An Overview of COVID-19 Infection
RELEASE DATE
April 1, 2022
EXPIRATION DATE
April 30, 2024
FACULTY
Micheline A. Goldwire, PharmD, MS, MA, BCPS
Professor and Director, Drug Information Services
Regis University School of Pharmacy
Denver, Colorado
FACULTY DISCLOSURE STATEMENTS
Dr. Goldwire has no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT
 Pharmacy
Pharmacy
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-22-043-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients’ conditions and possible contraindications or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
GOAL
To provide participants with an overview of coronavirus disease 2019 (COVID-19) as of March 3, 2022.
OBJECTIVES
After completing this activity, the participant should be able to:
- Outline the types of diagnostic tests available for detecting severe acute respiratory syndrome coronavirus 2.
- Discuss the evaluation of patients with symptomatic and asymptomatic COVID-19.
- Describe the current therapies granted Emergency Use Authorization for COVID-19.
- Identify patients eligible for COVID-19 vaccine and booster doses.
ABSTRACT: Coronavirus disease 2019 (COVID-19) has captured the world’s attention over the past 2 years as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has adapted to its environment, forming new variants and causing over 5.5 million deaths globally—including more than 950,000 in the United States—as of March 3, 2022. The FDA granted Emergency Use Authorization for non–FDA-approved therapies to treat and prevent COVID-19. Associations have developed guidelines for COVID-19 prevention and treatment, and the Infectious Diseases Society of America provides up-to-date information. Molecular, antigen, and serologic diagnostic tests designed to detect SARS-CoV-2 are available but differ in sensitivity and specificity. Because of their accessibility, pharmacists play an integral part in allaying patients’ concerns and curbing the spread of COVID-19.
Coronavirus disease 2019 (COVID-19) has commanded the world’s attention over the past couple of years as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has adapted to its environment, forming new variants and causing more than 5.5 million deaths worldwide to date. The FDA has granted Emergency Use Authorization (EUA) for non–FDA-approved therapies to treat and prevent COVID-19, and the Infectious Diseases Society of America has developed guidelines. The information presented here is current as of March 3, 2022.
INTRODUCTION
Variants
Viruses mutate over time as they adjust to environmental factors, which in some cases necessitates changes in the vaccines developed for prevention. The CDC monitors the circulation of SARS-CoV-2 and subsequent variants, which are classified as variants being monitored, variants of interest, variants of concern, or variants of high consequence.1 Over time, SARS-CoV-2, which causes COVID-19, has mutated into a number of variants, including B.1.1.7 (Alpha), B.1.617.2 (Delta), and B.1.1.529 (Omicron), the last two of which are considered variants of concern.2,3 The CDC has identified 10 variants being monitored.1 No variants of interest or of high consequence have been identified as of March 3, 2022.
TRANSMISSION, SYMPTOMS, MORBIDITY, AND MORTALITY
Transmission: Transmission of SARS-CoV-2 occurs through direct face-to-face contact with someone who is infected with the virus.4,5 Individuals are at high risk for becoming infected when they are within 6 feet of respiratory droplets released via a cough, sneeze, or oral secretions.4 Analysis of samples of nasopharyngeal, rectal, or anal secretions; placental cord blood; or neonatal amniotic fluid, along with immunoglobulin M serologic testing, can confirm vertical transmission with a pooled sampling proportion of 3.2% (95% CI, 2.2%-4.3%).6 Once infection occurs, viral shedding takes place 1 to 3 days before, and for up to 5 days after, symptom onset.4 Preventative therapies include physical distancing by at least 6 feet and mask-wearing.4,7
