A Pharmacist's Review of Nausea and Vomiting
RELEASE DATE:
January 2, 2019
EXPIRATION DATE:
January 31, 2021
FACULTY:
Troy J. Smith, PharmD, BSChE
Assistant Professor of Pharmacy
Timothy K. Fincher, RPh, PhD
Assistant Professor of Pharmacy
William Carey University School of Pharmacy
Hattiesburg, Mississippi
FACULTY DISCLOSURE STATEMENTS:
Drs. Smith and Fincher have no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT:
Pharmacy
 Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-19-004-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE:
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER:
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients' conditions and possible contraindications or dangers in use, review of any applicable manufacturer's product information, and comparison with recommendations of other authorities.
GOAL:
To review selected types of nausea and vomiting commonly encountered in retail and clinical practice and educate pharmacists regarding appropriate treatment.
OBJECTIVES:
After completing this activity, the participant should be able to:
- Recognize clinical situations involving nausea and vomiting in which a patient should seek further evaluation/emergency medical care.
- State appropriate treatment for patients undergoing chemotherapy.
- Discuss the risk of nausea and vomiting in a postoperative setting and recommend appropriate treatment for patients undergoing surgery.
- State appropriate treatment for pregnant patients.
ABSTRACT: Pharmacists are often approached with questions regarding nausea and vomiting (N/V). After obtaining a patient history, therapy should begin by isolating the suspected cause, assessing severity, and referring to emergency care if warranted. The pharmacist may then make an evidence-based recommendation for pharmacotherapy, if needed. In cases of simple N/V, in addition to avoiding triggers, a variety of pharmacologic agents are available for treatment. In chemotherapy-induced N/V, high-emetic risk chemotherapy drugs can be identified and appropriate treatment selected. In postsurgical N/V, the risk for emesis can be predicted and effective recommendations made to prevent and/or alleviate symptoms. For cases of recalcitrant pediatric N/V, there are some medications that may be used with caution in children older than age 2 years.
Nearly all people, from children to adults, occasionally experience nausea and vomiting (N/V). Nausea is the uncomfortable sensation that one feels prior to vomiting, whereas vomiting is the forceful expulsion of gastric contents through the mouth.1 This is not to be confused with regurgitation, the condition in which gastric contents are dispelled with little effort through the mouth from the pharynx.2
Most cases of simple N/V originate from bacterial or viral gastroenteritis, normally caused by the ingestion of a viral entity or bacterium that subsequently secretes harmful toxins, and are self-limiting. Symptoms usually begin 1 to 6 hours after ingestion and remain for 24 to 48 hours.1 Other common situations in which patients may experience N/V include pregnancy; cancer treatment, in which patients may experience chemotherapy-induced nausea and vomiting (CINV); and surgery, when patients may experience postoperative nausea and vomiting (PONV). Lesser N/V incidents may stem from psychiatric, neurologic, or metabolic disorders; opiate drug withdrawal; or iatrogenic causes, including N/V caused by the use of cannabinoids.2 Occasionally, patients may suffer from vestibular N/V or motion sickness. This article focuses on simple N/V, CINV, PONV, and N/V of pregnancy, and briefly discusses recalcitrant pediatric N/V, to assist the pharmacist with making clinical decisions pertaining to both simple, common presentations and those episodes requiring more complex therapy.
Etiology and Epidemiology
Understanding the origin of the patient's N/V is critical in determining the proper course of treatment. Although N/V is a common complaint, certain situations increase risk. Among patients receiving chemotherapy, for example, the incidence of N/V can vary significantly, depending on the regimen.3 The risk of emesis varies for postoperative surgery patients as well, depending on factors such as female gender, nonsmoking status, and the medications used to anesthetize.4 In pregnancy, nausea occurs in 50% to 80% of women, with vomiting occurring in nearly 50%.4 Almost all women experience N/V prior to 9 weeks gestation.4 Should the patient experience initial symptoms after this period, the pathology could be related to a host of other factors and require further evaluation.
