A Review of Coronavirus Disease 2019 (COVID-19) for Pharmacists
RELEASE DATE
August 1, 2020
EXPIRATION DATE
August 31, 2022
FACULTY
Allana J. Sucher, PharmD, BCPS, BCIDP
Professor of Pharmacy Practice
T. J. Sayre, PharmD, MPH, BCPP, BCPS
Assistant Professor of Pharmacy Practice
Kyle Dagen, PharmD Class of 2021
Taylor Vasas, PharmD Class of 2021
Regis University School of Pharmacy
Denver, Colorado
Elias B. Chahine, PharmD, FCCP, FASCP, FFSHP, BCPS, BCIDP
Professor of Pharmacy Practice
Palm Beach Atlantic University Gregory School of Pharmacy
West Palm Beach, Florida
FACULTY DISCLOSURE STATEMENTS
Dr. Chahine serves on the speakers’ bureaus of Merck & Co, Inc., and Paratek Pharmaceuticals, Inc. Drs. Sayre and Sucher received funding from Innovation Compounding for prior work on educational materials on COVID-19 and the role of hydroxychloroquine. Mr. Dagan and Mr. Vasas have no actual or potential conflict of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT
 Pharmacy
Pharmacy
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-20-071-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER:
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients’ conditions and possible contraindications or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
GOAL
To educate pharmacists about the clinical presentation, diagnostic tests, prevention measures, and treatment of COVID-19 based on current clinical practice guidelines.
OBJECTIVES
After completing this activity, the pharmacist should be able to:
- Identify the clinical presentation, time to onset of signs and symptoms, and modes of transmission of COVID-19.
- Describe diagnostic considerations and priorities for testing patients with suspected COVID-19.
- Compare and contrast guideline recommendations and the mechanisms of action of investigational therapeutic options used in the management of patients with COVID-19.
- State general measures for the public and measures for infection control in healthcare settings to prevent COVID-19.
ABSTRACT: Coronavirus disease 2019 (COVID-19) is a respiratory infection caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The most common symptoms of COVID-19 are fever, cough, and shortness of breath, with presentations ranging from absence of symptoms to severe infection requiring mechanical ventilation. COVID-19 has spread throughout the world and was declared a pandemic by the World Health Organization in March 2020. Current clinical management includes infection prevention measures and supportive care. Additionally, many investigational therapies are being used for the treatment of patients with this novel infection. Guidelines for the management of COVID-19 are being regularly updated based on rapidly emerging information. Pharmacists are in a key position to provide recommendations for appropriate preventive measures and to actively work with other healthcare professionals to ensure appropriate management of patients presenting with this infection.
On December 31, 2019, the World Health Organization (WHO) China Country Office was notified of a cluster of cases of pneumonia of an unknown origin in the city of Wuhan.1-3 The etiology was later identified as a new type of coronavirus, which was initially termed novel coronavirus (2019-nCoV) and was then named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1-5 The disease that the virus causes is named COVID-19, which stands for coronavirus disease 2019. The WHO characterized COVID-19 as a pandemic on March 11, 2020.6 As of July 16, 2020, there have been a total of 13,378,853 confirmed cases worldwide and 580,045 deaths.7 The first confirmed case in the United States was reported on January 20, 2020, and as of July 16, 2020, there have been a total of 3,483,832 cases and 136,938 deaths in the U.S.1,8 This article is an overview of COVID-19 and focuses on the treatment and prevention of the disease. As data are rapidly evolving, treatment guidelines and other resources should be consulted for the most updated information.
