Assessing the Current Antipsychotics Landscape
RELEASE DATE
May 4, 2020
EXPIRATION DATE
May 30, 2022
FACULTY
Tammie Lee Demler, BS, PharmD, MBA, BCGP, BCPP
Clinical Associate Professor, State University of New York at Buffalo
School of Medicine, Department of Psychiatry, Director of Psychiatric
Pharmacy Residency Programs, State University of New York at Buffalo
School of Pharmacy and Pharmaceutical Sciences
Buffalo, New York New York
FACULTY DISCLOSURE STATEMENTS
Dr. Demler has no actual or potential conflict of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT
 Pharmacy
Pharmacy
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-20-043-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER:
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients’ conditions and possible contraindications or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
GOAL
To educate pharmacists about the efficacy and tolerability of currently available antipsychotic agents and the appropriate use of these agents in individuals diagnosed with serious mental illness.
OBJECTIVES
After completing this activity, the participant should be able to:
- Identify currently available antipsychotics and their associated pharmacotherapeutic profiles.
- Recognize the associated side effects that differentiate these agents and opportunities to individualize treatment regimens.
- Review recent changes to FDA approvals, available formulations, and emerging safety information since 2015.
- Discuss opportunities for the pharmacist to ensure appropriate antipsychotic use and to improve the health of individuals diagnosed with mental illness.
ABSTRACT: Antipsychotic agents have been a part of our therapeutic arsenal for the past 70 years; however, the clinical benefits, emerging safety concerns, and availability of new agents continue to make medical news headlines. As pharmacists, keeping up with these advisories and the availability of new drug therapy is key to ensuring that patients with mental health conditions are receiving appropriate care. It is well recognized that individuals with serious mental illness can live longer, healthier lives when early intervention can prevent relapse and unnecessary hospitalization.
Antipsychotic agents were initially recognized in the 1950s, with chlorpromazine reported as first in class.1 This drug, along with others that came to market soon thereafter, were retrospectively termed first-generation antipsychotics (FGAs) when newer agents, such as clozapine (Clozaril), came to market in the 1980s as the initial second-generation antipsychotic (SGA) or atypical antipsychotic. The FGAs have also been referred to as conventional or typical antipsychotics.
Clozapine is the only antipsychotic agent that has repeatedly demonstrated benefit in treatment-resistant schizophrenia. Despite this, it is still considered vastly underutilized.2,3 Clozapine experienced an initially bumpy launch, with withdrawal from the market coming after its release due to its associated life-threatening blood dyscrasias and agranulocytosis. Once the FDA established a monitoring requirement, clozapine was again released to market and continues to be available today as the gold standard drug of choice for refractory schizophrenia.2-4
Fast forward to 2002, with the release of aripiprazole (Abilify), which at that time was considered a potential third-generation antipsychotic with dopamine partial agonist activities as its claim to fame. Since the release of aripiprazole, there has been a steady launch of SGAs with similar mechanisms of action, along with enhancements and modifications of preexisting formulations. Patent expirations over the past decade have also made long-standing brand-name products available as generic alternatives and accessible as more affordable medication-management interventions.
There has been little novelty in these new-to-market SGAs, with the exception of pimavanserin (Nuplazid), which was approved by the FDA in 2016 for the treatment of psychosis associated with Parkinson’s disease (PD).5 Granted breakthrough status in 2014 as a drug that demonstrated substantial improvement over currently available treatment for serious or lifethreatening conditions, the FDA allowed expedited development and review, leading to its faster approval.6 Pimavanserin owes its efficacy to serotonin (5HT) modulation through a combined effect of inverse agonism along with antagonism of 5HT2A and, to a lesser extent, 5HT2C. Because roughly half of patients with PD will experience hallucinations or delusions, and currently available dopamine antagonist antipsychotics (APS) only worsen the motor symptoms associated with PD, there was a tremendous need to establish alternative treatment interventions for PD psychosis (PDP). Before the availability of pimavanserin, quetiapine and clozapine were commonly prescribed for PDP.7
The FGAs are well recognized for their efficacy in controlling positive symptoms of schizophrenia, which include, but are not limited to, hallucinations and delusions, disorganized speech and thoughts, as well as bizarre behaviors considered to be the more visible and disruptive of symptoms. However, the use of FGAs left negative and executive symptoms largely unresolved.8,9 The mechanism of action of FGAs is almost entirely associated with dopamine antagonism, with the degree of modulation of dopamine varying based on the specific FGA used.
The SGAs are associated with much greater diversity of neurotransmitter effects; however, SGAs are generally recognized to share a balance of low-tomoderate D2 antagonism and high 5HT2A antagonism, with the exception of aripiprazole, brexpiprazole (Rexulti), cariprazine (Vraylar), and most recently, lumateperone (Caplyta), which share D2 partial agonist activity.10-14 It is also understood that the balance of this dopamine/serotonin ratio is the key to the reduced incidence of extrapyramidal symptoms (EPS) with the newer agents. The SGAs are frequently associated with a more anticholinergic profile, with the exception of some of the lower potency FGAs, such as chlorpromazine and thioridazine. These medications also have less EPS as a result of their unique receptor affinity profiles when compared with high-potency FGAs, such as haloperidol (Haldol) and fluphenazine (Prolixin).15,16
All APS agents, whether old or new, share similar efficacy when prescribed at relatively equivalent doses, and all APS share some common side effects while others are more likely reported in a specific class of APS.17 Huhn et al evaluated the safety and efficacy of 32 antipsychotic agents, and as expected, clozapine was still the most effective intervention, with a standard mean difference (SMD) in effect size when compared with placebo of -0.89 (94% credible interval [CrI], -1.08 to -0.71) versus placebo. It is important to note that while antipsychotic agents share similar efficacy, Huhn et al reported that olanzapine and risperidone may offer some additional benefit for certain patients (SMD, -0.56; 95% CrI, -0.62 to -0.48) and (SMD, -0.55; 95% CrI, -0.62 to -0.48), respectively.17 Therefore, it is important to review previously reported side effects to differentiate the benefit and potential role of newer APS as they become available.
