Assessing Aspirin for Primary CVD Prevention
RELEASE DATE:
February 1, 2019
EXPIRATION DATE:
February 28, 2021
FACULTY:
Jelena Lewis, PharmD
Assistant Professor of Pharmacy Practice
Chapman University School of Pharmacy
Irvine, California
Laressa Bethishou, PharmD, BCPS
Assistant Professor of Pharmacy Practice
Chapman University School of Pharmacy
Irvine, California
Laura V. Tsu, PharmD, BCPS, BCGP
Assistant Professor of Pharmacy Practice
Chapman University School of Pharmacy
Irvine, California
FACULTY DISCLOSURE STATEMENTS:
Drs. Lewis, Bethishou, and Tsu have no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT:
Pharmacy
 Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-19-006-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE:
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER:
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients' conditions and possible contraindications or dangers in use, review of any applicable manufacturer's product information, and comparison with recommendations of other authorities.
GOAL:
To provide the pharmacist with an overview of the role of aspirin for primary prevention and to review the most recent data about the use of aspirin for primary prevention.
OBJECTIVES:
After completing this activity, the participant should be able to:
- Review the mechanism of action of aspirin.
- Describe the role of aspirin for primary prevention.
- Recall current guideline recommendations for aspirin use for primary prevention.
- Discuss the most recent data regarding aspirin use for primary prevention.
ABSTRACT: Aspirin has been used for various indications for many years. It also may play a role in primary and secondary prevention of cardiovascular disease. The vast majority of evidence supports the use of aspirin for secondary prevention. However, for primary prevention, the use of aspirin is debatable. Three studies published recently address the issue of aspirin use in primary prevention. Based on the results of these studies, the use of aspirin for primary prevention remains controversial, and its use in this role should be individualized.
While several trials support the efficacy of daily aspirin therapy in secondary prevention of cardiovascular disease (CVD), the evidence supporting the benefit in primary prevention is inconclusive.1 The potential benefit in cardiovascular (CV) risk reduction must be weighed against the increased risk of bleeding. This article will discuss the role of aspirin in primary prevention, review the current guideline recommendations of aspirin use for primary prevention, and describe updates to evidence-based literature on the role of aspirin for primary prevention.
MECHANISM OF ACTION
Aspirin irreversibly blocks cyclooxygenase 1 and 2 (COX-1 and COX-2) enzymes, which results in the inhibition of thromboxane A2 (TXA2) and prostacyclin (PGI2) synthesis. The generation of TXA2 leads to platelet aggregation, vasoconstriction, and proliferation of vascular smooth-muscle cells and is proatherogenic.2 By inhibiting this pathway, aspirin reduces risk for myocardial infarction (MI) and thrombosis. In contrast to the TXA2 pathway, the generation of PGI2 leads to vasodilation and inhibition of both platelet aggregation and proliferation of vascular smooth-muscle cells.2 It is antiatherogenic, gut protective, and helps regulate renal blood flow.2 Since low-dose aspirin does not have a considerable effect on the PGI2 pathway or on the COX-2 inhibition, it does not impact renal function or raise blood pressure.2 However, because low-dose aspirin irreversibly blocks COX-1, this may increase risk of gastrointestinal (GI) bleeding due to inhibition of platelet aggregation by TXA2 and dose-dependent inhibition of PGI2, whose gut protective function may be negated when its synthesis is blocked.2 As a result, the use of antisecretory medications such as proton-pump inhibitors is recommended in patients who have a high risk of bleeding and who are taking aspirin daily.