Etiology
Coronaviruses are a large family of single-stranded RNA viruses that can be isolated from humans and various animal species.9 There are hundreds of coronaviruses circulating, and SARS-CoV-2 is the seventh coronavirus known to infect humans.9,10 Although coronaviruses typically cause mild-to-moderate upper respiratory tract illnesses, three novel coronavirus strains have caused more severe symptoms: severe acute respiratory syndrome coronavirus (SARS-CoV), which caused an outbreak starting in 2002; Middle East respiratory syndrome coronavirus (MERS-CoV), which caused an outbreak starting in 2012; and SARS-CoV-2, which is responsible for the current pandemic.9,11 The SARS-CoV-2 viral genome has been sequenced and was shown to be about 75% to 80% identical to SARS-CoV and more closely related to bat coronaviruses.12-14 Although these data suggest that SARS-CoV-2 infected humans from a bat reservoir, it is unknown if another animal species acted as an intermediate host between bats and humans.13
Transmission
SARS-CoV-2 appears to be a highly contagious virus.15 It is thought to spread mainly from person-to-person via respiratory droplets produced when an infected person coughs, sneezes, or talks among people in close contact.15 The CDC generally defines close contact as an individual within 6 feet for an extended period of time (approximately 15 minutes), while noting this definition is limited by other factors to consider such as proximity, duration of exposure, and whether the individual has symptoms.16 Transmission may also occur from direct contact of the eyes, nose, or mouth with infectious secretions or through self-inoculation after contact with a surface that is contaminated with respiratory droplets.15 An experimental study found that the virus can survive in aerosolized droplets for up to 3 hours after being coughed into the air.17 This study also indicated that fomite transmission is plausible, as SARS-CoV-2 remained viable for 4 hours on copper, 24 hours on cardboard, and 2 to 3 days on plastic or stainless steel.17 However, since this study was conducted under experimental conditions, the CDC states that it remains uncertain whether the virus can be transmitted by small respiratory particles and that airborne transmission over long distances is unlikely.15
Clinical Presentation
Individuals with COVID-19 may remain asymptomatic or display symptoms ranging from mild to severe.18-20 If symptoms develop, they usually manifest 2 to 14 days after exposure to the virus, with a median time of onset of 4 to 5 days after virus exposure.19 Symptoms of COVID-19 include cough, fever, chills, shortness of breath/difficulty breathing, fatigue, muscle or body aches, headache, sore throat, new loss of taste or smell, nasal congestion, diarrhea, or nausea/vomiting.18,19 The CDC is also investigating reports of multisystem inflammatory syndrome in children.19 Clinicians should be aware of warning signs of COVID-19 (difficulty breathing, bluish lips or face, chest pain/pressure, new-onset confusion, and inability to wake or stay awake) that require immediate medical attention.18
Although anyone can develop a severe infection, adults aged 65 years and older, those who reside in a nursing home or long-term-care facility, and those of all ages with underlying medical conditions (e.g., chronic lung disease, moderate-to-severe asthma, heart conditions, immunocompromised status, severe obesity, diabetes mellitus, liver disease, chronic kidney disease on dialysis) are at increased risk of severe illness.20,21