ADRS AND SIDE EFFECTS DRIVE SELECTION
Differentiating Adverse Effects
All APS are associated with varying degrees of side
effects and adverse drug reactions (ADRs), ranging
from benign, common, and expected to more rare,
serious, and even life-threatening. Although the differentiation between ADRs and side effects can be challenging, it is recognized that both of these factors can
lead to decreased medication adherence and, eventually,
even the discontinuation of treatment. Thus, the choice
of APS agent is generally based on expected side effects,
since the efficacy is comparable when APS are dosed at
equivalent doses (TABLE 1).17-19
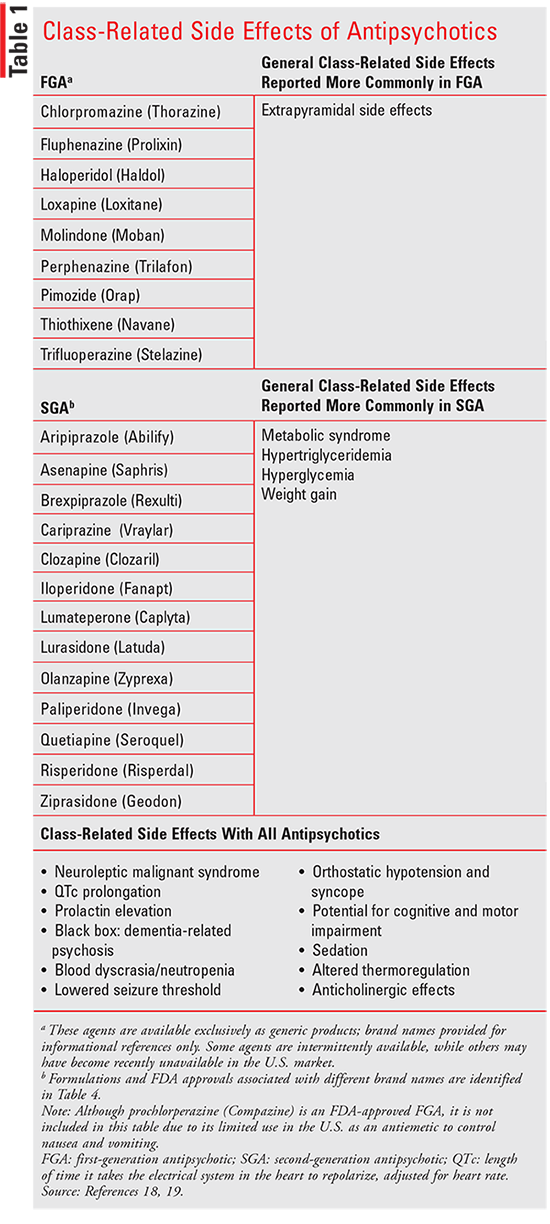
GENERAL CLASS-RELATED SIDE EFFECTS
Extrapyramidal Symptoms
EPS is an umbrella term that describes a variety of druginduced movement disorders that include dystonia,
akathisia, pseudoparkinsonism, and tardive dyskinesia.20 Because the FGAs are well known to cause EPS
and to worsen preexisting abnormal movements, the
SGAs have become a popular choice. In addition to a
number of other risk factors associated with SGAs,
patients who have experienced EPS when exposed to
FGAs in the past are more likely to experience EPS with
the SGAs.17 It is estimated that roughly 30% of patients
managed on an FGA will develop EPS, and in some
studies, this percentage is even higher.17
In behavioral health, it is important to establish a process of surveillance that monitors any emergence of EPS, and it is imperative to confirm the diagnosis of EPS in the presence of early indicators. Patients with psychiatric conditions may exhibit bizarre movements that are actually behaviorally associated. The Abnormal Involuntary Movements Scale (AIMS) is a method of establishing baseline movements with periodic routine monitoring to assess changes over time and with medication-regimen modifications.21
Symptoms of dystonia, which include prolonged abnormal muscular contractions, most often occurs at 48 hours and up to 5 days after drug exposure, either with initial treatment or with dose increases. These contractions can include spasm of the neck muscles, making swallowing and breathing more challenging.21 Pseudoparkinsonism is a constellation of symptoms that includes slow movement or difficulty initiating movement (akinesia and bradykinesia), tremor, pill rolling, cogwheel rigidity, slowed speech, and masked facial expressions, whereas akathisia is an abnormal movement that involves pacing, shuffling, and other restless behavior that would otherwise be considered purposeless movement. Responding to internal and external restlessness can even equate to foot tapping and reported compulsion to stay in continuous motion.