Aspirin has been studied in doses of as low as 75 mg and as high as 650 mg for primary and secondary prevention. When aspirin is taken once daily, even at low doses, it has a cumulative antithrombotic effect.3 This is because it irreversibly blocks the cyclooxygenase pathway.3 Therefore, when used for CVD prevention, the most commonly used dose of aspirin in the United States is 81 mg. Lower aspirin doses may pose less of a risk for GI bleeding. However, certain patients at a high risk for GI bleeding are recommended to take an antisecretory medication, such as a proton-pump inhibitor, even when taking low-dose aspirin.4,5 Based on the systematic evidence review for the United States Preventive Services Task Force (USPSTF) done for the years 2008 to 2015 on aspirin for primary prevention of CV events, higher doses of aspirin do not provide greater benefit.6
ROLE OF ASPIRIN IN PRIMARY PREVENTION
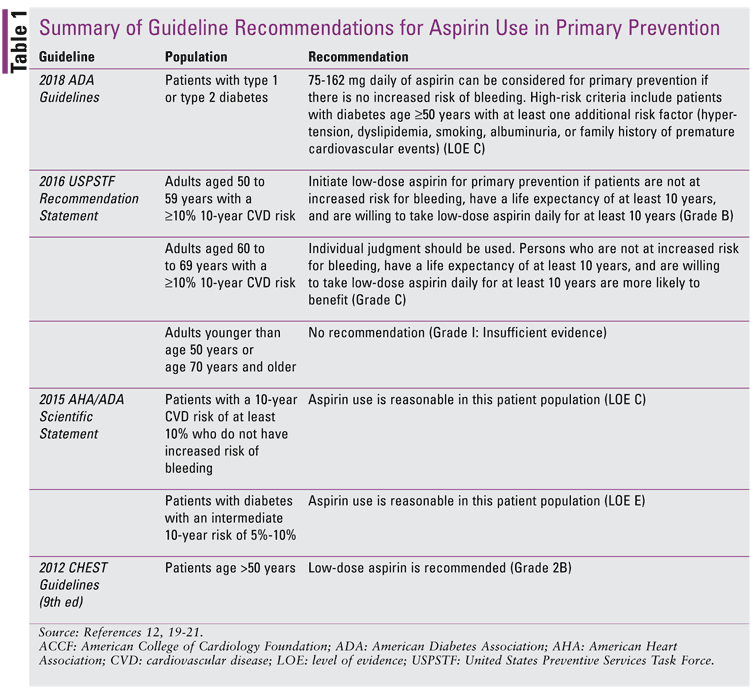
Aspirin has been manufactured and marketed since 1899, and it has mainly been used as an anti-inflammatory, antipyretic, and analgesic medication.2 It also plays a role in primary and secondary prevention of CVD. Primary prevention is defined as a means to prevent the onset of disease such as CVD, while secondary prevention aims to prevent the already existing disease from becoming worse. In the case of aspirin, secondary prevention refers to helping reduce CVD progression. Although the use of aspirin for secondary prevention has been proven to be beneficial, its use for primary prevention remains controversial because the risk of bleeding may outweigh the benefit of prevention of CVD.7
Because the current guidelines are inconsistent in recommending aspirin for primary prevention and there has been a decrease in the use of aspirin for primary prevention in the U.S., it is evident that more research needs to be done in this area.
Numerous trials have evaluated the efficacy of aspirin for primary prevention. One of the more noteworthy of these studies was the Antithrombotic Trialists' Collaboration, which evaluated the risk reduction of serious vascular events such as MI, stroke, or vascular death in patients without CVD using long-term aspirin versus control. This trial was a meta-analysis that evaluated six primary prevention trials and totaled 95,000 patients in the aspirin group.8 The findings indicated a risk reduction of 0.51% for the aspirin group versus 0.57% for the control group per year (P = .0001).8 This represents a 12% proportional reduction in serious vascular events in the aspirin group.8 This was mainly due to reduction in nonfatal MIs, as the effect on stroke and vascular mortality was nonsignificant.8 Nonetheless, there was an increase of 0.03% per year in major GI and extracranial bleeds in the aspirin group (0.10% in aspirin group vs. 0.07% in placebo group, P <.0001).7,8 The doses of aspirin used in the evaluated trials were 75 mg daily, 100 mg daily, 100 mg on alternate days, 325 mg daily on alternate days, and 500 mg daily.8
Since the publication of this meta-analysis, other randomized trials of aspirin for primary prevention of CVD were done, and none of these trials concluded that aspirin should be used routinely for primary prevention of CVD.2 One of these trials was the Japanese Primary Prevention Project (JPPP), which included 14,464 patients aged 60 to 85 years who presented with hypertension, dyslipidemia, or diabetes mellitus and who were randomized to either aspirin 100 mg daily or no aspirin.9 After 5 years, the researchers concluded that the primary outcome event rate, which was death from CV causes, did not differ significantly between the two groups.9 However, there was an increased risk in extracranial hemorrhage requiring transfusion or hospitalization in the aspirin group.9 A recent metaanalysis from 2016, which also included the JPPP trial, showed that aspirin prevents MI events (relative risk [RR] 0.78%; 95 CI, 0.65-0.94) and increases risk for major and intracranial bleeding; however, it does not reduce total stroke or all-cause vascular death.10
Since the publication of the 2009 USPTF recommendations, aspirin use for the role of primary prevention in the primary-care setting has decreased.11 Because the current guidelines are inconsistent in recommending aspirin for primary prevention and there has been a decrease in the use of aspirin for primary prevention in the U.S., it is evident that more research needs to be done in this area.7,11,12 Current guideline recommendations for aspirin use in primary prevention are listed in TABLE 1. The most recent studies addressing these issues and published in 2018 are discussed in the remainder of this article and summarized in TABLE 2.
