risk of HF exacerbation was a class effect. The EXAMINE trial (2015) showed that patients receiving alogliptin had a higher incidence of HF-related hospitalizations versus placebo, but this outcome did not attain statistical significance.18 Nevertheless, the 2018 ADA Standards of Care lists alogliptin as having a potential HF risk.20
TECOS (2015) did not show an increased risk of HF in patients taking sitagliptin for treatment of type 2 diabetes.19 Nevertheless, in 2017, the FDA added an warning to Januvia's package insert that advised prescribers to weigh the risks and benefits of sitagliptin in patients at risk for HF before initiating therapy.3 The potential CV and renal effects of linagliptin have been investigated in the CAROLINA and CARMELINA trials; however, as noted earlier, findings have not yet been published.
In these recent studies, the potential risk of hospitalization for HF was significantly increased only in patients taking saxagliptin. Therefore, HF should not be considered a class effect for DPP-4 inhibitors. However, as a precaution, patients with risk factors should be monitored for signs of new-onset HF at each follow-up appointment.41
Pancreatitis: All DPP-4 inhibitors available in the U.S. have reported postmarketing cases of acute fatal and nonfatal pancreatitis. As a result, several post hoc analyses have investigated the risk of developing pancreatitis as a result of DPP-4 inhibitor therapy. A pooled analysis of 22 studies examining linagliptin therapy revealed only two events of acute pancreatitis, neither of which was fatal.42 SAVOR-TIMI reported that more patients treated with saxagliptin were diagnosed with acute pancreatitis compared with those receiving placebo, but the difference was not significant (relative risk [RR], 1.87; 95% CI, 0.84-4.20).17 EXAMINE and TECOS similarly found an increased incidence of pancreatitis (RR, 1.49; 95% CI, 0.61-3.63 and RR, 1.92; 95% CI, 0.96-3.85, respectively).18,19 Although individually these three studies yielded nonsignificant results, a combined analysis suggested a significantly increased RR of 79% and an absolute risk of 0.13% for acute pancreatitis (RR, 1.79; 95% CI, 1.13-2.81).43
Although causative effect has not been established, DPP-4 inhibitor therapy should be discontinued immediately if a patient presents with signs of pancreatitis, such as severe abdominal pain, nausea, vomiting, and weight loss.3,30
Arthralgia: In phase III clinical trials, patients receiving linagliptin and metformin combination therapy had a higher rate of patient-reported arthralgia than patients receiving sulfonylurea and metformin combination therapy (8.1% vs. 6.1%).44 Subsequently, there have been postmarketing reports of severe cases of joint pain in patients receiving DPP-4 inhibitor therapy. Case reports have indicated that development of severe or disabling arthralgia may occur after days or years following of DPP-4 inhibitor initiation.
Laboratory-Value Effects: Saxagliptin may decrease absolute lymphocyte count. This effect seems to be dose-related, as patients receiving 5 mg daily showed a mean reduction of about 100 cells/mcL, and those receiving 2.5 mg daily did not have a decrease in lymphocyte count.11
Place in Therapy
In the ADA's 2018 Standards of Medical Care in Diabetes, DPP-4 inhibitors are indicated as an add-on option for patients without clinical atherosclerotic CV disease (ASCVD) who are unable to reach goal A1C after taking metformin for 3 months.20 In agreement with the ADA, the American Association of Clinical Endocrinologists (AACE) lists DPP-4 inhibitors as an alternative in patients with an A1C under 7.5% in whom the use of metformin is contraindicated; however, GLP-1 receptor agonists and SGLT2 inhibitors are more strongly recommended over DPP-4 inhibitors.45