Symptoms: After contact, initial symptoms appear within 2 to 14 days, with nearly all patients developing symptoms within 12 days.5,8 Common symptoms include fever or chills, cough, shortness of breath or difficulty breathing, fatigue, muscle or body aches, headache, new loss of smell or taste, sore throat, congestion or runny nose, nausea/vomiting, and diarrhea.8 However, some people with COVID-19 are asymptomatic, and some patients may exhibit severe symptoms (hypoxia, pneumonia). A meta-analysis of 44 studies (N = 26,884 patients) concluded that the most common symptoms associated with COVID-19 with a high degree of specificity (true negatives) or sensitivity (true positives) are fever, loss of smell or taste, cough, chest tightness, nausea/vomiting, and diarrhea.9
The Infectious Diseases Society of America (IDSA) recommends that asymptomatic patients be tested for SARS-CoV-2: 1) regardless of exposure if they are immunocompromised or prior to transplantation (hematologic or solid organ); 2) with no known exposure if they are admitted to the hospital in geographical areas of high COVID-19 prevalence; or 3) if they are undergoing major surgery or bronchoscopy.10 Symptomatic patients exhibiting at least one of the most common symptoms (e.g., cough, shortness of breath/difficulty breathing, fever) should be tested for SARS-CoV-2.10 The IDSA does not make a recommendation for or against testing asymptomatic patients with cancer or autoimmune diseases prior to initiation of immunosuppressive therapy.10
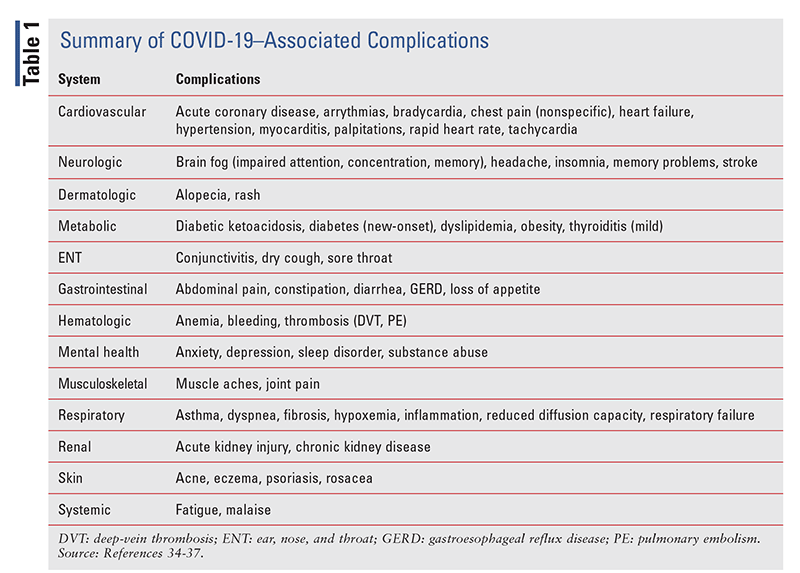
Morbidity: Complications of COVID-19 vary according to patient comorbidities, and nearly every organ system can be adversely affected by the virus. A retrospective study that assessed medical records for 973 patients with COVID-19 who were admitted to the ICU from March 1, 2020, to March 1, 2021, showed that upon ICU discharge, 67.4% of patients had at least one new medical condition, and the mean number of medications increased from five at admission to eight at discharge.11 Residual effects of COVID-19 may linger for months after the initial infection, and these patients may be known as “COVID long haulers.”3 The most common effect is trouble breathing, but fatigue, chest pain, and neurologic disorders have also been reported. TABLE 1 highlights COVID-19–associated complications.
Mortality: As of March 3, 2022, COVID-19 has been linked to 5,977,416 deaths worldwide, including 954,594 in the United States, according to the Johns Hopkins University of Medicine Coronavirus Resource Center (https://coronavirus.jhu.edu/map.html). Mortality in patients with COVID-19 is often associated with older age and the presence of comorbidities such as chronic lung disease, cardiovascular disease, hypertension, and diabetes, but younger patients without comorbidities may also be at risk for severe outcomes such as multiorgan failure and death.10 A meta-analysis of data from 42 studies (N = 423,117 patients) from December 2019 to August 2020 found that risk factors for increased mortality in hospitalized patients with COVID-19 included older age (odds ratio [OR] = 2.61; 95% CI, 1.75-3.47); male sex (OR = 1.45; 95% CI, 1.41-1.51); current-smoker status (OR = 1.42; 95% CI, 1.01-1.83); and comorbidities, specifically chronic obstructive pulmonary disease (OR = 1.58; 95% CI, 1.08-2.07), cardiovascular disease (OR = 1.83; 95% CI, 1.50-2.17), diabetes (OR = 1.52; 95% CI, 1.36-1.69), hypertension (OR = 1.57; 95% CI, 1.27-1.87), obesity (OR = 1.34; 95% CI, 1.17-1.52), acute kidney injury (OR = 1.87; 95% CI, 1.48-2.26), and increased d-dimer (OR = 10.49; 95% CI, 1.80-19.18).12
TESTING AND SCREENING
Diagnostic tests can provide evidence of the presence of SARS-CoV-2 infection. Three types of tests are available for diagnosis of COVID-19: molecular, antigen, and serologic (antibody).