RECENT DATA ARRIVE
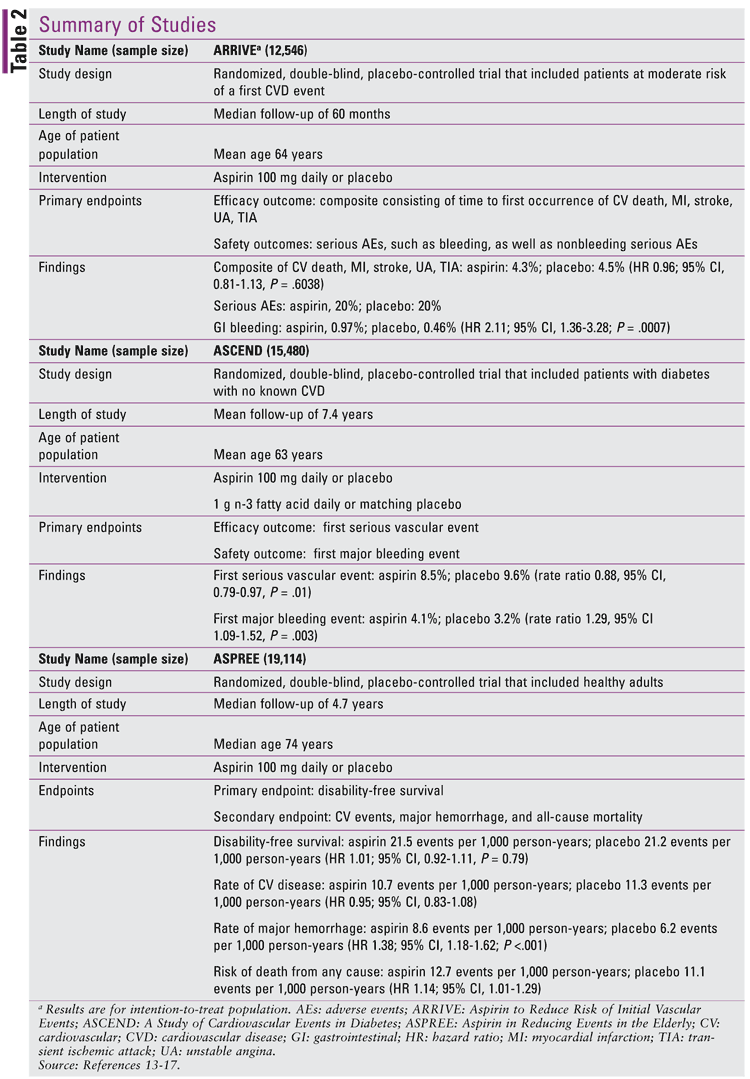
The Aspirin to Reduce Risk of Initial Vascular Events (ARRIVE) study was a randomized, double-blind, placebo-controlled trial done across seven countries (six in Europe and the U.S.).13
Study Design
This study included 12,546 patients at moderate risk of a first CV event who were randomly assigned to taking either aspirin 100 mg daily or placebo. Patients were excluded if they had diabetes, if they were taking antiplatelet therapy, or if they had a previous CV event (stroke, MI, coronary artery angioplasty or stenting), vascular intervention, coronary artery bypass, significant arrhythmias, or congestive hearth failure. Patients at a high risk for bleeding, such as those with history of bleeding ulcers, taking nonsteroidal anti-inflammatory medications frequently, or taking anticoagulants, were also excluded from the study. The mean age of patients was 64 years, and 30% of patients were female.
At baseline, the mean Framingham 10-year coronary heart disease risk score was 14% for both aspirin and placebo groups, and the mean estimate ACC/ AHA 10-year athersclerotic CVD risk score was 17% for both groups. Analyses were done for intention-totreat and per-protocol group.