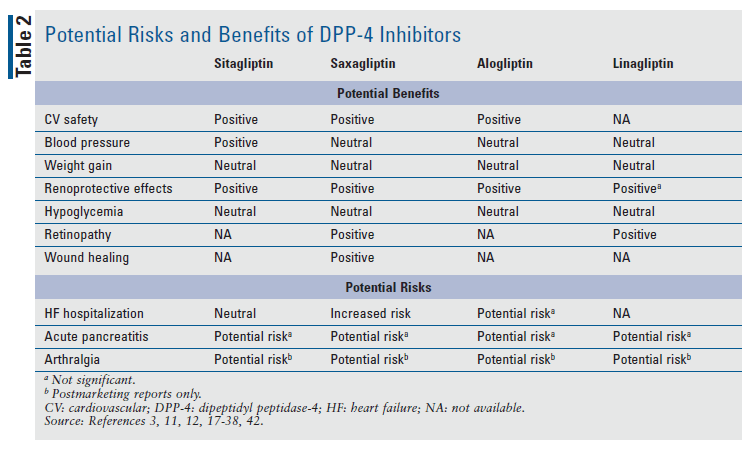
A barrier to DPP-4 inhibitor use has been the lack of evidence of CV risk reduction in patients with clinical ASCVD. SGLT2 inhibitors, such as empagliflozin and canagliflozin, have similar A1C-lowering properties (0.5%-0.8%) and out-of-pocket costs (except for alogliptin, which is available generically), but have the added advantages of CV risk reduction, BP-lowering, and weight loss.45 Products that are a combination of a DPP-4 inhibitor and an SGLT2 inhibitor (sitagliptin/ertugliflozin, saxagliptin/dapagliflozin, and linagliptin/empagliflozin) may be used to further lower A1C and provide increased CV benefit in patients who are not meeting A1C goals on dual therapy. Further research must be conducted on the additional potential benefits of DPP-4 inhibitors before the recommendation for DPP-4 inhibitors over SGLT2 inhibitors or GLP-1 agonists as a second-line agent for patients with type 2 diabetes can be strengthened. TABLE 2 summarizes the potential benefits and risks of each DPP-4 inhibitor.4,6,20,44
Combination of DPP-4 Inhibitors and GLP-1 Agonists: Because both DPP-4 inhibitors and GLP-1 agonists increase incretin, there has been interest in learning whether this combination could result in a synergistic A1C-lowering effect. It has been postulated that DPP-4 inhibition would work with incretin mimetics to increase the half-life and activity of GLP-1 in achieving glucose control.46 However, a switch study examining triple therapy with exenatide, sitagliptin, and metformin found a clinically insignificant additional lowering of A1C (0.3%) after 20 weeks of follow-up.46
Based on a lack of robust data regarding the safety and efficacy of simultaneous use of DPP-4 inhibitors and GLP-1 agonists, the combination is not recommended. The FDA, ADA, and AACE do not support the use of this combination in patients with type 2 diabetes.46
Drug-Drug Interactions: Saxagliptin is a strong CYP3A4 inhibitor, so its labeling recommends limiting saxagliptin to 2.5 mg daily in patients taking other strong CYP3A4 inhibitors, such as ketoconazole, based on the risk of increased saxagliptin levels.11 Linagliptin is a strong CYP3A4 inducer; therefore, its combination with a strong CYP3A4 inducer, such as rifampin, should be avoided if possible.12 If this combination is used, the patient should be closely monitored to determine linagliptin's efficacy. As previously mentioned, because DPP-4 inhibitors can increase the risk of hypoglycemia when added to insulin or a sulfonylurea, a dose decrease of sulfonylurea and/or insulin should be considered prior to initiation of a DPP-4 inhibitor. Slight increases in digoxin may be observed in patients taking sitagliptin 100 mg for 10 or more days; however, the labeling for sitagliptin recommends appropriate monitoring and does not suggest dosage adjustments to either sitagliptin or digoxin.3 No significant CYP450 interactions were observed in clinical trials of alogliptin; therefore, alogliptin is preferred in patients taking CYP450 inhibitors or inducers.6,30
Pregnancy: All DPP-4 inhibitors are considered Pregnancy Category B because safety in humans has not been proven in adequate and well-controlled studies. Insulin is the drug of choice in patients with diabetes who are pregnant.20 However, manufacturers of DPP-4 inhibitors available in the U.S. state that these products may be used if clearly needed to lower BG.3,6,11,12,30
Patient Counseling Points
Sitagliptin, saxagliptin, alogliptin, and linagliptin should be taken once daily and may be given without regard to meals.3,11,12,30 These simple administration instructions may be beneficial for patients who are nonadherent or have a high pill burden.