Classification of severity of illness is based on clinical judgment derived from number and severity of symptoms, as well as the need for hospitalization and respiratory support.18-20 It is estimated that approximately 80% of infections are asymptomatic or mild, 15% of infections are severe (characterized by dyspnea, hypoxia, or >50% lung involvement on imaging), and 5% of patients develop a critical infection characterized by respiratory failure, shock, or multiorgan system dysfunction.19 For those with severe infection that requires hospitalization, complications include pneumonia, hypoxemic respiratory failure/acute respiratory distress syndrome, sepsis/septic shock, hypercoagulability/thrombotic complications, cardiomyopathy and arrhythmia, and acute kidney injury, as well as complications from prolonged hospitalization.19
Diagnostic Considerations
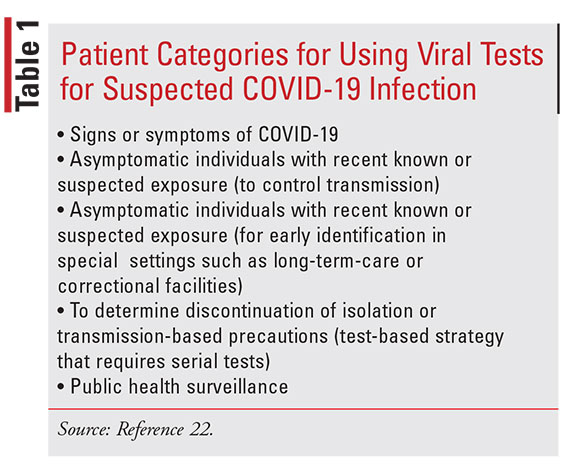
SARS-CoV-2 virus testing and COVID-19 diagnosis are rapidly evolving in response to the pandemic. According to CDC guidance, clinicians should use their judgment to determine if a patient has signs and symp- toms consistent with COVID-19. Other considerations for testing include epidemiologic factors such as local community transmission. Categories for testing patients with suspected COVID-19 infection with viral tests are included in TABLE 1.22
For initial testing, the CDC recommends collecting an upper-respiratory specimen (e.g., nasopharyngeal swab, oropharyngeal swab, or nasal wash/aspirate) to test for the presence of SARS-CoV-2. The CDC also states that testing lower-respiratory-tract specimens (e.g., sputum, bronchoalveolar lavage, or tracheal aspiration) is an option if available, but it does not recommend the induction of sputum.23
Diagnostic tests fall into two broad categories: viral tests to detect SARS-CoV-2 nucleic acid or antigen, and serology tests for detection of IgM and IgG antibodies.22,24 Viral tests such as reverse transcriptase polymerase chain reaction (RT-PCR) are currently the most frequently used diagnostic tests for COVID-19, but there are some limitations to these tests. Although a positive result is most likely accurate, false-negative results are possible since the sensitivity of the test is variable and is affected by the quality of the sample and the viral load.24,25 Additionally, viral RNA load may be highest at or before symptom onset, and there are variable detection rates over the course of an individual’s illness.24,26 Finally, detection of viral RNA does not equate to live virus, so its presence does not necessarily indicate that the virus is capable of causing disease.24 Serology testing is not recommended to diagnose acute illness since antibodies typically become detectable 1 to 3 weeks after the onset of symptoms.22,27 Antibody testing may be used in addition to a viral test to support the diagnosis of individuals who present late in the course of illness.24,27 Although the presence of antibodies may offer some level of protection from reinfection, it is not definitively known if a positive serologic test indicates protective immunity from future infection, the concentration of antibodies required to protect from reinfection, or the duration of protection.27 Therefore, at the time of writing, the CDC states that antibody tests should not be used to make decisions about returning to the workplace or grouping individuals in congregate settings.27