Pathophysiology
Knowing the pathophysiology of N/V and the mechanisms of action of the various medications available to treat it provides the pharmacist with the insight needed to make a recommendation. Antiemetic medications have been designed to antagonize the receptors known for causing N/V. These receptors are located in the vomiting center of the brain, the chemoreceptor trigger zone, and the gastrointestinal tract, which includes cholinergic, histaminic, dopaminergic serotonergic, neurokinin (NK), and benzodiazepine receptors.2
Patient Assessment
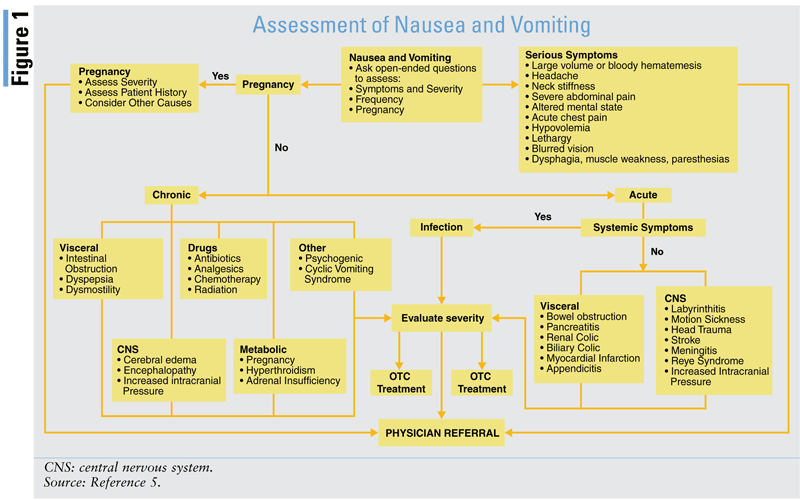
The pharmacist should have a detailed history of the patient's condition. If the N/V is acute, that is, lasting for less than 1 month, it could be suggestive of a drug-related cause, food poisoning, pancreatitis, gastroenteritis, or a gallbladder infection. If the patient complains of pain, then an obstruction may be present and the patient should be instructed to seek emergency care. If chronic, that is, longer than 1 month, the origin of N/V could be pregnancy, gastroesophageal reflux disease (GERD), gastroparesis, or metabolic disorder, or it may be drug-related.1
FIGURE 1 is a flowchart devised to help the pharmacist understand the cause of the patient's N/V and to guide therapy.5
Prevention
In many cases of N/V, prevention consists of avoiding potentially causative drugs and observing dietary restrictions. Eating smaller, more frequent meals, avoiding spicy and/or fried foods, and following a bland diet may assist with preventing symptoms.2
PHARMACOLOGIC TREATMENT OF SIMPLE N/V
In cases of simple N/V, there are a variety of medications to recommend. However, the practitioner should be aware of the potential side effects associated with these and make the appropriate selection based on the patient's presentation.
Antacids: Some antacid medications available to treat symptoms of simple N/V contain magnesium or calcium. The pharmacist should be aware that magnesium products are associated with diarrhea, whereas calcium products are associated with constipation. The pharmacist should ask the patient if he or she currently has either of these symptoms and advise accordingly.2
Antihistamine-Anticholinergics: Antihistamine-anticholinergic medications are used when the cause of N/V is motion sickness or vertigo. These medications exert their effect by blocking the muscarinic and histaminic receptors in the vomiting center. Examples include dimenhydrinate, diphenhydramine, hydroxyzine IM (unlabeled), meclizine, and the scopolamine transdermal patch. Use these medications with special caution in elderly patients, owing to drowsiness that may contribute to falls and to an increased risk of complications in those patients with benign prostatic hyperplasia, narrow-angle glaucoma, and asthma.2
Benzodiazepines: The benzodiazepines alprazolam and lorazepam may be used for anticipatory N/V. Side effects include sedation, appetite changes, and memory impairment.2
Butyrophenones: The butyrophenone haloperidol has been used to treat N/V, but mostly in a palliative-care setting and is not considered first-line treatment for noncomplicated N/V, owing to its sedating and extrapyramidal side effects and for the increased risk of a prolonged QT interval.2
Histamine (H2) Antagonists: H2 antagonists may be recommended for N/V related to GERD or heartburn. However, it is prudent to avoid cimetidine due to the potential inhibitory drug interactions. Monitor by assessing for symptom relief.2
Other: Metoclopramide has been found to be useful in treating N/V for patients suffering from diabetic gastroparesis by causing a quicker emptying of gastric contents. Side effects may include asthenia, headache, and somnolence.2
Phenothiazines: Phenothiazines chlorpromazine, prochlorperazine, and promethazine are commonly employed to treat simple N/V. Minor side effects include drowsiness, headache, and blurry vision. Potentially more serious side effects include tardive dyskinesia. Prochlorperazine has also shown effectiveness in treating CINV but should be used cautiously with patients at risk for a prolonged QT interval. Assess patient for decreased incidents of N/V. If promethazine is used IV, care must be taken to dilute and administer the infusion properly to avoid extravasation.2
OVERVIEW OF CINV
Factors that increase the risk of CINV include young age, female gender, patient history of low alcohol intake, N/V related to pregnancy, and previous CINV experience.2
There are three basic categories of CINV: acute, which occurs within 24 hours of chemotherapeutic administration; delayed, which may also occur within 24 hours but can last several days after treatment; and anticipatory, which occurs prior to receiving chemotherapy.