DPP-4 inhibitors are generally well tolerated and have a low incidence of side effects. Patients may experience headache or cold symptoms, such as sore throat, runny nose, and congestion.3,11,12,30 DPP-4 inhibitors have not been shown to cause weight gain or hypoglycemia unless used in combination with other antihyperglycemic agents, such as insulin or sulfonylureas.20
Patients should be advised to seek medical attention immediately if they experience severe joint pain, severe abdominal pain, increased shortness of breath, or swelling in the legs, as these signs and symptoms could indicate the presence of a serious adverse event.3,11,12,30
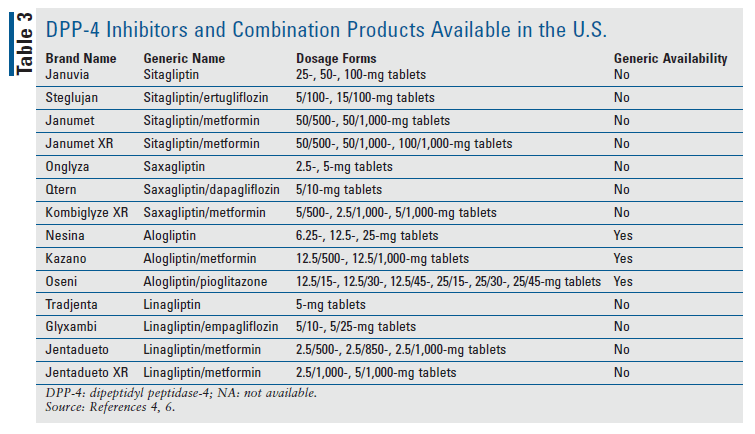
Most agents in this drug class are available in branded form only. Therefore, patients with inadequate or no insurance coverage may have affordability concerns. Alogliptin is currently the only DPP-4 inhibitor in the U.S. that is available generically, which may result in a significant cost savings over its brand-name counterparts. However, these medications are available in combination with metformin or other antihyperglycemic agents, which may provide the advantage of decreased pill burden for patients with uncontrolled diabetes. TABLE 3 outlines the DPP-4 inhibitors and combination products available in the U.S.4,6
Conclusion
In general, DPP-4 inhibitors are effective at promoting moderate lowering of A1C and are associated with few adverse events. These agents are recommended as second-line add-on therapy in patients without clinical ASCVD. DPP-4 inhibitors are not associated with increased CV risk; however, there is a lack of data supporting CV risk reduction in patients with clinical ASCVD. Potential benefits associated with DPP-4 inhibitors include lower risk of hypoglycemia, neutral effect on weight, renoprotective effects, and lowering of BP. Potential negative effects of DPP-4 inhibitors include increased rates of hospitalization for acute HF, pancreatitis, and arthralgia. DPP-4 inhibitors lack significant clinical benefits over newer anti-diabetic agents (i.e., GLP-1 agonists and SGLT2 inhibitors); however, alogliptin offers a potential cost benefit.
REFERENCES
- Deacon CF. A review of dipeptidyl peptidase-4 inhibitors. Hot topics from randomized controlled trials. Diabetes Obes Metab. 2018;20(suppl 1):34-46.
- FDA Drug Safety Communication: FDA warns that DPP-4 inhibitors for type 2 diabetes may cause severe joint pain. www.fda.gov/Drugs/DrugSafety/ucm459579.htm. Accessed July 24, 2018.
- Januvia (sitagliptin) package insert. Whitehouse Station, NJ: Merck & Co, Inc; February 2018.
- Lexi-Drugs Online. Hudson, OH: Lexi-Comp, Inc; 2018. http://online.lexi.com. Assessed July 17, 2018.
- Mulvihill EE, Drucker DJ. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr Rev. 2014;35:992-1019.
- Facts & Comparisons eAnswers. Hudson, OH: Wolter Kluwer; 2018. http://online.factsandcomparisons.com. Assessed July 18, 2018.
- Aschner P, Kipnes MS, Lunceford JK, et al. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006;29(12):2632-2637.
- DeFronzo RA, Fleck PR, Wilson CA, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes and inadequate glycemic control: a randomized, double-blind, placebo-controlled study. Diabetes Care. 2008;31(12):2315-2317.
- Sjöstrand M, Wei C, Cook W, et al. Assessment of saxagliptin efficacy: meta-analysis of 14 phase 2 and 3 clinical trials. Diabetes Ther. 2017;8(3):587-599.
- Del Prato S, Barnett AH, Huisman H, et al. Effect of linagliptin monotherapy on glycaemic control and markers of β-cell function in patients with inadequately controlled type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab. 2011;13(3):258-267.
- Onglyza (saxagliptin) package insert. Wilmington, DE: AstraZeneca Pharmaceuticals LP; April 2018.
- Tradjenta (linagliptin) package insert. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; August 2017.
- Hinnen DA. Therapeutic options for the management of postprandial glucose in patients with type 2 diabetes on basal insulin. Clin Diabetes. 2015;33(4):175-180.
- Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care. 2009;32(suppl 2):S151-S156.
- Lyu X, Zhu X, Zhao B, et al. Effects of dipeptidyl peptidase-4 inhibitors on beta-cell function and insulin resistance in type 2 diabetes: meta-analysis of randomized controlled trials. Sci Rep. 2017;7:44865.
- Center for Drug Evaluation and Research. Guidance for industry: diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. www.fda.gov/Downloads/Drugs/Guidances/ucm071627. pdf. Accessed August 6, 2018.
- Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317-1326.
- Zannad F, Cannon CP, Cushman WC, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385(9982):2067-2076.
- Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232-242.
- American Diabetes Association. 8. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41(suppl 1):S73-S85.
- Hattori S. Sitagliptin reduces albuminuria in patients with type 2 diabetes. Endocr J. 2011;58(1):69-73.
- Sakai Y, Suzuki A, Mugishima K, et al. Effects of alogliptin in chronic kidney disease patients with type 2 diabetes. Intern Med. 2014;53(3):195-203.