These EPS manifestations are generally more severe and occur with greater frequency with the use of highpotency FGAs, especially when higher doses are used and/or long-acting injections (LAIs) are administered. In the event this painful movement disorder occurs, discontinuation and/or dose decrease of the offending APS can be considered, as well as treat ment with the administration of anticholiner gic agents, such as diphenhydramine, benztropine, or trihexyphenidyl. The initiation of an alternative APS with a lower affinity (less strong) dopamine antagonism is a reasonable therapeutic plan for patients who have experienced dystonic reactions.12 Acute laryngeal dystonia is observed more commonly in younger males, but all patients treated with high-potency agents, especially those administered in depot long-acting formulations, are at risk for this ADR.21
Tardive dyskinesia (TD) is an abnormal involuntary movement and disfiguring condition that generally emerges after longer duration of antipsychotic use and is reported to worsen with stress and lessen to some degree at rest and when the individual is sleeping. These movements include the classic hallmark symptoms of tongue thrusting, lip smacking, grimacing, and chewing. Additionally, TD can also feature a more global symptom expression and include limb twisting and bodily rocking motions. Although considered rare, TD is a potentially permanent condition. The risks of TD increase for older women and individuals who have received high-potency APS for long duration and at high dose (daily and cumulative); however, the use of any antipsychotic agent is also considered a potential risk.22
Although prevention is key, TD may emerge unpredictably and will require urgent intervention. Prior to the launch of agents that selectively inhibit vesicular monoamine transporter 2 (VMAT2) in presynaptic neurons, such as deutetrabenazine (Austedo) and valbenazine (Ingrezza), treatment was limited to discontinuation of the offending agent since anticholinergic agents may actually mask the symptoms of TD, allowing a more severe case to develop without appropriate detection. While the VMAT2 inhibitors have demonstrated improvement in patients with TD, not all patients respond to treatment and, thus, these agents are not considered a cure for TD. Clozapine has been considered an additional intervention for patients who develop TD since there is evidence supporting switching to clozapine in order to reduce the risk of developing or reducing the severity of existing tardive dyskinesia.22,23
GENERAL SGA SIDE EFFECTS
Metabolic Symptoms
Although weight gain has been reported with certain
FGAs, the SGAs are well known for their metabolic
adverse effects, which include hypertriglyceridemia,
hyperglycemia, and weight gain. These side
effects are frequently linked to the development
of type 2 diabetes mellitus, which is among the
most frequently reported adverse outcome,
with clozapine and olanzapine most strongly
associated with metabolic side effects and the
development of metabolic syndrome.24 Individuals diagnosed with serious mental illness
(SMI) have a greater risk of premature all-cause
mortality than their age-matched counterparts
in the general population without SMI. An
examination of epidemiological studies reveals
that individuals with SMI experience a reduced
life expectancy up to 24 years shorter than the
general population. This is likely due to a multitude of factors, but predominately cardiovascular disease (CVD) associated with poor lifestyle choices, such as smoking and sedentary
behaviors, as well as medication-induced
risks.25,26 Essential consideration must be given to identify high-risk patients and carefully selection of an APS
with a lowest risk of metabolic side effects in order to
reduce the likely iatrogenic contribution to metabolic
syndrome and CVD.
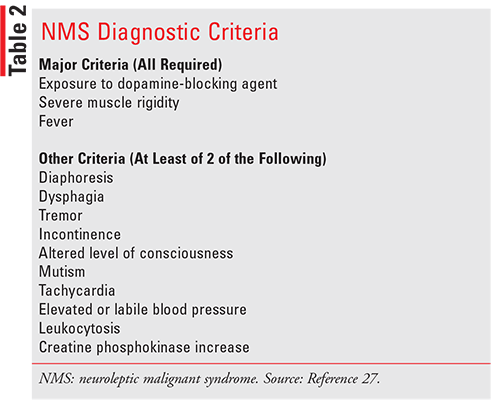
Neuroleptic Malignant Syndrome
Neuroleptic malignant syndrome (NMS) is a severe
adverse reaction that results from exposure to medications with strong dopamine receptor-antagonist properties. Although this reaction was originally reported
after APS first became available and has been associated with the use of both FGA and SGAs ever since,
NMS has also been reported to occur with a variety of
dopaminergic medications, including rapid withdrawal
of such drugs. Earlier reports revealed the incidence of
NMS to be as high as 3.2% of individuals exposed to
APS; however, more recently, with the availability of
newer SGAs with lower potential to cause NMS and increased awareness of the risks, the incidence has
reportedly declined to as low as 0.02%.27 This unpredictable and potentially fatal syndrome is characterized
by muscle rigidity, altered mental status, and autonomic
instability, as well as other clinical indicators, such as
elevated creatine phosphokinase (CPK), rhabdomyolysis with myoglobinuria, and acute renal failure, and it
is a medical emergency that can lead to death if not
urgently treated (TABLE 2).27
QTc Prolongation
Prolongation of the QT interval may lead to malignant
arrhythmia, torsades de pointes, and sudden cardiac
death. There are numerous medical and psychiatric
agents that are known to impact the QT interval, which
is also commonly referred to as the QTc interval when
corrected for heart rate. Therefore, it is important for
clinicians to be aware of drugs they prescribe which can
contribute to further prolongation. Antipsychotic agents
have varying degrees of potential to prolong the QTc,
and it important to remember that even those SGAs that
are well known to have a greater capacity than others
within class, such as ziprasidone (Geodon), may be
superseded by others within the FGA class, such as haloperidol. The risk of drug-induced QTc prolongation is
even greater with concomitant use of drugs with a shared
capacity to prolong the QTc interval and/or when used
in patients who have electrolyte abnormality, congenital
prolongation of the QT interval, or history of cardiac
arrhythmias.28,29
Prolactin Elevation
Medications such as APS that antagonize dopamine
D2 receptors can also increase prolactin levels, which
can persist throughout treatment when the medications are administered chronically. This side effect has
provoked continued debate about the clinical consequences of prolonged medication-induced hyperprolactinemia, which could result in reduced pituitary
gonadotropin secretion due to suppressed hypothalamic gonadotropin-releasing hormone (GnRH).30
Although impaired reproductive function is one concern, the more common patient reports of associated gynecomastia and erectile dysfunction are particularly concerning for male patients and amenorrhea and other menstrual abnormalities for female patients, with potential changes in bone density a concern for both. And although debate has ensued over the potential risk of breast cancer linked to prolonged prolactin elevation, the epidemiologic evidence is considered too limited to be conclusive and, therefore, does not support avoidance of APS when its use is deemed medically necessary.30
The degree to which antipsychotic agents can influence prolactin is highly variable. For example, in adult premarketing clinical trials, the incidences of adverse events related to abnormal prolactin levels for sublingual asenapine (Saphris) were 0.4% versus 0% for placebo. This compares with ranges as high as 49% for risperidone (Risperdal), which exhibits comparable D2 blockade as that seen in the FGAs.30,31
Mortality in Elderly Patients With
Dementia-Related Psychosis
The FDA issued its first wave of requirements
for updated SGA labeling to include a black box
warning (BBW) in 2005 that warned to avoid
the use of APS in patients with dementia-related
psychosis. This BBW was expanded to include
the FGAs just a few years later. This safety
warning was based on an analyses of 17 placebo-controlled trials examining patients with
dementia-related psychosis who were also taking SGAs. This analysis revealed an up to 1.7
times higher risk of death in the drug-treated
patients compared to those treated with placebo. The deaths were most often of cardiovascular origin, such as sudden death or heart failure, or infection, such as pneumonia.32 Even the
product label for pimavanserin now carries this
same BBW.5,33
The absolute observance of this BBW has represented challenges, however, when there are often no alternatives for patients experiencing behavioral and psychological symptoms of dementia (BPSD), which can include psychosis. Frequently, a shared decision must be made with caregivers to accept the lesser risk of potential harm of BBW when there is greater benefit with the use of APS to provide potential harm of BBW stability for the invidual who otherwise represents a danger to self or others. Inadequately treated BPSD can lead to unnecessary earlier institutionalization when caregiver stress is maximized.