Results
The primary efficacy outcome was a composite outcome consisting of time to first occurrence of CV death, MI, stroke, unstable angina, or transient ischemic attack (TIA).13 Safety outcomes were only assessed for the intention-to-treat population, and these included serious adverse events such as bleeding (including hemorrhagic stroke and GI bleeding) as well as nonbleeding serious adverse events. Patients were enrolled from July 2007 to November 2016 and had a median follow-up of 60 months. The primary efficacy outcome for the intention-to-treat population occurred in 4.3% of the aspirin group versus 4.5% in the placebo group (hazard ratio [HR] 0.96; 95% CI, 0.81-1.13, P = .6038).
In line with previous studies, the ARRIVE study results demonstrated that the use of aspirin was not beneficial in patients with a moderate risk for coronary artery disease and that aspirin was not associated with lower CV events.
Secondary outcomes included a composite of the time to first occurrence of CV death, MI, or stroke, time to first occurrence of these events individually, as well as time to first occurrence of unstable angina, time to first occurrence of TIA, and time to first occurrence in any death. These all showed a P value that was nonsignificant between the two groups for the intention-to-treat population. The incidence of serious adverse events was about 20% for both groups. Gastrointestinal bleeding occurred in 0.97% of the aspirin-treated group versus 0.46% in the placebo group (HR 2.11; 95% CI, 1.36-3.28; P = .0007). Mild GI bleeding was the most common adverse event on the severity scale of mild, moderate, and severe.
ASCEND
Aspirin use in patients with diabetes was evaluated in A Study of Cardiovascular Events in Diabetes (ASCEND).14
Study Design
This trial included 15,480 patients with either type 1 or type 2 diabetes with no history of CVD who were randomized to either aspirin 100 mg daily or matching placebo, along with either 1 gram of n-3 fatty acid once daily or placebo.14 Patients were excluded if they had a clear indication or contraindication to aspirin, or a condition that may have affected medication adherence for at least 5 years. All patients were recruited by screening questionnaires in the United Kingdom and were only included if they completed an initial run-in period of 8 to 10 weeks with adherence to the trial regimen. The mean age of patients in this study was 63 years, and approximately 62% were males. Most patients had type 2 diabetes (94%), and the median duration of diabetes was 7 years. Most patients (82%) had a low or moderate vascular risk score.
In the ASPREE Study, which evaluated the role of aspirin in primary prevention of CVD in the geriatric patient population, participants were required to be free from documented CV and cerebrovascular disease. Results demonstrated that the use of aspirin was not beneficial in patients with a moderate risk for coronary artery disease and that aspirin was not associated with lower CV events.
Results
The primary efficacy outcome was the first vascular event, which included MI, stroke, or TIA, or death from any vascular cause, excluding any confirmed intracranial bleeding.14 The primary safety outcome was the first major bleeding event, which included intracranial hemorrhage, sight-threatening bleeding event in the eye, GI bleeding, or other serious bleeding.
Patients were enrolled from June 2005 to July 2011 and were followed up for a mean of 7.4 years. A significantly lower percentage of patients developed a first vascular event in the aspirin group compared to the placebo group (8.5% vs. 9.6%; 95% CI, 0.79-0.97; P = .01). An exploratory analysis shows that this benefit was mainly seen in the first 5 years of therapy. However, the aspirin group also had a higher rate of bleeding (4.1% vs. 3.2%; 95% CI, 1.09-1.52, P = .003), which was consistent over time. Most bleeding events were in the GI tract (41.3%), followed by sight-threatening bleeding (21.1%) and intracranial bleeding (17.2%). The study also reported no difference in the development of GI cancer as a secondary outcome, which affected approximately 2.0% of participants in each group.
ASPREE
The Aspirin in Reducing Events in the Elderly (ASPREE) trial evaluated the role of aspirin in primary prevention of CVD in the geriatric patient population.15-17
Study Design
This randomized double-blind, placebo-controlled trial investigated whether 100 mg of enteric-coated aspirin daily would prolong the lifespan of older adults.15-17 The study was conducted in Australia and the United States. Between 2010 and 2014, community-dwelling, relatively healthy elderly adults aged 70 years or older (or age 65 years and older among blacks and Hispanics in the U.S.) were enrolled in this primary prevention study. A total of 19,114 persons were enrolled, with 9,525 patients assigned to receive aspirin and 9,589 to receive placebo. Study participants were required to be free from any chronic illness that would limit survival to less than 5 years and free from documented CV and cerebrovascular disease. Patients were excluded if they had a diagnosis for dementia, known high risk of bleeding, or a contraindication to aspirin.