Molecular Testing
Molecular (viral) tests, also known as nucleic acid amplification tests, amplify (i.e., make more copies of) viral genetic material, which then causes a reaction that indicates the result. One such test, polymerase chain reaction (PCR), detects ribonucleic acid (RNA) from a current or recent exposure to SARS-CoV-2. With SARS-CoV-2 infection, genetic material is present in the patient’s nasal or throat secretions.13 The PCR test converts singlestranded RNA into double-stranded DNA by reverse transcription. Short sequences called primers attach to the DNA strands, and as this occurs, fluorescent dyes attach to the DNA, providing a marker. This test detects exposure more accurately than the antigen test.10
Antigen Testing
Antigen testing, also referred to as the rapid test, is available for patient at-home use. The antigen test detects viral proteins from a nasal or throat swab and provides results within minutes. False-negative results are more common than false-positive results; however, specimen integrity impacts the result. This test is less accurate than molecular testing.10
Serologic Testing
This type of test assesses the blood for the presence of antibodies to SARS-CoV-2, which indicates a past infection. The presence of antibodies does not denote current infection and may cause false-positive results. Antibody testing does not replace virologic testing, and it should not be used to establish the presence or absence of infection.14 The IDSA recommends serologic testing for: 1) patients with a high suspicion of COVID-19 when molecular testing is negative and at least 2 weeks have passed since symptom onset, 2) assessment of multisystem inflammatory syndrome in children, and 3) conducting serology surveillance studies.10
The Pharmacist’s Role in Testing
Even before COVID-19 was declared a global pandemic, the U.S. government developed the Federal Retail Pharmacy Program for COVID-19 Vaccination to provide expanded access to COVID-19 vaccines at no charge to people nationwide.15 This program includes 21 retail pharmacies (more than 41,000 locations) as well as longterm-care pharmacies. As of January 12, 2022, community pharmacies have reported more than 215.9 million COVID-19 vaccinations administered by staff.15
Pharmacies may offer point-of-care (POC) testing provided that a Clinical Laboratory Improvement Amendment (CLIA) waiver from the Center for Medicare and Medicaid Services is obtained. With a CLIA waiver, pharmacists are permitted to order and administer FDA-authorized SARS-CoV-2 tests. Interestingly, the number of pharmacies with CLIA waivers nearly doubled (from 17.94% to 27.63%) between 2015 and 2020.16 Tests designed for at-home use do not need a CLIA waiver, whereas tests granted EUA status that are intended for use outside the home require a CLIA waiver.17 To increase testing, the federal government granted pharmacists the ability to order and administer COVID-19 tests.18,19 Despite this federal law, individual state laws may preclude direct pharmacist involvement with testing. According to a review of state laws, 11 states have laws precluding pharmacists from independently performing CLIA-waived testing and pharmacists are required to test in collaboration with a physician.20
Throughout the pandemic, pharmacists have been instrumental in delivering quality care. In a patient-perception survey of 2,200 Hispanic or lower-income patients, pharmacist POC services for COVID-19 were well received: 98% of respondents agreed or strongly agreed that they were satisfied with the testing services, 97.6% stated that their access to healthcare services was improved, 88% to 90% felt more knowledgeable about COVID after speaking to the pharmacist, and 97.6% were willing to undergo testing again.21 Pharmacists have also delivered care via telehealth.22
Regulation, Accelerated Approval, and Emergency Use
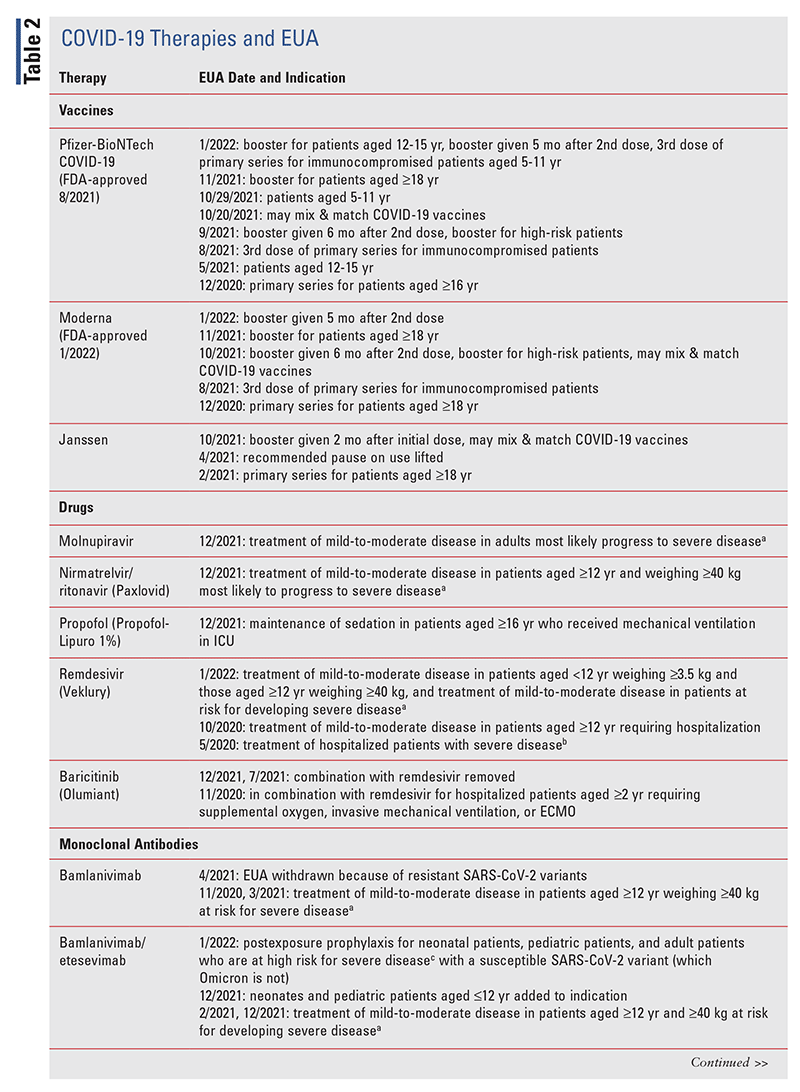
The FDA acted quickly to grant EUA for needed therapies. According to the Food, Drug, and Cosmetic Act, the FDA may issue an EUA in the presence of chemical, biological, radiological, and nuclear threats, thereby allowing non–FDA-approved therapies to be manufactured and administered.23 In February 2020, the Health and Human Services Secretary declared that the circumstances surrounding the COVID-19 pandemic warranted the use of an EUA, which became effective in March 2020. Specific indications for therapies granted EUA constantly change as new information becomes available and as the prevalence of SARS-CoV-2 variants shifts.