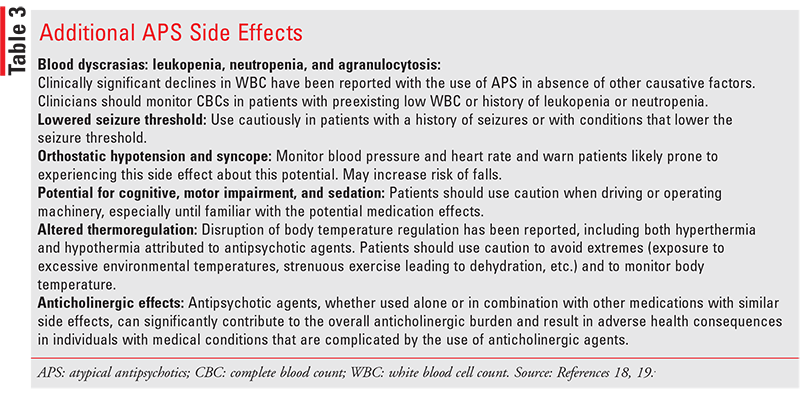
Antipsychotic agents approved for the adjunctive treatment of major depressive disorder have an additional BBW that warns of the risk of worsening depression and/or the emergence of suicidal ideation and behavior (suicidality) in individuals taking medication to treat depression.13 Additional classrelated side effects of APS are listed below, referencing package labels from illustrative examples of both the FGAs and SGAs, in TABLE 3.
FDA APPROVAL UPDATES
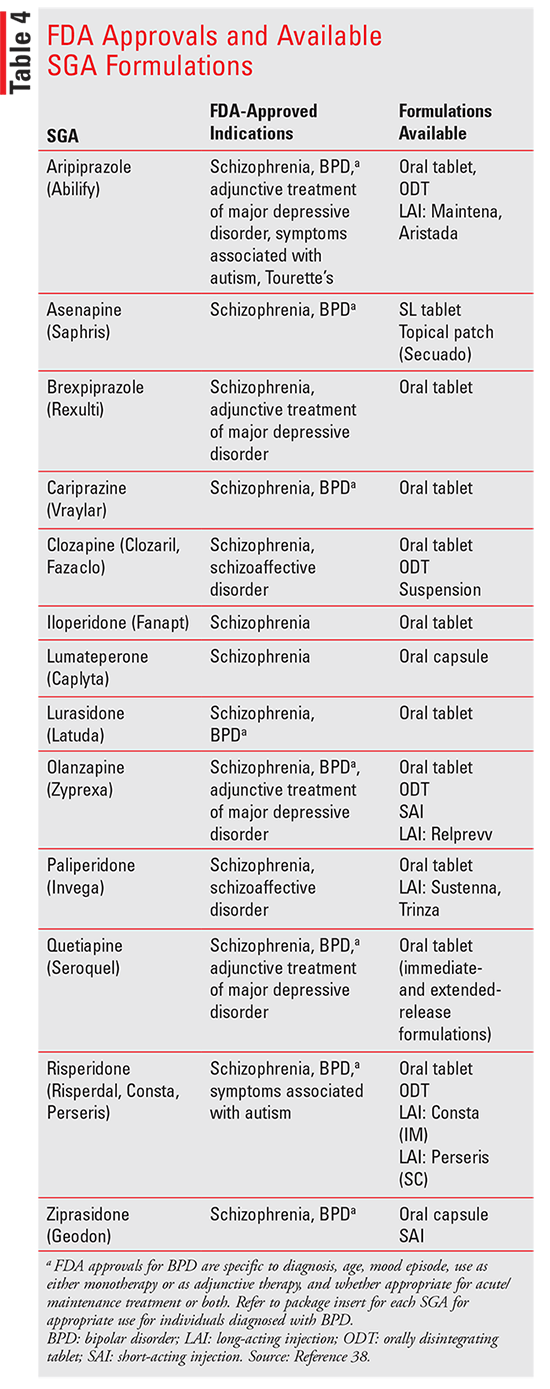
Asenapine (Saphris)
Approved in 2009 for the treatment of schizophrenia,
asenapine was granted FDA approval for a supplemental
new drug application in 2015 as monotherapy for the
acute treatment of manic or mixed episodes of bipolar I
disorder in pediatric patients aged 10 to 17 years.19
Cariprazine (Vraylar)
Initially approved for both schizophrenia and bipolar
disorder in 2015, cariprazine (Vraylar) received
expanded approval for the treatment of bipolar depression in 2019. This recent approval builds on the 2017
trial that demonstrated some unique clinical benefits of
using cariprazine to reduce negative symptoms of schizophrenia.14 When compared with risperidone in patients
randomly assigned to either drug for 26 weeks, patients
who received cariprazine experienced a greater mean
reduction in negative symptoms on the Positive and
Negative Syndrome Scale compared with risperidonetreated patients, with a small to medium effect size. Cariprazine did not have a differential effect on positive
symptoms, depression, or EPS but did improve social
functioning when compared with risperidone.34
Lurasidone (Latuda)
First approved in 2010 for schizophrenia, lurasidone
received FDA approval as both monotherapy and
adjunctive therapy in adults with bipolar depression
just 3 years later. In 2017, lurasidone received FDA
approval for the treatment of adolescents with schizophrenia and in 2018 was approved for the treatment
of bipolar depression in pediatric patients aged 10 to
17 years.35 Most FDA approvals of APS used for
bipolar disorder were initially for the acute treatment
of mania, with the indication expanding to more
complicated presentations such as the acute treat ment
of manic or mixed episodes and/or the maintenance
monotherapy treatment of bipolar I disorder. Because
there were limited APS options with demonstrated
efficacy for the depressive phase of bipolar disorder,
this additional approval for lurasidone was notable.36
Paliperidone (Invega)
Initially approved for schizophrenia in 2009, paliperidone, the metabolite of risperidone, received FDA
expanded-use approval for schizoaffective disorder in
late 2014, which allowed it to be promoted for this
unique therapeutic indication in 2015. Along with clozapine, which also shares this FDA indication, paliperidone is now officially approved to treat this specific
hybrid of symptoms of both schizophrenia and bipolarlike symptoms (TABLE 4).37
EMERGING SAFETY WARNINGS
Drug-induced dermatologic side effects are not a new challenge in the medication management of psychiatric illness. Rashes such as Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are considered delayed-type hypersensitivity reactions that, if left untreated or inadequately treated, can result in death. There is a great degree of overlap with the manifestation of either SJS/TEN with a prodromal phase that includes flu-like symptoms of malaise and fever, followed by skin and mucous membrane lesions along with other systemic symptoms.39 In December 2014, the FDA issued a new warning for a serious dermatologic condition called drug reaction with eosinophilia and systemic symptoms (DRESS) associated with the use of ziprasidone. Like SJS/ TEN, DRESS is also associated with fever and generalized rash along with swollen lymph glands. With a hallmark elevation of eosinophils, clinicians may use this key indicator to assist in identifying potential cases of DRESS and to avoid a delay in diagnosis, which could translate into further inflammation and damage to organs, such as the heart, liver, lungs, kidney or pancreas, and which could result in death.40 In 2016, the FDA expanded this same warning for DRESS for individuals who have taken olanzapine, which is available in a number of products and formulations (Zyprexa, Zyprexa Zydis, Zyprexa Relprevv, and Symbyax).41 DRESS is not a new phenomenon. Previously, this side effect was reported with the use of aromatic anticonvulsants (e.g., carbamazepine, phenytoin, and phenobarbital), sulfonamides, allopurinol, and, over the past decade, even with the use of lamotrigine.4
Unusual and Uncontrollable
(Compulsive) Urges