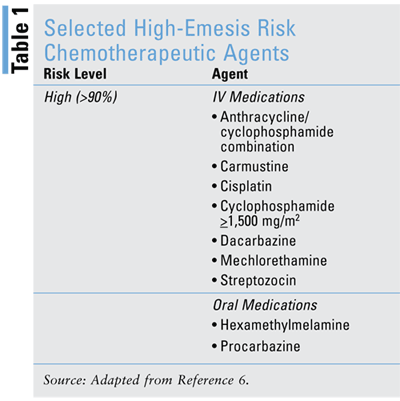
Furthermore, the emetic risk of chemotherapeutic agents may be classified as high (emesis risk >90% without antiemetics); moderate (emesis risk 30% to 90% without antiemetics); low (emesis risk 10% to 30% without antiemetics); and minimal (emesis risk <10% without antiemetics). See TABLE 1 for selected high-emetic-risk chemotherapeutic agents.
Pharmacologic Treatment of CINV
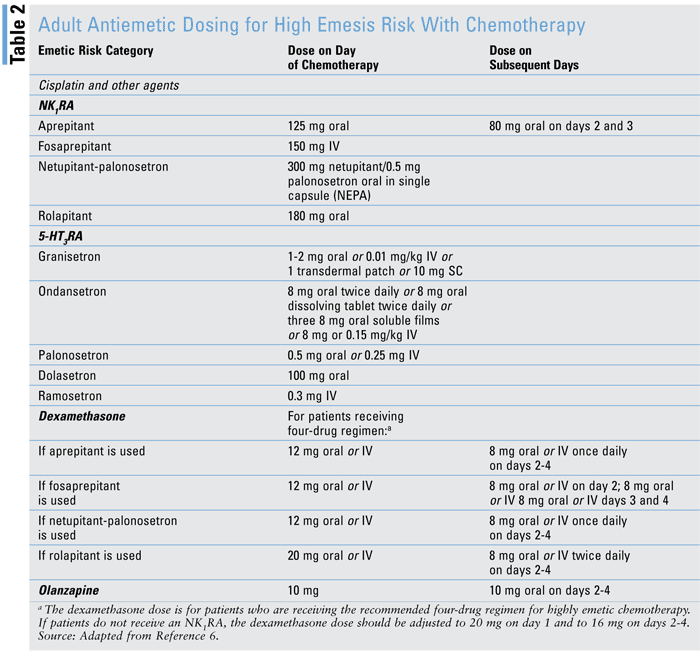
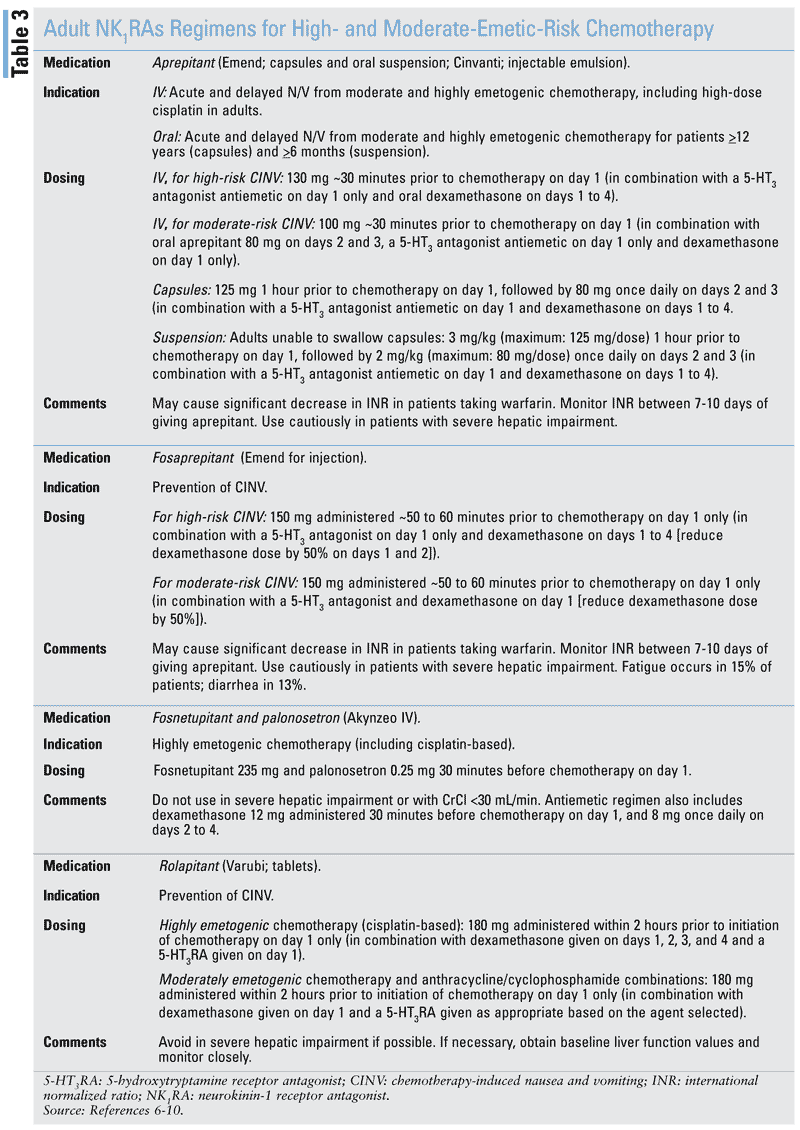
The goal of pharmacologic treatment for CINV is to eliminate symptoms during the period of chemotherapy treatment. The practitioner should determine the risk of emesis associated with therapy and begin with the corresponding antiemetic medication.2 TABLE 2 lists selected agents used to treat adults at high risk of CINV.6 TABLE 3 describes neurokinin-1 receptor antagonist (NK1RA) regimens for high- and moderate-emetic-risk chemotherapy. Refer to the American Society of Clinical Oncology Guidelines for recommendations for antiemetic regimens corresponding to all CINV risk groups.6
5-Hydroxytryptamine Receptor Antagonists (5-HT3RAs): The 5-HT3RAs are well established as the most effective class of medications for preventing CINV; however, the practitioner should be aware that QT prolongation is a contraindication to their use. Data suggest that these agents are interchangeable. However, among these, palonosetron presents with the least QT prolongation at a rate of <1%.6 Regarding dolasetron, use of the IV formulation for CINV was banned by the FDA in 2010 due to increased QT interval and torsades de pointes, but the oral dosage is still used (see TABLE 2).3
Other medications that may be considered are metoclopramide, lorazepam, and cannabinoids. Metoclopramide should be reserved for refractory cases of CINV. Lorazepam is useful as adjunctive therapy but should not be used as a stand-alone treatment. Evidence remains insufficient to support the use of cannabinoids in this setting.6
OVERVIEW OF PONV
The pathophysiology of PONV involves the complex neurotransmitter cascade, culminating in emesis. There are five basic pathways associated with PONV. These are the chemoreceptor trigger zone, the vagal mucosal pathway, neuronal pathways from the vestibular system, afferent reflexes of the cerebral cortex, and midbrain afferents.3
When assessing a patient's risk for PONV, influential factors include the patient's status and history, and preoperative, intraoperative, and postoperative factors.3
Regarding patient factors, the risk of PONV is increased by female gender, a history of motion sickness, nonsmoking status, age less than 50 years, and having a condition that delays gastric emptying, such as diabetes mellitus or hypothyroidism.3