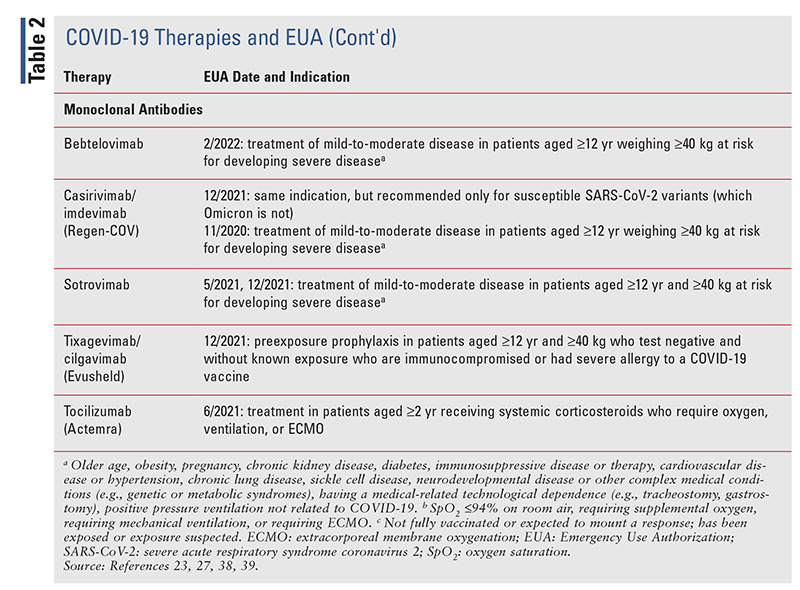
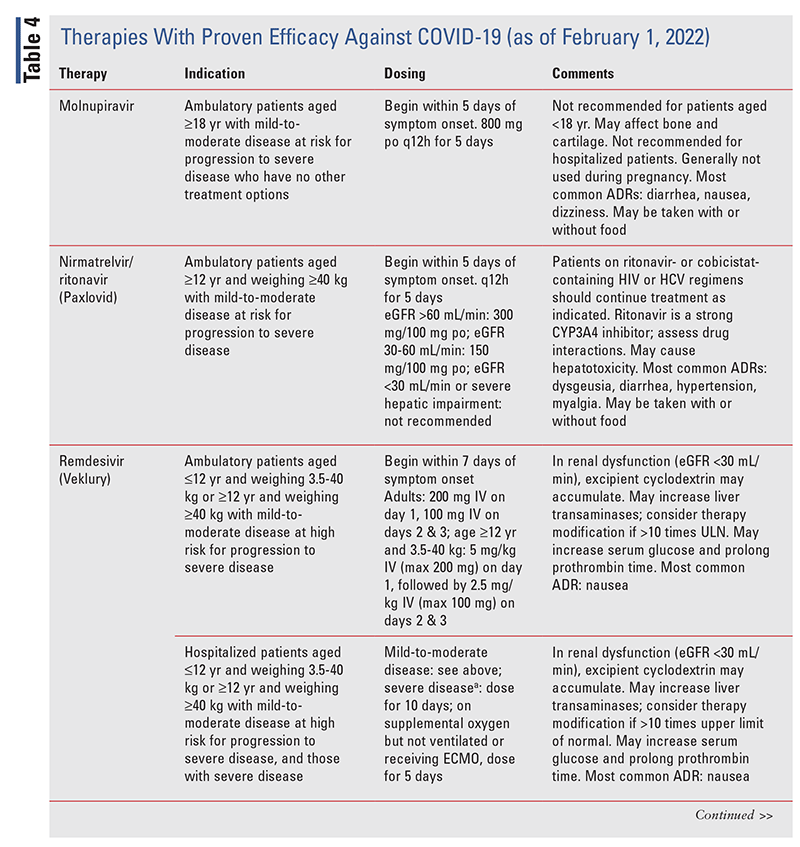