The FDA responded to reports of impulsivity associated with the use of aripiprazole that resulted in
updated labeling to advise of this warning in 2016.
Although compulsive behaviors have been linked to
dopamine agonist drugs, this was a newly recognized
side effect of these dopamine partial agonist antipsychotic agents. This impulsivity was reported to manifest
as strong, unusual urges that cannot be controlled and
have included compulsive gambling, sexual urges,
shopping, and binge eating.43 The FDA later required
brexpiprazole (Rexulti), also a partial dopamine agonist first approved in 2015, to include the same warning
in its package insert in 2018.13
New Aripiprazole Formulations
Aripiprazole is an SGA that experienced significant
changes over the past few years. Initially available only
in oral dosage forms, this SGA also later became available as a short-acting injection (SAI). However, with the
change to generic status, the SAI was phased out. New
LAI formulations became available with Maintena, a
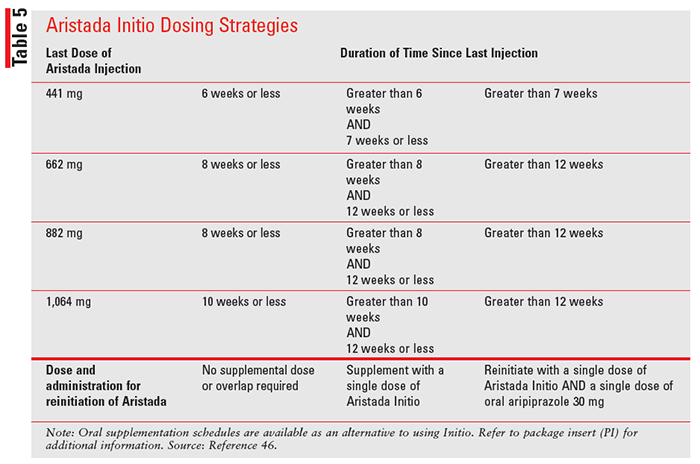
once-monthly injection, and later with aripiprazole lauroxil (Aristada), a prodrug of aripiprazole. Aristada is
administered every 2 months, and Aristada Initio, the
newest formulation of aripiprazole approved by the FDA
in July 2018, can be used to initiate Aristada or to restart
an interrupted Aristada regimen.12,44-46 Both Maintena
and Aristada require oral overlap, which is a period of
continued oral dosing during initial weeks of LAI administration. However, studies comparing the 1-day Initio
initiation regimen to the standard 21-day oral overlap
regimen when administered with Aristada LAI demonstrated comparable aripiprazole exposure and supported
the use of Initio as an alternative approach that was safe,
effective, and efficient.47 Additionally, when Initio is
used, there is opportunity to significantly shorten, and
sometimes entirely eliminate, the need for the oral supplementation, allowing the achievement of clinical efficacy without the worry of adherence to the concomitant
oral overlap. The Aristada Initio formulation contains a
dispersion of smaller nanocrystalline particles of aripiprazole lauroxil, allowing quicker dissolution and faster
release of active drug into plasma (TABLE 5).46
Aripiprazole tablets are also available with an ingestible event marker (IEM), which is a sensor that tracks the ingestion of the medication through a signal received from a sensor embedded in the tablet which is detected by a patch worn on the patient’s left side and transmitted to a smartphone app. Known as Abilify MyCite (aripiprazole tablets with sensor), this product records the time that an aripiprazole IEM tablet was taken, the activity level evidenced by the number of steps taken by the patient per day and the amount of time the individual sleeps.45 A patient can also record the reason that a dose was not taken, note how well he/she rested, and capture overall mood with an emoji.48 According to the manufacturer, the MyCite app has not been established to improve adherence or modify dosage, and the tracking data are not in real time. Therefore, the app should not be used for real or emergency monitoring because detection may be delayed or not occur at all (TABLE 6).45
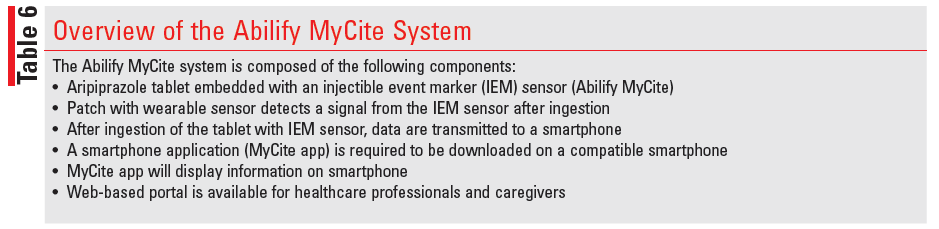
Although ingestion tracking data are most often detectable within a half hour, it is recommended that an additional window of up to 2 hours be considered in some cases. In the event there is no detection, repeat dosing is not recommended. Pharmacists should also reassure patients that a detection failure does not require a change in app or upgrade to their existing smartphone. In addition to the standard patch application procedures, which advise not applying to irritated, cracked, or inflamed skin, the MyCite patch should be placed as instructed by the app to the left side of the body, just above the lower edge of the rib cage, and should be changed weekly or sooner if necessary. According to the manufacturer, patch removal and replacement will be prompted by the app.45
New Asenapine Formulation
As an alternative to the sublingual (SL) formulation,
asenapine is now available as a topical patch that is
reapplied every 24 hours to either the hip, abdomen,
upper arm, or upper back area (TABLE 7).30 Asenapine (Secuada) is an alternative route of administration that
provides an advantage to individuals who otherwise
did not prefer the SL formulation because they have
experienced mouth soreness or found the flavor distasteful, or for those who experienced suboptimal treatment results due to inappropriately swallowing the SL
tablets instead of placing under tongue.30 Certain