- Takeda H, Sasai N, Ito S, et al. Efficacy and safety of alogliptin in patients with type 2 diabetes: analysis of the ATTAK-J study. J Clin Med Res. 2016;8(2):130-140.
- Ott C, Raff U, Schmidt S, et al. Effects of saxagliptin on early microvascular changes in patients with type 2 diabetes. Cardiovasc Diabetol. 2014;13:19.
- Jax T, Stirban A, Terjung A, et al. A randomised, active- and placebo- controlled, three-period crossover trial to investigate short-term effects of the dipeptidyl peptidase-4 inhibitor linagliptin on macro- and microvascular endothelial function in type 2 diabetes. Cardiovasc Diabetol. 2017;16(1):13.
- Evans M, Schweizer A, Foley JE. Blood pressure and fasting lipid changes after 24 weeks' treatment with vildagliptin: a pooled analysis in >2,000 previously drug-naïve patients with type 2 diabetes mellitus. Vasc Health Risk Manag. 2016;12:337-340.
- Marfella R, Sasso FC, Rizzo MR, et al. Dipeptidyl peptidase 4 inhibition may facilitate healing of chronic foot ulcers in patients with type 2 diabetes. Exp Diabetes Res. 2012;2012:892706.
- Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007;298(2):194-206.
- Goldstein BJ, Feinglos MN, Lunceford JK, et al. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care. 2007;30:1979-1987.
- Nesina (alogliptin) package insert. Deerfield, IL: Takeda Pharmaceuticals America, Inc; December 2016.
- Fujita H, Taniai H, Murayama H, et al. DPP-4 inhibition with alogliptin on top of angiotensin II type 1 receptor blockade ameliorates albuminuria via up-regulation of SDF-1a in type 2 diabetic patients with incipient nephropathy. Endocr J. 2014;61(2):159-166.
- Mosenzon O, Leibowitz G, Bhatt DL, et al. Effect of saxagliptin on renal outcomes in the SAVOR-TIMI 53 trial. Diabetes Care. 2017;40(1):69-76.
- Groop PH, Cooper ME, Perkovic V, et al. Linagliptin lowers albuminuria on top of recommended standard treatment in patients with type 2 diabetes and renal dysfunction. Diabetes Care. 2013;36:3460-3468.
- Forst T, Michelson G, Diessel S, et al. Microvascular effects of the inhibition of dipeptidylpeptidase IV by linagliptin in nondiabetic hypertensive patients. J Hypertens. 2016;34(2):345-350.
- Berndt-Zipfel C, Michelson G, Dworak M, et al. Vildagliptin in addition to metformin improves retinal blood flow and erythrocyte deformability in patients with type 2 diabetes mellitus—results from an exploratory study. Cardiovasc Diabetol. 2013;12:59.
- Long M, Cai L, Li W, et al. DPP-4 inhibitors improve diabetic wound healing via direct and indirect promotion of epithelial-mesenchymal transition and reduction of scarring. Diabetes. 2018;67(3):518-531.
- Frykberg RG. Diabetic foot ulcers: pathogenesis and management. Am Fam Physician. 2002;66(9):1655-1662.
- Packer M. Have dipeptidyl peptidase-4 inhibitors ameliorated the vascular complications of type 2 diabetes in large-scale trials? The potential confounding effect of stem-cell chemokines. Cardiovasc Diabetol. 2018;17(1):9.
- Schaffer C, Buclin T, Jornayvaz FR, et al. Use of dipeptidyl-peptidase IV inhibitors and bullous pemphigoid. Dermatology. 2017;233(5):401-403.
- Yoshiji S, Murakami T, Harashima SI, et al. Bullous pemphigoid associated with dipeptidyl peptidase-4 inhibitors: a report of five cases. J Diabetes Investig. 2018;9(2):445-447.
- FDA Drug Safety Communication: FDA adds warnings about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin. www.fda.gov/Drugs/DrugSafety/ucm486096.htm. Accessed September 19, 2018.
- Lehrke M, Marx N, Patel S, et al. Safety and tolerability of linagliptin in patients with type 2 diabetes: a comprehensive pooled analysis of 22 placebo-controlled studies. Clin Ther. 2014;36(8):1130-1146.
- Tkác I, Raz I. Combined analysis of three large interventional trials with gliptins indicates increased incidence of acute pancreatitis in patients with type 2 diabetes. Diabetes Care. 2017;40(2):284-286.
- Haak T, Meinicke T, Jones R, et al. Initial combination of linagliptin and metformin improves glycaemic control in type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metabol. 2012;14(6):565-574.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2018 executive summary. Endocr Pract. 2018;24(1):91-120.
- PL detail-document. Combining a GLP-1 agonist and a DPP-4 inhibitor for type 2 diabetes. Pharm Lett/Presc Lett; August 2012.