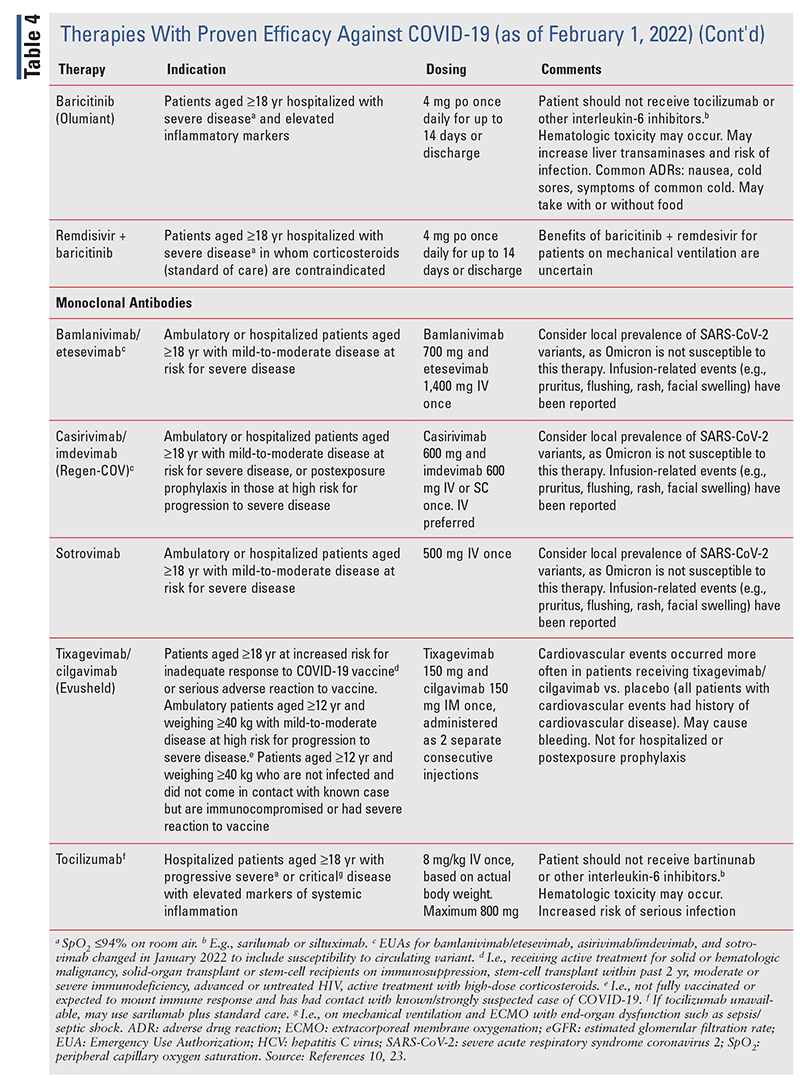
TABLE 2 lists the EUA changes made during the COVID19 pandemic.