adverse drug reactions reported during clinical trials
were fewer with the patch and others greater. For example, reports of somno lence, including sedation, lethargy, and hypersomnia were fewer with the patch;
however, there were a greater number of reports of
weight gain associated with the patch. Dosage-formulation specific ADRs were obviously absent in the alternative product label, such as oral hypoesthesia only for
SL tablets and application-site reaction only for the
topical patch.

Paliperidone (Invega)
Initially approved exclusively for oral administration,
the extended-release formulation not only limited the
dosing-regimen options but the presence of the
osmotic tablet shell in the stool, reported by the manufacturer as an expected consequence of elimination,
was thought to represent additional barriers and challenges for some patients taking this medication. This
limited product availability was expanded with the
launch of the extended-release Invega Sustenna LAI,
approved in 2009 for monthly administration. The
2009 news of the first parenteral paliperidone option
was superseded by the launch in 2015 of the first, and
cur rently only, LAI that can be administered every 4
months (quarterly). The four-times-a-year paliperidone palmitate, Trinza, requires initial treatment with
Sustenna prior to first use.49
CHANGES TO FDA-REQUIRED MONITORING
Clozapine REMS
Blood dyscrasias such as leukopenia and neutropenia
have been reported with the use of all APS. However,
clozapine has been the antipsychotic most commonly
recognized for dyscrasias. Agranulocytosis is defined as
an absolute neutrophil count (ANC) below 500/mm3 and has been reported to occur in up to 1% of all patients
taking clozapine. Although the greatest risk of developing agranulocytosis is reported to occur between weeks
6 and 18 of the first 6 months of treatment, patients may
still develop blood dyscrasias anytime during the treatment and are therefore required to be monitored by FDA
guidelines. Because possible risk factors for developing
dyscrasia include preexisting low blood counts, the FDA
requires an acceptable baseline WBC/ANC value that
applied to all patients initiated on clozapine. In the past,
patients with an established history of low WBC threshold and/or ANC were considered to be permanently
nonrechallengeable and not approved to receive clozapine therapy again in the future.50
In 2015, the FDA updated guidelines to reflect additional consideration of the recognized ethnic differences that contribute to seemingly low, but not harmful, blood counts in certain ethnicities when compared with accepted neutrophil ranges in the general population.51 Despite what appears clinically to be neutropenia, this benign ethnic neutropenia is not associated with medical illness or anticipated untoward events resulting from a decreased baseline neutrophil count. In further recognition of the importance of continuing an uninterrupted clozapine regimen, the FDA also discontinued the supplemental monitoring interventions required in the event of an intermittent substantial drop of WBC or ANC. These interventions frequently resulted in an unnecessary and often temporary interruption of therapy, often prompting a change to an undesirable, more frequent, monitoring schedule.50
New Role, New Pathway
Earlier studies have explored the modulation of the glutamate system as a novel pathway in the treatment of
schizophrenia and whether alterations in the glutamatergic neurotransmission may have an impact on downstream neurochemical systems, such as dopamine.52 The
use of glycine, D-serine, N-acetylcysteine, and sarcosine
in individuals diagnosed with schizophrenia has demonstrated varying degrees of benefit as well as adverse
effects.53,54 These N-methyl-D-aspartate (NMDA) receptor antagonists are associated with positive symptoms,
including hallucinations and delusions, as well as emotional detachment/withdrawal that closely resembles
what are considered negative symptoms observed in
schizophrenia. Stone et al, however, hypothesize that
dopamine antagonism and NMDA antagonism could
offer two unique pharmacotherapeutic interventions that
target different symptoms of the illness.55,56 Studies investigating drugs that increase NMDA receptor activity via
the glycine site by either synaptic glycine reuptake inhibition were found to be modestly effective on both positive
and negative symptoms when used as augmentation
strategies in schizophrenia.57