There are many commercially available COVID-19 tests, and the FDA continues to issue Emergency Use Authorizations (EUA) for tests.27,28 Additionally, alternative methods and integrative technology for testing are being developed.28,29 Pharmacists, in partnership with other healthcare providers, are well positioned to bolster COVID-19 testing expansion through improved access and distribution of tests. National chain pharmacies and other ambulatory care settings have mobilized pharmacists to order and administer COVID-19 testing under authorization from the Public Readiness and Emergency Preparedness Act.30 The CDC states that healthcare providers (including pharmacists) considering testing those with possible COVID-19 should coordinate testing through public health laboratories or work with laboratories using viral tests granted an EUA by the FDA.22
Treatment
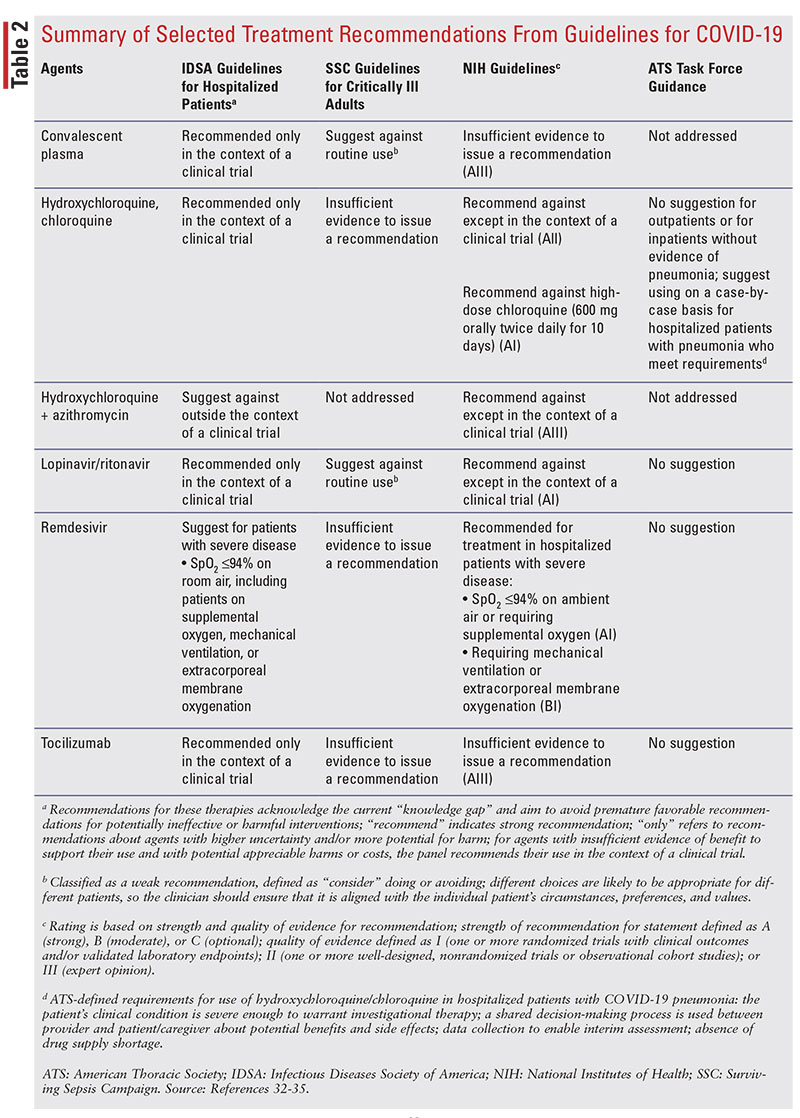
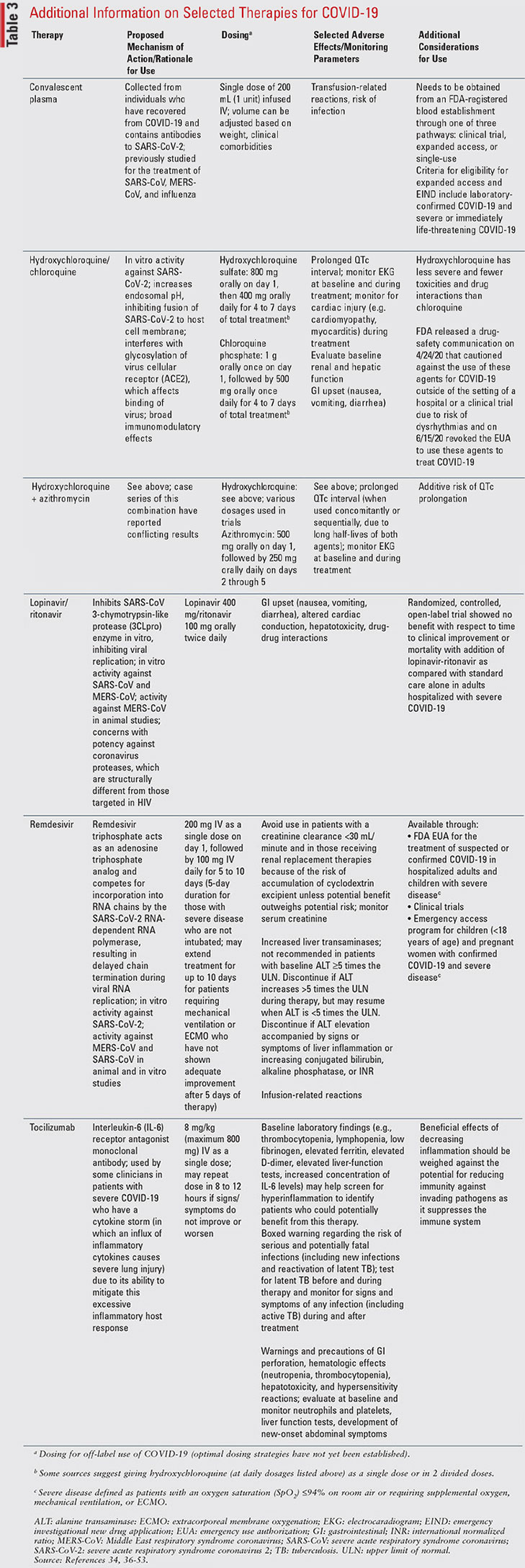
Currently, there are no FDA-approved agents for the treatment or prevention of COVID-19.31 Clinical management includes infection prevention measures as well as supportive care. The agents discussed in this section are all currently considered investigational for the treatment of COVID-19.31 This article does not include a comprehensive list of all agents but focuses on summarizing recommendations for agents with reasonable data that are included in the Infectious Diseases Society of America (IDSA) guidelines on the treatment and management of COVID-19 in hospitalized patients; the Surviving Sepsis Campaign (SSC) guidelines on the management of critically ill adults with COVID-19; COVID-19 Treatment Guidelines from the National Institutes of Health (NIH); and the American Thoracic Society (ATS)–led task force on interim guidance on management of COVID-19.32-35 TABLE 2 is a summary of these recommendations, and TABLE 3 provides additional information about mechanism of action, dosing, adverse effects, and considerations for use of these agents.32-53
In order to address unanswered questions regarding safety and efficacy of potential treatments for COVID-19, the IDSA guideline panel expressed the overarching goal that patients be recruited into ongoing trials, and the NIH guideline panel recommends that agents, including those already approved for another indication, be studied in well-designed controlled clinical trials.32,34 Providers can enroll a patient in a clinical trial at www.clinicaltrials.gov, which includes information on clinical trials being conducted to assess the safety and efficacy of various treatments for COVID-19, both in the U.S. and internationally.31,34 Providers can also access and prescribe an investigational agent or one approved for another indication through a variety of other mechanisms, including an EUA, Emergency Investigational New Drug application, (EIND) compassionate-use program from a drug manufacturer, expanded-access program from the drug manufacturer, or off-label use.34
As data are rapidly evolving, resources and treatment guidelines are frequently being updated and should regularly be consulted for the most up-to-date information. Additionally, the reader is referred to the full text of the guidelines for details from the clinical trials that led to the guideline recommendations.