Preoperative: It is uncertain whether preoperative fasting is a risk factor. Anxiety is not thought to be clinically relevant in predicting PONV.3
Intraoperative: The provider should be aware of the operative procedures that pose higher risk. Cholecystectomy and gynecologic and laparoscopic surgeries pose an increased risk of PONV. Procedures that are longer in duration are also associated with increased risk.3
The selection of anesthesia plays a major role. Medications associated with a higher incidence of PONV include nitrous oxide, ether, cyclopropane, etomidate, and ketamine. Also, pressure changes in the middle ear stimulate the vestibular system and may bring about emesis. Fewer incidents of PONV occur with propofol, an increasingly popular choice among providers. The incidence of PONV from use of neuromuscular reversal agents is not clear at this time.3
Postoperative: Postoperative determinants of N/V include pain, ambulation, and the use of opioids in a dose-dependent manner. Supplemental oxygen is no longer recommended for PONV.3
The Apfel scoring system is frequently used to predict PONV risk. It takes into account four factors: female gender, history of PONV and/or motion sickness, being a nonsmoker, and postoperative use of opioids. Patients are evaluated according to these criteria and are given a point for each affirmative answer. If the patient scores 0, 1, 2, 3, or 4, the chance of emesis is approximately 10%, 20%, 30%, 40%, and 80%, respectively.11
Pharmacologic Treatment of PONV
Developing a strategy to prevent PONV is dependent upon the risk. If the risk is low, no antiemetic therapy is needed. However, if the risk is deemed moderate or greater, monotherapy or combination therapy should be initiated, with attention given to potential side effects in susceptible patients. If the therapy is not effective, consider adding an agent from a different therapeutic class.3
5-HT3RAs
Ondansetron: Ondansetron 4 mg given IV push is recommended at the conclusion of surgery. If administering the oral disintegrating ondansetron tablet, an 8 mg orally disintegrating tablet is recommended.3
Dolasetron: Dolasetron may be given at the conclusion of surgery at a dose of 12.5 mg IV for PONV.3
Granisetron: 3 mg IV should be given in combination with dexamethasone 8 mg IV.3
Palonosetron: Palonosetron is a second-generation 5-HT3RA with a longer half-life of approximately 40 hours. The approved dose is 0.075 mg IV; it will continue to alleviate symptoms for approximately 24 hours.3
Antihistaminic-Anticholinergic Agents