Lumateperone has high binding affinity for serotonin 5HT2A receptors and moderate binding affinity for dopamine D2 receptors and serotonin transporters, which it is capable of inhibiting.This APS has been promoted as unique among the SGAs based on its moderate binding affinity for dopamine D1 and D4, as well as reduced dopamine signaling due to its partial agonist activity at presynap tic dopamine D2 receptors, resulting in reduced pre synaptic release of dopamine along with antagonistic activity at postsynaptic dopamine D2 receptors.58,59 Lumateperone has low binding affinity for muscarinic and histaminergic recep tors and adrenergic alpha1A and alpha1B recep tors.11 The pharmocodynamic consequence of targeting dopamine (D1) receptors and D1 activation is increased glutamatergic NMDA GluN2B receptor phosphoryla tion.58-60 This is significant since NMDA-mediated glutamate signaling appears to be impaired in patients who have schizophrenia. The recommended dosage of lumateperone is 42 mg once daily with food, and although dose adjustments are recommended for patients on moderate or strong CYP3A4 inhibitors or inducers and for those with mild hepatic impairment, lumateperone is not recommended in individuals with moderate or severe impairment.11
Risperidone Monthly SC Injection
Risperidone monthly extended-release injectable suspension (Perseris) for SC abdominal administration
was approved by the FDA in 2018 for adults with
schizophrenia.61 The previous LAI formulation of
risperidone (Risperdal Consta) required more frequent administration of every 2 weeks. As with any
LAI, oral challenge to establish tolerability is a necessary safety measure; however, unlike Consta, supplemental oral overlap is not required or recommended.
Patients must be advised they should expect a lump to develop after administration that should diminish after a few weeks. They should also be warned that pressure from massaging, rubbing, or resulting from wearing tight-fitting clothing or belts can cause excessive drug release from the injection site and, thus, should be avoided to reduce the risk of potential adverse effects. Because reconstitution of Perseris is challenging, the clinician administering the injec tion should carefully review the instructions prior to use.61
ROLE OF THE PHARMACIST
Pharmacists have an important and unique opportunity to educate patients on the wide array of medications available to treat mental illness and to emphasize the importance of adherence to a regimen once a selection has been made. Pharmacists also provide a valuable resource to promote wellness initiatives that can improve cardiovascular health as an additional intervention to enhance quality of life and prolong life expectancy in this vulnerable patient population. Pharmacists can assist prescribers in selecting optimal agents and monitoring for ongoing safety and efficacy as well as reinforcing the importance of using antipsychotics for appropriate indications, even in cases where the risks noted on BBWs must be weighed against the potential benefits in patients who are otherwise a danger to themselves or others.
CONCLUSION
Pharmacists are among the most accessible healthcare professionals and are available to provide encouragement, offer support, and reduce stigma as an essential part of the allied healthcare team. With the recent wave of increased awareness of mental health conditions, coupled with the availability of new medications to treat these disorders, the general public must be encouraged to seek help when they or others in their care express or exhibit sudden changes in mood, behaviors, thoughts, and feelings and to talk to their pharmacist about both medical and mental health medications.
Pharmacists have an important and unique opportunity to educate their patients on the wide array of medications available to treat mental illness and to emplasize the importance of careful adherence to a regimen once a selection has been made.
References
- Bryan J. How chlorpromazine improved the treatment of schizophrenic patients. Acute Pain. 2019;10.
- Roerig J. L. Clozapine augmentation strategies. Mental Health Clinician. 2019;9(6):336-348.
- Gee SH. Giving the gold standard the cold shoulder: delay to clozapine use in treatment-resistant schizophrenia. Doctoral dissertation, King’s College London. 2019.
- Agranulocytosis. Changes for clozapine monitoring in the United States. Molecular Psychiatry. 2016;21:858-860.
- Nuplazid [package insert]. San Diego, CA: Acadia Pharmaceuticals Inc; 2016.
- FDA. Fact Sheet: Breakthrough Therapies. www.fda.gov/regulatory-information/food-and-drug-administration-safety-and-innovation-act-fdasia/fact-sheet-breakthrough-therapies. Accessed February 29, 2020.
- Meltzer HY, Mills R, Revell S, et al. Pimavanserin, a serotonin2A receptor inverse agonist, for the treatment of Parkinson’s disease psychosis. Neuropsychopharmacology. 2010;35(4):881-892.