OTHER TREATMENT CONSIDERATIONS
ACE Inhibitors, ARBs, and NSAIDs
Because angiotensin-converting enzyme 2 (ACE2)
has been shown to be the human cell receptor for SARS-CoV-2, one letter published in a journal raised
a hypothetical concern that agents that increase ACE2
expression (e.g., angiotensin-converting enzyme [ACE]
inhibitors, angiotensin receptor blockers [ARBs], ibuprofen) may increase the risk for or worsen the course
of COVID-19.54 However, according to the American
Heart Association, the Heart Failure Society of America, and the American College of Cardiology, there
are no experimental or clinical data that demonstrate
either beneficial or adverse outcomes with background
use of ACE inhibitors or ARBs.55 These societies
released a joint statement that recommends continuation of these agents in those who have an indication
and that any renin angiotensin aldosterone system–related treatments should not be added or removed
beyond actions based on standard clinical practice.55
This is supported by the NIH guidelines, which recommend continuing ACE inhibitors or ARBs for cardiovascular disease (or other indications) in those with
COVID-19.34
The FDA released a statement in March 2020 indicating that there is no scientific evidence establishing an association between ibuprofen and worsening COVID-19 and encouraged patients to take all medications in accordance with label instructions and as directed by a healthcare professional.56 The NIH guidelines recommend no preference for an antipyretic agent in those with COVID-19 and recommend continuing therapy for those previously directed to take an NSAID.34
PREVENTION
General Prevention in the Community
To prevent transmission in the community, the CDC
recommends avoiding close contact with others by
staying at least 6 feet apart from other individuals
(including household members who are ill), avoiding
mass/group gatherings, using a cloth face cover when
going out in public in order to protect others, cleaning
and disinfecting frequently touched surfaces on a daily
basis, washing hands often with soap and water for at
least 20 seconds, using hand sanitizer with at least 60%
alcohol if soap and water are not readily available, and
avoiding touching the face with unwashed hands.57
The Environmental Protection Agency (EPA) has compiled a list of disinfectants that are effective against the
SARS-CoV-2 virus, which is available on the EPA website, www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2.58
Infection Prevention
in Healthcare Settings
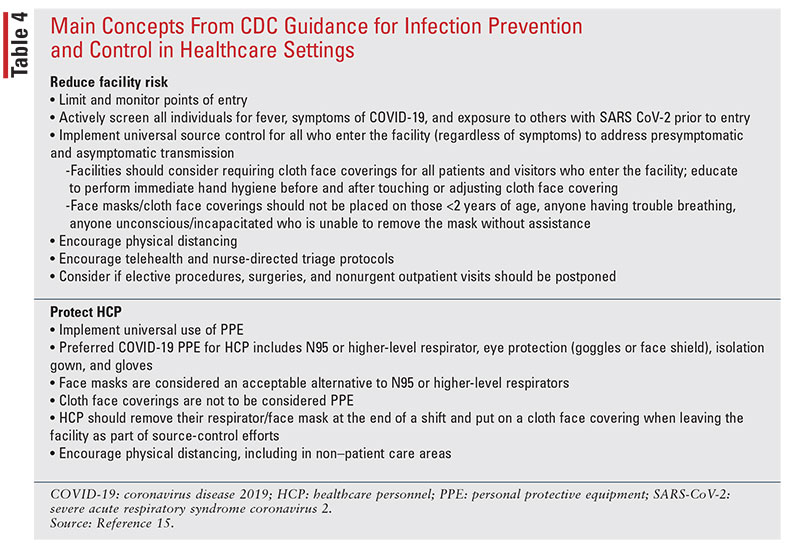
The CDC continues to update guidance for infection
prevention and control in healthcare settings based on
information about COVID-19, community transmission, identified infections in healthcare personnel
(HCP), as well as shortages of items such as respirators,
face masks/eye protection, gloves, and gowns. This
information is available on the CDC website, www.cdc.gov/coronavirus/2019-ncov/infection-control/control-recommendations.html. The main concepts from the
CDC guidance document are included in TABLE 4.15
For HCP caring for or entering the room of a patient with known or suspected COVID-19, personal protective equipment (PPE) should include a respirator or face mask, eye protection, gown, and gloves in addition to standard precautions. If there are shortages of respirators, face masks are considered to be an acceptable alternative and available respirators should be prioritized for procedures likely to generate respiratory aerosols. Cloth face coverings are not considered to be PPE and should not be worn by HCP for the care of patients with known or suspected COVID-19 or other situations that require PPE. If there are gown shortages, gowns should be prioritized for high-contact patient-care activities, including aerosol-generating procedures or anticipated splashes/sprays.15