VACCINES
Efficacy
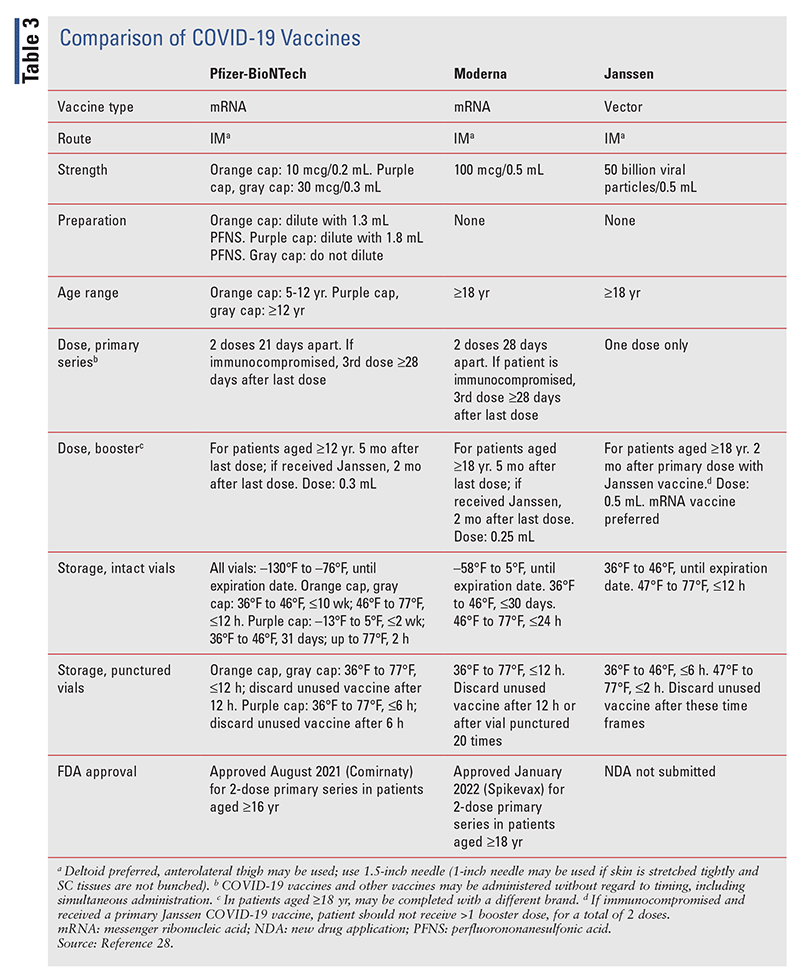
The efficacy of the COVID-19 vaccines wanes over time, necessitating subsequent doses. An analysis of the Israel Ministry of Health database found that in 4,696,865 patients aged 16 years or older who had received two doses of BNT162b2 (Pfizer-BioNTech) at least 5 months earlier, those who received a booster dose had a 10 times lower rate of confirmed infection than those who did not receive a booster dose.24 TABLE 3 describes the EUA-approved COVID-19 vaccines.
In a case-control study that assessed the association of SARS-CoV-2 symptoms with vaccination secondary to the Delta or Omicron variant in patients who either received two or three doses of the Pfizer-BioNTech or Moderna vaccine or were unvaccinated, receipt of three doses increased the odds of protection against both variants, with more protection afforded against Delta.25 Compared with unvaccinated patients, patients who received three vaccine doses had 67.3% greater odds of protection against Omicron (OR = 0.33; 95% CI, 0.31-0.35) and 93.5% greater protection against Delta (OR = 0.065; 95% CI, 0.059-0.071). Receipt of three doses provided more protection than two doses, with 66% greater odds against Omicron (OR = 0.34; 95% CI, 0.32-0.36), and 84% greater odds against Delta (OR = 0.16; 95% CI, 0.14-0.17).
An interim analysis of data from 243 adolescents aged 12 to 17 years who completed the primary vaccine series with the Pfizer-BioNTech vaccine (2 doses >14 days before) demonstrated 92% effectiveness.26 Receipt of the Janssen COVID-19 vaccine showed that a booster dose increased preprimary vaccination antibody titers eightfold and approximately higher than prebooster levels.27 Results show that vaccination protects against symptomatic COVID-19.
Safety
The COVID-19 vaccines are contraindicated in patients with a history of severe allergic reaction after a previous dose. The Janssen vaccine is contraindicated in patients who had thrombosis with thrombocytopenia syndrome following a previous Janssen COVID-19 vaccine; recently, cases of immune thrombocytopenia have been reported.27,28 Caution should be exercised in any patient who has experienced an immediate allergic reaction to any vaccine or injectable therapy, including allergy shots; any patient who experienced an immediate nonsevere allergic reaction to a previous dose of COVID-19 vaccine within 4 hours of administration; or any patient with a moderate-to-severe illness.
Vaccine Adverse Event Reporting System data for the Pfizer-BioNTech COVID-19 vaccine showed that nonserious events (n = 4,149) were often reported (97%) in children aged 5 to 11 years.29 Adverse events included mild-to-moderate local and systemic reactions. Concerningly, the most common adverse events were related to administration errors (product preparation, 22.3%; incorrect dose, 16.3%; underdosing, 7.8%).
Patients who complete their primary series rarely develop severe outcomes, based on a review of 1,228,664 patients who completed primary vaccination from December 2020 to October 2021.30 Severe outcomes and deaths were rare (0.015% and 0.0033%, respectively). Risk factors for severe outcomes included older age (>65 years), immunosuppression, diabetes mellitus, and chronic disease of the kidney, liver, heart, lung, or nervous system. All patients with severe outcomes (n = 1,748) had at least one risk factor, and 78% of patients who died had at least four risk factors. The most commonly occurring adverse events were local injection-site reactions, fever, headache, fatigue, chills, myalgias, and arthralgias.30
VACCINE HESITANCY
Vaccines are effective only when they are administered, and successful immunization programs require high rates of vaccination. In the age of social media, the spreading of misinformation about vaccine effectiveness has fueled public mistrust, and the current political environment also has contributed to SARS-CoV-2 vaccine mistrust.31 A recent systematic review demonstrated that vaccine hesitancy was highest among the black/African American population and in pregnant or breastfeeding women, and that vaccine hesitancy was driven by lack of education and understanding of the vaccine-development process.32
Pharmacists are poised to combat misinformation, as 90% of the population lives within 5 miles of a community pharmacy.33 One proposed method for engaging in vaccine conversations with patients is the ASPIRE framework33:
• Assume a desire for vaccination and anticipate patient’s questions.