Though rarely reported, the class side effects are dry mouth, constipation, drowsiness, urinary retention, and blurry vision. Extreme caution should be used in pregnant patients. Dimenhydrinate may cause an oxytocic effect.12 Promethazine may inhibit platelet aggregation in newborns.13
Scopolamine: Scopolamine, as a transdermal patch, is useful for preventing PONV for patients utilizing patient-controlled anesthesia. Apply 2 to 4 hours prior to the beginning of anesthesia.6
Dimenhydrinate: The dose is 1-2 mg/kg given IV.3
Meclizine: The dose is 50 mg orally along with ondansetron 4 mg IV. Meclizine is useful because it has a longer duration of action than ondansetron alone.3
Promethazine: The dose is 12.5-25 mg given IV. Of note, SC administration is contraindicated. It may be given as a deep IM injection, or as an infusion at a concentration of 25 mg/mL, not exceeding a rate of 25 mg/min.3
Dopamine antagonist: Metoclopramide, given at a dose of 10 mg IV, may assist with PONV but is considered only weakly antiemetic and is associated with extrapyramidal symptoms, headache, dizziness, and sedation.3
NK1RA: Aprepitant, dosed at 40 mg, was shown to be more effective than ondansetron for preventing vomiting at 24 and 48 hours and for reducing nausea during that time. The half-life of aprepitant is relatively long at 40 hours.3
Corticosteroid: Due to a slower onset of action, dexamethasone at a dose of 8 mg is administered with induction of anesthesia. But care should be exercised because of an increased risk of infection from the immunosuppressive actions of dexamethasone. Care should also be taken in patients with type 2 diabetes, as dexamethasone has shown to increase blood sugar. Note that dexamethasone is contraindicated in labile diabetic patients.3
N/V OF PREGNANCY
Most pregnant women experience symptoms of N/V prior to 9 weeks gestation. However, women with abnormal presentations, such as symptoms of N/V beyond 9 weeks gestation, abdominal tenderness, fever, or significant dehydration (consistent with hyperemesis gravidarum) should be referred for further evaluation.4 The pathology of N/V related to pregnancy is not well understood.4
Complimentary/Alternative Treatment
Trials evaluating the efficacy of acupuncture in N/V related to pregnancy have shown no improvement versus placebo. Ginger is an herbal medication that has shown to be both superior to placebo, at a dose of 125 to 250 mg every 6 hours, and comparable to pyridoxine at relieving symptoms.6 Ginger ale or tea may also be used as adjunctive therapy.4
Pharmacologic Treatment
Treatment of N/V in pregnancy is aimed at relieving symptoms while minimizing the risk of adverse drug effects on the mother and fetus.