The CDC has placed an increased emphasis on early identification and implementation of source control. Home care is preferable if possible and if hospitalization is not medically necessary. If admitted, patients with known or suspected infection should be cared for in a single-person room with the door kept closed and a dedicated bathroom, while airborne-infection isolation rooms should be reserved for patients undergoing aerosol-generating procedures. In order to limit HCP exposure and conserve PPE, the CDC states that facilities can consider designating facility units and dedicated HCP to care for known or suspected patients during their shift.15
Vaccine Development
Currently, there are no vaccines approved for COVID-19.57 There are several trials being conducted to evaluate the safety and efficacy of vaccine candidates for COVID-19; information is available at www.clinicaltrials.gov.
In the U.S., vaccine candidates have entered phase I/II clinical trials using either RNA or DNA technology. An open-label, dose-ranging study of the safety and immunogenicity of a 2-dose schedule of mRNA-1273 is currently being investigated with an estimated completion date of September 20, 2021.59 Another randomized, placebo-controlled study is being conducted to evaluate the safety, tolerability, immunogenicity, and potential efficacy of up to four different RNA vaccine candidates against COVID-19, with an estimated completion year of 2023.60 There is also an open-label trial of a DNA vaccine candidate, INO-4800, to assess the safety, tolerability, and immunogenicity of two doses of vaccine, administered intradermally followed by electroporation, with an estimated completion date of April 2021.61 Finally, the Bacille Calmette-Guérin (BCG) vaccine is being evaluated for use among healthcare workers.62 Traditionally used as a vaccine to prevent tuberculosis, there is experimental evidence indicating that the BCG vaccine may have nonspecific effects on the immune system, potentially against other respiratory tract infections.62
The Pharmacist’s Role
Pharmacists play an integral role in the management of patients with COVID-19. Pharmacists are in a key position to educate the public and other healthcare professionals about updated guidelines and rapidly emerging information related to drug therapy. Pharmacists should educate the public about appropriate measures to prevent the spread of infection and can work with infection control personnel in healthcare facilities to ensure that appropriate infection prevention recommendations are in place.
Authors’ note: At press time, a preliminary report of a recent study suggested that a 10-day course of dexa-methasone 6 mg once daily given to hospitalized patients with COVID-19 reduced deaths by one-third in ventilated patients and one-fifth in patients receiving oxygen only. No benefit was observed in those who did not require respiratory support.63 IDSA and NIH guidelines were updated on June 25, 2020. IDSA guidelines suggest glucocorticoids for hospitalized patients with severe COVID-19 (Sp02 <94% on room air or requiring supplemental oxygen, mechanical ventilation, or ECMO) and suggest against the use of glucocorticoids for hospitalized patients with- out hypoxemia requiring supplemental oxygen. NIH guidelines recommend using dexamethasone in patients with COVID-19 who are mechanically ventilated or require supplemental oxygen, and recommend against the use of dexamethasone in patients with COVID-19 who do not require supplemental oxygen.32,34
REFERENCES
- Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929-936.
- World Health Organization. Pneumonia of unknown cause—China. January 5, 2020. www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/. Accessed April 22, 2020.
- World Health Organization. Novel coronavirus—China. January 12, 2020 www.who.int/csr/don/12-january-2020-novel-coronavirus-china/ en/. Accessed April 22, 2020.
- Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720.
- CDC. Coronavirus disease 2019 (COVID-19): situation summary. www.cdc.gov/coronavirus/2019-ncov/cases-updates/summary.html. Accessed April 21, 2020.