• Share key facts to counter misinformation.
• Present strong recommendations to vaccinate and stories about vaccination experiences.
• Initiate discussion; proactively address questions about adverse effects.
• Respond to questions and actively listen.
• Empathize with the patient’s concerns.
When speaking with patients, the pharmacist should be mindful that positive, presumptive language directed at the patient’s own health and the health of others may minimize vaccine hesitancy.31 Paraphrased examples include31:
• Affirming values: “I can tell that you truly care about [taking proper care of your health; protecting your loved ones’ health; protecting your community]. Getting the COVID-19 vaccine helps you do that.”
• Explaining motivation for misinformation: “I understand your concern about [adverse effects; vaccine safety; need for vaccine; timing of vaccine]. This is a common misperception that has been fueled by popular media.”
• Repeating factual information: “There is extensive evidence regarding [documented COVID-19 vaccine safety; the mild, temporary character of adverse effects; the need for individuals to get vaccinated as soon as the vaccine is available to them; the vaccine’s importance in protecting people and ending the pandemic].”
GUIDELINES FOR PREVENTION AND TREATMENT
Various associations have developed guidelines for the prevention and treatment of COVID-19, and the IDSA provides up-to-date information on prevention and treatment.10 During the course of the COVID19 pandemic, several medications whose use for this purpose was not supported by scientific evidence widely gained traction, including hydroxychloroquine with or without azithromycin, ivermectin, famotidine, and fluvoxamine. These therapies are not recommended by the IDSA.10 Therapies with proven efficacy are described in TABLE 4.
CONCLUSION
The COVID-19 pandemic continues to challenge the medical community in many ways, largely because therapies must keep evolving as SARS-CoV-2 mutates and thereby prolongs its circulation. The potential for infection remains despite the existence of therapies for prevention, and the number of deaths is rising even as therapies are developed. The use of non–FDA-approved therapies through the EUA program has provided the necessary tools to combat the virus and, it is to be hoped, curb the pandemic.
REFERENCES
- CDC. SARS-CoV-2 variant classifications and definitions. www. cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html. Accessed January 26, 2022.
- Eyre DW, Taylor D, Purver M, et al. Effect of Covid-19 vaccination on transmission of alpha and delta variants. N Engl J Med. 2022;386(8):744-756.
- Eroglu B, Nuwarda RF, Ramzan I, Kayser V. A narrative review of COVID-19 vaccines. Vaccines (Basel). 2021;10(1):62.
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782-793.
- Meyerowitz EA, Richterman A, Gandhi RT, Sax PE. Transmission of SARS-CoV-2: a review of viral, host, and environmental factors. Ann Intern Med. 2021;174(1):69-79.
- Kotlyar AM, Grechukhina O, Chen A, et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am J Obstet Gynecol. 2021;224(1):35.e3-53.e3.
- CDC. COVID-19: quarantine and isolation. www.cdc.gov/ coronavirus/2019-ncov/your-health/quarantine-isolation.html. Accessed February 25, 2022.
- CDC. Symptoms of COVID-19. www.cdc.gov/coronavirus/2019ncov/symptoms-testing/symptoms.html. Accessed January 27, 2022.
- Struyf T, Deeks JJ, Dinnes J, et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease. Cochrane Database Syst Rev. 2020;7(7):CD013665.
- Infectious Diseases Society of America. IDSA guidelines on the treatment and management of patients with COVID-19. www.idsociety.org/COVID19guidelines. Accessed January 25, 2022.
- Mohammad RA, Nguyen CT, Costello PG, et al. Medication changes and diagnosis of new chronic illnesses in COVID-19 intensive care unit survivors. Ann Pharmacother. 2022 Jan 13;10600280211063319.
- Dessie ZG, Zewotir T. Mortality-related risk factors of COVID19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis. 2021;21(1):855.
- National Human Genome Research Institute. Understanding COVID-19 PCR testing. www.genome.gov/about-genomics/factsheets/Understanding-COVID-19-PCR-Testing. Accessed January 27, 2022.
- CDC. Interim guidelines for COVID-19 antibody testing. www. cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html. Accessed January 27, 2022.
- CDC. The Federal Retail Pharmacy Program for COVID-19 vaccination. www.cdc.gov/vaccines/covid-19/retail-pharmacy-program/ index.html. Accessed March 14, 2022.