First-line Therapy: Vitamin B6, also known as pyridoxine, is considered first-line therapy for relieving symptoms, with or without doxylamine.2,4 The combination of vitamin B6 and doxylamine is effective therapy and has been shown to reduce symptoms by 70% and to be safe during the first trimester.4 The two medications are available in a combination product, Diclegis. Diclegis contains 10 mg of doxylamine and 10 mg of pyridoxine.
Secondary Therapy: The phenothiazine promethazine is available for rectal administration, but the practitioner should be cautious when giving this medication concurrently with dopamine antagonists owing to an increased risk of extrapyramidal side effects, and with 5-HT3RAs, because of an increased incidence of QT prolongation.4
Ondansetron, a 5HT3RA, is safe and effective for use during pregnancy. It may be given every 6 hours and is available as an orally disintegrating tablet. Adverse effects include headache, gastrointestinal distress, and fatigue.4 However, the pharmacist should recognize other drug therapies that are contraindications to ondansetron. Some of these medications include anticholinergics, diuretics, HIV protease inhibitors, hydroxyzine, erythromycin, azithromycin, amitriptyline, and haloperidol.4
The antihistamines diphenhydramine, meclizine, and dimenhydrinate have been shown to be safe and more effective than placebo. However, side effects such as drowsiness, dizziness, and headache are a concern.4
The antiemetics chlorpromazine and prochlorperazine have proved helpful. Promethazine is often employed but has sedating properties; it is available for rectal application.4
Regarding promotility agents, metoclopramide is the only drug in this class used in N/V associated with pregnancy. It may be used either alone or in combination with other agents. The FDA has issued a black box warning for this medication owing to its association with tardive dyskinesia; it is recommended that the drug not be given for more than 12 weeks.4
Refractory N/V in Pregnancy
Methylprednisolone is considered a last-resort therapy and is the only corticosteroid medication that has been studied for this purpose. It should not be utilized prior to 10 weeks gestation owing to an increased risk of cleft lip and should not be used for longer than 6 weeks.4
For women who have endured a prolonged period of N/V and cannot tolerate oral rehydration, IV fluid with dextrose and vitamins is recommended. Give thiamine prior to administration to avoid Wernicke encephalopathy.4
Acid-reducing medications such as H2 receptor antagonists and proton pump inhibitors have been shown safe and effective for N/V in pregnancy and may be used for symptoms caused by gastric ulcers.4
If the patient cannot tolerate oral rehydration and has failed to respond to outpatient management, recommend hospitalization.4
N/V IN INFANTS AND CHILDREN
It is critical for the pharmacist to have an accurate and in-depth patient history when evaluating N/V in infants and children. Patterns of N/V should be noted, as well as fever, anorexia, abdominal pain, distension, or the presence of diarrhea, with or without blood. The pharmacist should ask the parent if the child cries inconsolably or has shown significant changes in weight. The child should be examined for rashes, jaundice, and dehydration.14
Most cases of N/V originate from acute viral gastroenteritis. However, there are occasions when this is not the case. Refer to a physician if the child presents with yellow or green emesis, projectile vomiting, abdominal pain, bloody stools or no stools, fever, decreased bowel sounds, or recent head trauma. These are indicative of possibly more serious conditions, with some requiring surgery.