- World Health Organization. Rolling updates on coronavirus disease (COVID-19). www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen. Accessed April 22, 2020.
- World Health Organization. Coronavirus disease (COVID-2019) situation reports. www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/. Accessed July 16, 2020.
- CDC. Coronavirus disease 2019 (COVID-19): cases in U.S. www. cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed July 16, 2020.
- National Institute of Allergy and Infectious Diseases (NIADH). Coronaviruses. www.niaid.nih.gov/diseases-conditions/coronaviruses. Accessed April 24, 2020.
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-733.
- Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470-473.
- Perlman S. Another decade, another coronavirus. N Engl J Med. 2020;382:760-762.
- Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565-574.
- Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270-273.
- CDC. Interim infection prevention and control recommendations for patients with suspected or confirmed coronavirus disease 2019 (COVID-19) in healthcare settings. www.cdc.gov/coronavirus/2019-ncov/infection-control/control-recommendations.html. Accessed June 19, 2020.
- CDC. Public health guidance for community-related exposure. www.cdc.gov/coronavirus/2019-ncov/php/public-health-recommendations.html. Accessed May 24, 2020.
- van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564-1567.
- CDC. Symptoms of coronavirus. www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed May 24, 2020.
- CDC. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients. html. Accessed May 24, 2020.
- World Health Organization. Q&A on coronaviruses (COVID-19).
www.who.int/news-room/q-a-detail/q-a-coronaviruses. Accessed May
24, 2020.
- CDC. People who are at higher risk for severe illness. www.cdc. gov/coronavirus/2019-ncov/need-extra-precautions/people-at-higher-risk.html. Accessed May 24, 2020.
- CDC. Overview of testing for SARS-CoV-2. www.cdc.gov/ coronavirus/2019-ncov/hcp/testing-overview.html. Accessed June 19, 2020.
- CDC. Interim guidelines for collecting, handling, and testing clinical specimens for COVID-19. www.cdc.gov/coronavirus/2019-nCoV/lab/guidelines-clinical-specimens.html. Accessed May 24, 2020.
- Patel R, Babady E, Theel ES, et al. Report from the American Society for Microbiology COVID-19 International Summit, 23 March 2020: value of diagnostic testing for SARS-CoV-2/COVID-19. mBio. 2020;11(2):e00722-20.
- West CP, Montori VM, Sampathkumar P. COVID-19 testing: the threat of false-negative results [Epub ahead of print April 9, 2020]. Mayo Clin Proc.
- He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;10.1038/s41591- 020-0869-5.
- CDC. Interim guidelines for COVID-19 antibody testing. www. cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines. html. Accessed May 24, 2020.
- American Pharmacists Association. COVID-19: demystifying testing for the SARS-CoV-2 virus: frequently asked questions about COVID-19 testing. www.pharmacist.com/sites/default/files/audience/APhACOVID- 19DemystifyingTesting0420_web.pdf. Accessed May 24, 2020.
- FDA. Emergency use authorizations for medical devices. www.fda.gov/medical-devices/emergency-situations-medical-devices/emergency-use-authorizations#covid19ivd. Accessed May 24, 2020.
- US Department of Health & Human Services. HHS statements on authorizing licensed pharmacists to order and administer COVID-19 tests. www.hhs.gov/about/news/2020/04/08/hhs-statements-on-authorizing-licensed-pharmacists-to-order-and-administer-covid-19-tests. html. Accessed May 24, 2020.
- CDC. Information for clinicians on investigational therapeutics for patients with COVID-19. www.cdc.gov/coronavirus/2019-ncov/hcp/therapeutic-options.html. Accessed May 24, 2020.
- Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19. www.idsociety.org/COVID19guidelines. Accessed June 25, 2020.
- Alhazzani W, Møller MH, Arabi YM, et al. Surviving sepsis campaign: Guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med. 2020;10.1097/ CCM.0000000000004363.
- National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines. https://covid19treatmentguidelines.nih.gov/. Accessed June 25, 2020.
- Wilson KC, Chotirmall SH, Bai C, et al. COVID 19: Interim guidance on management pending empirical evidence. From an American Thoracic Society-led international task force. www.thoracic.org/covid/covid-19-guidance.pdf. Accessed May 24, 2020.
- Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and pro- jection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS- CoV-2). Clin Infect Dis. [Epub ahead of print, March 9, 2020].
- Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019- nCoV) in vitro. Cell Res. 2020;30(3):269-271.