- Klepser NS, Klepser DG, Adams JL, et al. Impact of COVID-19 on prevalence of community pharmacies as CLIA-Waived facilities. Res Social Adm Pharm. 2021;17(9):1574-1578.
- FDA. In vitro diagnostics EUAs—molecular diagnostic tests for SARS-CoV-2. www.fda.gov/medical-devices/coronavirus-disease2019-covid-19-emergency-use-authorizations-medical-devices/in-vitrodiagnostics-euas-molecular-diagnostic-tests-sars-cov-2. Accessed January 28, 2022.
- U.S. Department of Health & Human Services. Guidance for licensed pharmacists, COVID-19 testing, and immunity under the PREP Act. www.hhs.gov/sites/default/files/authorizing-licensed-pharmacists-to-order-and-administer-covid-19-tests.pdf. Accessed January 26, 2022.
- Hess K, Bach A, Won K, Seed SM. Community pharmacists roles during the COVID-19 pandemic. J Pharm Pract. 2020 Dec 15;897190020980626.
- Doucette J, Lavino J. Ability of community pharmacists to independently perform CLIA-waived testing—a multistate legal review. Explor Res Clin Soc Pharm. 2021;2:100024.
- Patel J, Christofferson N, Goodlet KJ. Pharmacist-provided SARS-CoV-2 testing targeting a majority-Hispanic community during the early COVID-19 pandemic: results of a patient perception survey. J Am Pharm Assoc (2003). 2022;62(1):187-193.
- Segal EM, Alwan L, Pitney C, et al. Establishing clinical pharmacist telehealth services during the COVID-19 pandemic. Am J Health Syst Pharm. 2020;77(17):1403-1408.
- FDA. Emergency Use Authorization. www.fda.gov/emergencypreparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization#coviddrugs. Accessed January 26, 2022.
- Bar-On YM, Goldberg Y, Mandel M, et al. Protection against Covid-19 by BNT162b2 booster across age groups. N Engl J Med. 2021;385(26):2421-2430.
- Accorsi EK, Britton A, Fleming-Dutra KE, et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 Omicron and Delta variants. JAMA. 2022;327(7):639-651.
- Lutrick K, Rivers P, Yoo YM, et al. Interim estimate of vaccine effectiveness of BNT162b2 (Pfizer-BioNTech) vaccine in preventing SARS-CoV-2 infection among adolescents aged 12–17 years—Arizona, July–December 2021. MMWR Morb Mortal Wkly Rep. 2021;70(5152):1761-1765.
- FDA. Coronavirus disease 2019 (COVID-19). www.fda.gov/ emergency-preparedness-and-response/counterterrorism-and-emerging-threats/coronavirus-disease-2019-covid-19#eua. Accessed January 30, 2022.
- CDC. COVID-19 vaccine quick reference guide for healthcare professionals. www.cdc.gov/vaccines/covid-19/downloads/covid19vaccine-quick-reference-guide-2pages.pdf. Accessed January 30, 2022.
- Hause AM, Baggs J, Marquez P, et al. COVID-19 vaccine safety in children aged 5–11 years—United States, November 3–December 19, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(5152):17551760.
- Yek C, Warner S, Wiltz JL, et al. Risk factors for severe COVID-19 outcomes among persons aged ≥18 years who completed a primary COVID-19 vaccination series—465 health care facilities, United States, December 2020–October 2021. MMWR Morb Mortal Wkly Rep. 2022;71(1):19-25.
- Finney Rutten LJ, Zhu X, Leppin AL, et al. Evidence-based strategies for clinical organizations to address COVID-19 vaccine hesitancy. Mayo Clin Proc. 2021;96(3):699-707.
- Yasmin F, Najeeb H, Moeed A, et al. COVID-19 vaccine hesitancy in the United States: a systematic review. Front Public Health. 2021;9:770985.
- Shen AK, Tan ASL. Trust, influence, and community: why pharmacists and pharmacies are central for addressing vaccine hesitancy. J Am Pharm Assoc (2003). 2022;62(1):305-308.
- Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601-615.
- Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594(7862):259264.
- Jain U. Effect of COVID-19 on the organs. Cureus. 2020;12(8):e9540.
- Barrett CE, Koyama AK, Alvarez P, et al. Risk for newly diagnosed diabetes >30 days after SARS-CoV-2 infection among persons aged <18 years—United States, March 1, 2020–June 28, 2021. MMWR Morb Mortal Wkly Rep. 2022;71(2):59-65.
- FDA. Letter of authorization—Pfizer-BioNTech COVID-19 vaccine. January 3, 2022. www.fda.gov/media/150386/download. Accessed January 26, 2022.
- FDA. Moderna COVID-19 vaccine letter of authorization. January 31, 2022. www.fda.gov/media/144636/download. Accessed January 31, 2022.