Results
Median age was 74 years, and the patient population was predominantly white, with 8.7% nonwhite patients.15-17 In Australia, recruitment involved collaboration with the primary care physician, whereas in the U.S., recruitment was community based through academic health centers. Participants were contacted by phone quarterly and asked to attend in-person visits annually. The trial was terminated at a median of 4.7 years of follow-up after it was determined there would be no benefit with regards to the primary endpoint. The rate of adherence to the assigned intervention was 62.1% in the aspirin group and 64.1% in the placebo group in the final year of trial participation.
Disability-Free Survival in the Healthy Elderly: The primary endpoint was a composite of death, dementia, or persistent physical disability.15 The rate of the primary endpoint was 21.5 events per 1,000 person years in the aspirin group and 21.2 per 1,000 person years in the placebo group (HR 1.01; 95% CI, 0.92-1.11; P = .79). Among participants who had a primary endpoint event, death was the most common first event, accounting for 50% of events. Dementia was the next most common, accounting for 30% of events, and persistent physical disability was the least common, accounting for 20% of events. The lack of effect was consistent across all baseline subgroups as well, except frailty, in which the effect was unclear.
Cardiovascular Events and Bleeding in the Healthy Elderly: Secondary endpoints included major bleeding and cardiovascular disease (fatal coronary heart disease, nonfatal MI, stroke, or hospitalization for heart failure).16 After a median of 4.7 years of follow-up, the rate of CVD was 10.7 events per 1,000 person years in the aspirin group and 11.3 events per 1,000 person-years in the placebo group (HR 0.95; 95% CI, 0.83-1.08). The rate of major hemorrhage was significantly higher in the aspirin group, with 8.6 events per 1,000 person-years compared with 6.2 events per 1,000 person-years in the placebo group (HR 1.38; 95% CI 1.18-1.62; P <.001). Major hemorrhagic events primarily involved upper GI and intracranial bleeding.
All-Cause Mortality in the Healthy Elderly: The evaluation of all-cause mortality in the healthy elderly was another secondary endpoint evaluated in this study. A total of 1,052 deaths occurred during the 4.7 years of follow-up.17 The risk of death from any cause was 12.7 events per 1,000 person-years in the aspirin group and 11.1 events per 1,000 person-years in the placebo group (HR 1.14; 95% CI 1.01-1.29).17 The higher mortality in the aspirin group was primarily attributed to cancer, accounting for 1.6 excess deaths per 1,000 person-years. Cancer-related death occurred in 3.1% of the participants in the aspirin group and in 2.3% of those in the placebo group (HR 1.31; 95% CI, 1.10-1.56). The impact of major hemorrhage only contributed minimally. Other primary prevention trials have not shown similar results with regard to increased mortality in cancer patients taking aspirin.
SUMMARY OF RECENT DATA ASSOCIATED WITH ASPIRIN FOR PRIMARY CVD PREVENTION
The ARRIVE study demonstrated that the use of aspirin did not prove to be beneficial in patients who have a moderate risk for coronary artery disease and that aspirin use was not associated with lower CV events. Increased risk of GI bleeding occurred in the aspirin group; however, the events were mild in severity.
The ASCEND trial showed that while aspirin significantly reduced the risk for a first vascular event in patients with diabetes who have a low or moderate vascular risk, its benefits were offset by the significantly increased risk for bleeding complications.
The role of aspirin is much better defined for secondary prevention compared with primary prevention of CV events because the benefits of aspirin for secondary events outweigh the risks for bleeding. As a result, the use of aspirin for primary prevention should be individualized based on a patient's preference, risk factors, and financial barriers.
The ASPREE study showed that aspirin did not prolong independent living in the healthy elderly, such as a life free of dementia or persistent physical disability. Furthermore, in this study, rates of bleeding events increased in healthy adults aged 70 years and older who took aspirin, and there was no reduction in CV events in these patients. There was a higher death rate in the aspirin-treated group, and this was primarily attributed to death due to cancer. These results are interesting, as aspirin has been shown to reduce cancer risk.18 Until we have further analysis of this data and additional studies on this topic, the results should be interpreted with caution.
Collectively, these studies showed that the use of low-dose aspirin in patients without history of ASCVD may pose greater risk for bleeding than provide a benefit for primary prevention of CVD. Clinicians should focus on modifiable risk factors, such as blood pressure, cholesterol, smoking cessation, and weight loss prior to recommending aspirin for primary prevention. For patients who are already taking aspirin for primary prevention, the risks and benefits should be discussed with regard to whether aspirin should be continued. Aspirin may be beneficial for primary prevention in patients with high risk for CV events (i.e., 10-year ASCVD risk of 20% or higher or patients with diabetes and high vascular risk score). Age and bleeding risk should be taken into consideration, and therapy should be individualized based on a patient's risk factors.