- FDA. Letter revoking EUA for chloroquine phosphate and hydroxychloroquine sulfate. June 15, 2020. www.fda.gov/media/138945/download. Accessed June 16, 2020.
- FDA. Fact sheet for health care providers, emergency use authorization (EUA) of hydroxychloroquine sulfate supplied from the strategic national stockpile for treatment of COVID-19 in certain hospitalized patients. www.fda.gov/media/136537/download. Accessed June 16, 2020.
- FDA. Fact sheet for health care providers. Emergency use authorization (EUA) of remdesivir (GS-5734). www.fda.gov/media/137566/ download. Accessed June 16, 2020.
- FDA. FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems. www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-covid-19-outside-hospital-setting-or. Accessed April 24, 2020.
- Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19 N Engl J Med. 2020;382:1787-1799.
- Yao TT, Qian JD, Zhu WY, Wang Y, Wang GQ. A systematic review of lopinavir therapy for SARS coronavirus and MERS corona- virus—a possible reference for coronavirus disease-19 treatment option J Med Virol. 2020;92:556-563.
- Mehta P, McAuley DF, Brown M, et al. HLH Across Specialty Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033-1034.
- Fu B, Xu X, Wei H. Why tocilizumab could be an effective treatment for severe COVID-19. J Transl Med. 2020;18(1):164.
- Henderson LA, Canna SW, Schulert GS, et al. On the alert for cytokine storm: immunopathology in COVID-19. Arthritis Rheumatol. 2020;10.1002/art.41285.
- Ritchie AI, Singanayagam A. Immunosuppression for hyperin- flammation in COVID-19: a double-edged sword? Lancet. 2020;395:1111.
- FDA. Recommendations for investigational COVID-19 convales- cent plasma. www.fda.gov/vaccines-blood-biologics/investigational- new-drug-ind-or-device-exemption-ide-process-cber/recommendations- investigational-covid-19-convalescent-plasma. Accessed May 24, 2020.
- Zhao Q, He Y. Challenges of convalescent plasma therapy on COVID-19. J Clin Virol. 2020;127:104358.
- COVID-19 expanded access program. www.uscovidplasma.org/. Accessed May 24, 2020.
- Gilead. Remdesivir emergency use. www.gilead.com/remdesivir. Accessed May 24, 2020.
- Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov. 2020;19(3):149-150.
- Lexi-Comp Online, Hudson, OH: Wolters Kluwer Clinical Drug Information, Inc.; 2020.
- Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4):e21.
- Bozkurt B, Kovacs R, Harrington B. HFSA/ACC/AHA statement addresses concerns re: using RAAS antagonists in COVID-19. www.acc.org/latest-in-cardiology/articles/2020/03/17/08/59/hfsa-acc-aha-statement-addresses-concerns-re-using-raas-antagonists-in-covid-19. Accessed April 18, 2020.
- FDA. FDA advises patients on use of non-steroidal anti-inflammatory drugs (NSAIDs) for COVID-19. www.fda.gov/drugs/drug-safety- and-availability/fda-advises-patients-use-non-steroidal-anti-inflammatory-drugs-nsaids-covid-19. Accessed May 24, 2020.
- CDC. How to protect yourself & others. www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html. Accessed May 24, 2020.
- Environmental Protection Agency. List N: disinfectants for use against SARS-CoV-2. www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2. Accessed May 24, 2020.
- Safety and immunogenicity study of 2019-nCoV vaccine (mRNA- 1273) for prophylaxis of SARS CoV-2 infection (COVID-19). www. clinicaltrials.gov/ct2/show/NCT04283461. Accessed May 24, 2020.
- Study to describe the safety, tolerability, immunogenicity, and potential efficacy of RNA vaccine candidates against COVID-19 in healthy adults. www.clinicaltrials.gov/ct2/show/NCT04368728. Accessed May 24, 2020.
- Safety, tolerability and immunogenicity of INO-4800 for COVID- 19 in healthy volunteers. www.clinicaltrials.gov/ct2/show/NCT04336410. Accessed May 24, 2020.
- BCG vaccine for health care workers as defense against COVID- 19 (BADAS). www.clinicaltrials.gov/ct2/show/NCT04348370. Accessed May 24, 2020.
- RECOVERY Collaborative Group. Horby P, Lim WS, Emberson J, et al. Dexamethasone in hospitalized patients with Covid-19—preliminary report N Engl J Med. 2020;10.1056/NEJMoa2021436. [Published online ahead of print, July 17, 2020].