Pharmacologic Treatment
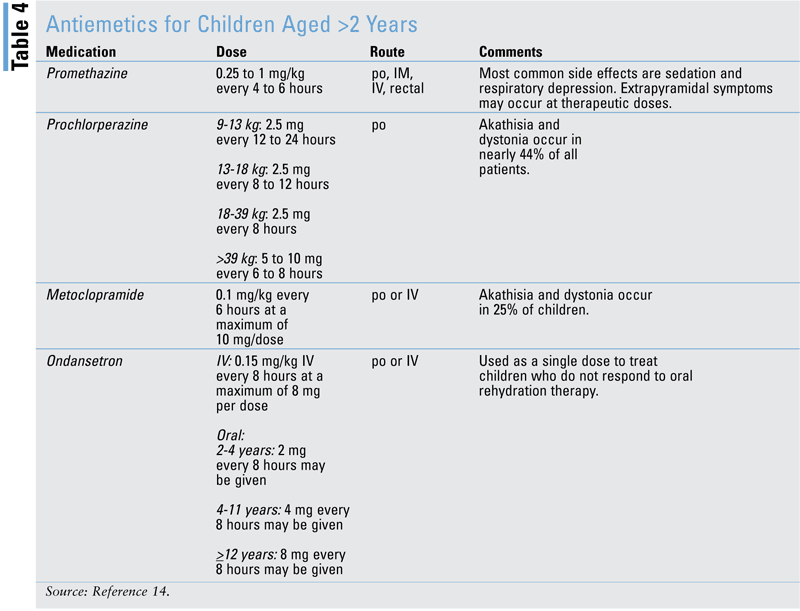
Rehydration is crucial.14 Fewer medications are used to treat N/V in infants and children, owing to a lack of data to prove safety and efficacy in this patient population. However, if the N/V is recalcitrant, there are some medications that may be used with caution.14 TABLE 4 presents medications that may be used when treating children older than age 2 years.
PHARMACIST'S ROLE
Obtaining an adequate patient history and knowing the origin of the symptoms are paramount concerning the treatment and counseling of patients with N/V. The pharmacist should be aware of pharmacologic and nonpharmacologic options to communicate to the patient and the provider as well as factors that warrant referral to emergency care or further evaluation. With proper evaluation and counseling, the patient suffering from N/V may find resolution of their symptoms. In some cases, serious conditions can be caught early and overall outcomes for patients can be improved.
REFERENCES
- Scorza K, Williams A, Phillips D, Shaw J. Evaluation of nausea and vomiting. Am Fam Physician. 2007;76(1):76-84.
- Hylton Gravatt L, Donohoe K, Dipiro C. In: DiPiro ST, Talbert RC, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 10th ed. New York, NY: McGraw-Hill Education; 2017:497-508.
- Shaikh SI, Nagarekha D, Hegade G, Marutheesh M. Post operative nausea and vomiting: a simple yet complex problem. Anesth Essays Res. 2016;10(3):388-396.
- The American College of Obstetricians and Gynecologists. Nausea and vomiting of pregnancy. Obstet Gynecol. 2018;131;1:e15-e30.
- Henderson MC, Tierney LM, Jr, Smetana GW. eds. The Patient History: An Evidence-Based Approach To Differential Diagnosis. New York, NY: McGraw-Hill; 2012. http://accessmedicine.mhmedical.com/ content.aspx?bookid=500§ionid=41026539. Accessed November 12, 2018.
- Hesketh PJ, Kris MG, Basch E. Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017;35:3240-3261.
- Aprepitant. Lexi-Drugs. Lexicomp. Riverwoods, IL: Wolters Kluwer Health, Inc. www.wolterskluwercdi.com/lexicomp-online. Accessed November 26, 2018.
- Fosaprepitant. Lexi-Drugs. Lexicomp. Riverwoods, IL: Wolters Kluwer Health, Inc. www.wolterskluwercdi.com/lexicomp-online. Accessed November 26, 2018.
- Netupitant and palonosetron. Lexi-Drugs. Lexicomp. Riverwoods, IL: Wolters Kluwer Health, Inc. Riverwoods, IL. www.wolterskluwercdi. com/lexicomp-online. Accessed November 26, 2018.
- Rolapitant. Lexi-Drugs. Lexicomp. Riverwoods, IL: Wolters Kluwer Health, Inc. www.wolterskluwercdi.com/lexicomp-online. Accessed November 26, 2018.
- Ebell M. Predicting postoperative nausea and vomiting. Am Fam Physician. 2007;75(10):1537-1538.
- Dimenhydrinate. Lexi-Drugs. Lexicomp. Riverwoods, IL: Wolters Kluwer Health, Inc. www.wolterskluwercdi.com/lexicomp-online. Accessed December 3, 2018.
- Promethazine. Lexi-Drugs. Lexicomp. Riverwoods, IL: Wolters Kluwer Health, Inc. www.wolterskluwercdi.com/lexicomp-online. Accessed December 3, 2018.
- Consolini D. Nausea and vomiting in infants and children. Merck Manual. www.merckmanuals.com/professional/pediatrics/symptoms-ininfants-and-children/nausea-and-vomiting-in-infants-and-children. Accessed: September 13, 2018.