CONCLUSION
The role of aspirin is much better defined for secondary prevention compared with primary prevention of CV events. This is because the benefits of aspirin for secondary events outweigh the risks for bleeding, while in primary prevention this risk-benefit assessment of aspirin is inconclusive. The three studies published in 2018 and discussed throughout this article attempted to better define aspirin's role for primary prevention of CV events. However, all three studies were in line with many of the previously published studies, which found that the benefits of aspirin do not outweigh the risks when it comes to primary prevention. The use of aspirin for primary prevention should be individualized based on a patient's preference, risk factors, and financial barriers. The decision to use aspirin for primary prevention, especially for patients at low risk for CVD, remains a decision that should be made after a thorough patient-provider discussion.
REFERENCES
- Ferreiro JL, Angiolillo DJ. Diabetes and antiplatelet therapy in acute coronary syndrome. Circulation. 2011;123:798-813.
- Capodanno D, Angiolillo DJ. Aspirin for primary cardiovascular risk prevention and beyond in diabetes mellitus. Circulation. 2016;134:1579-1594.
- Patrono C. Low-dose aspirin in primary prevention: cardioprotection, chemoprevention, both, or neither? Eur Heart J. 2013;34:3403-3411.
- Lanas A, Bajador E, Serrano P, et al. Nitrovasodilators, lowdose aspirin, other nonsteroidal antiinflammatory drugs, and the risk of upper gastrointestinal bleeding. N Engl J Med. 2000;343:834-839.
- Bhatt DL, Cryer BL, Contant CF, et al; COGENT Investigators. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363:1909-917.
- Guirguis-Blake JM, Evans CV, Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:804-813.
- Piepoli MF, Hoes AW, Agewall S, et al. ESC Scientific Document Group. 2016 European guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37:2315-2381.
- Baigent C, Blackwell L, Collins R, et al. Antithrombotic Trialists' (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849-1860.
- Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA. 2014;312:2510-2520.
- Raju N, Sobieraj-Teague M, Bosch J, et al. Updated metaanalysis of aspirin in primary prevention of cardiovascular disease. Am J Med. 2016;129:e35-e36.
- Van't Hof JR, Duval S, Walts A, et al. Contemporary primary prevention aspirin use by cardiovascular disease risk: impact of US Preventive Services Task Force Recommendations, 2007-2015: a serial, cross-sectional study. J Am Heart Assoc. 2017;6.
- Bibbins-Domingo K. U.S. Preventive Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164:836-845.
- Gaziano JM, Brotons C, Coppolecchia R, et al. ARRIVE Executive Committee. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392:1036-1046.
- Bowman L, Mafham M, Wallendszus K, et al. ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379:1529-1539.
- McNeil JJ, Woods RL, Nelson MR, et al. ASPREE Investigator Group. Effect of aspirin on disability-free survival in the healthy elderly. N Engl J Med. 2018;379:1499-1508.
- McNeil JJ, Wolfe R, Woods RL, et al; ASPREE Investigator Group. Effect of Aspirin on Cardiovascular Events and Bleeding in the Healthy Elderly. N Engl J Med. 2018;379:1509-1518.
- McNeil JJ, Nelson MR, Woods RL, et al; ASPREE Investigator Group. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379:1519-1528.
- Mills EJ, Wu P, Alberton M, et al. Low-dose aspirin and cancer mortality: a meta-analysis of randomized trials. Am J Med. 2012;125:560-567.
- American Diabetes Association. Cardiovascular disease and risk management: standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S86-S104.
- Fox CS, Golden SH, Anderson C, et al. American Heart Association Diabetes Committee of the Council on Lifestyle and Cardiometabolic Health, Council on Clinical Cardiology, Council on Cardiovascular and Stroke Nursing, Council on Cardiovascular Surgery and Anesthesia, Council on Quality of Care and Outcomes Research, and the American Diabetes Association. Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation. 2015;132:691-718.
- Vandvik PO, Lincoff AM, Gore JM, et al. Primary and secondary prevention of cardiovascular disease: antithrombotic therapy and prevention of thrombosis, 9th ed. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(Suppl 2):e637S-e668S.