- American Psychiatric Association. Diagnostic and Statistical Manual of
Mental Disorders (DSM-5). Arlington, VA: American Psychiatric Association; 2013. - Marder S, Fleischhacker WW, Earley W, et al. Efficacy of cariprazine across symptom domains in patients with acute exacerbation of schizophrenia: pooled analyses from 3 phase II/III studies. European Neuropsychopharmacology. 2019;29(1):127-136.
- Chokhawala K, Stevens L. Antipsychotic medications. In StatPearls. StatPearls Publishing. 2019.
- Caplyta [package insert]. New York, NY: Intra-Cellular Therapies, Inc; 2019.
- Aristada [package insert]. Waltham, MA: Alkermes, Inc; 2018.
- Rexulti [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc; 2018.
- Vraylar [package insert]. Parsippany, NJ; Actavis Pharma, Inc; 2019.
- Bergman H, Soares‐Weiser K. Anticholinergic medication for antipsychotic‐induced tardive dyskinesia. Cochrane Database of Systematic Reviews, 2018(1).
- D'Abreu A, Akbar U, Friedman JH. Tardive dyskinesia: epidemiology. J Neurological Sciences. 2018;389:17-20.
- Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. J. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. The Lancet. 2019;394(10202):939-951.
- Haldol [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2017.
- Saphris [package insert]. Whitehouse Station, NJ: Merck & Co., Inc; 2009.
- Fredericks D, Norton JC, Atchison, C, et al. Parkinson’s disease and Parkinson’s disease psychosis: a perspective on the challenges, treatments, and economic burden. The Am J Managed Care. 2017;23(5 Suppl):S83-S92.
- D’Souza RS, Hooten WM. Extrapyramidal symptoms (EPS) [Updated November 29, 2019]. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2020. www.ncbi.nlm.nih.gov/books/NBK534115.
- Anderson KE, Stamler D, Davis MD, et al. Deutetrabenazine for treatment of involuntary movements in patients with tardive dyskinesia (AIM-TD): a double-blind, randomised, placebo-controlled, phase 3 trial. The Lancet Psychiatry. 2017;4(8):595-604.
- Pardis P, Remington G, Panda, R., et al. Clozapine and tardive dyskinesia in patients with schizophrenia: a systematic review. J Psychopharmacology. 2019;33(10):1187-1198.
- Anderson KE, Stamler D, Davis MD, et al. Deutetrabenazine for treatment of involuntary movements in patients with tardive dyskinesia (AIM-TD): a double-blind, randomised, placebo-controlled, phase 3 trial. The Lancet Psychiatry. 2017;4(8):595-604.
- Hirsch L, Yang J, Bresee L, et al. Second-generation antipsychotics and metabolic side effects: a systematic review of population-based studies. Drug Safety. 2017;40(9):771-781.
- Penninx BW, Lange SM. Metabolic syndrome in psychiatric patients: overview, mechanisms, and implications. Dialogues in Clinical Neuroscience. 2018;20(1):63.
- Grundy SM. Metabolic syndrome update. Trends in Cardiovascular Medicine. 2016;26(4):364-373.
- Berman BD. Neuroleptic malignant syndrome: a review for neurohospitalists. The Neurohospitalist. 20111(1):41-47.
- Geodon [package insert]. New York, NY: Pfizer, Inc; 2014.
- Aronow WS, Shamliyan TA. Effects of atypical antipsychotic drugs on QT interval in patients with mental disorders. Annals of Translational Medicine. 2018;6(8):147.
- Secuada [package insert]. Miami, FL: Noven Therapeutics, LLC; 2019.
- Saphris [package insert]. Irvine, CA: Allergan USA, Inc; 2017.
- FDA expands mortality warnings on antipsychotic drugs. www.fdanews.com/articles/107752-fda-expands-mortality-warnings-on-antipsychotic-drugs. Accessed February 29, 2020.
- FDA approves first drug to treat hallucinations and delusions associated with Parkinson’s disease. www.fda.gov/news-events/press-announcements/fda-approves-first-drug-treat-hallucinations-and-delusions-associated-parkinsons-disease. Accessed February 29, 2020.
- Németh G, Laszlovszky I, Czobor P, et al. Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomised, double-blind, controlled trial. The Lancet. 2017;389(10074):1103-1113.
- Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc; 2018.
- Jauhar S, Young AH. Controversies in bipolar disorder; role of second-generation antipsychotic for maintenance therapy. International Journal of Bipolar Disorders. 2019;7(1):10.
- Paliperidone Titusville, NJ: Janssen Pharmaceuticals, Inc; 2017.
- FDA: drug approvals and databases. www.fda.gov/drugs/development-approval-process-drugs/drug-approvals-and-databases. Accessed February 29, 2020.
- Lerch M, Mainetti C, Beretta-Piccoli BT, Harr T. Current perspectives on Stevens-Johnson syndrome and toxic epidermal necrolysis. Clinical Reviews in Allergy & Immunology. 2018;54(1):147-176.
- FDA Drug Safety Communication: FDA reporting mental health drug ziprasidone (Geodon) associated with rare but potentially fatal skin reactions. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communicationfda-reporting-mental-health-drug-ziprasidone-geodon-associated-rare.
- FDA Drug Safety Communication: FDA warns about rare but serious skin reactions with mental health drug olanzapine (Zyprexa, Zyprexa Zydis, Zyprexa Relprevv, and Symbyax). www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-warns-about-rare-serious-skin-reactions-mental-health-drug.
- Han SH., Hur MS, Youn HJ, et al. Drug reaction with eosinophilia and systemic symptom syndrome induced by lamotrigine. Annals of Dermatology. 2017;29(2):206-209.
- FDA Drug Safety Communication: FDA warns about new impulse-control problems associated with mental health drug aripiprazole (Abilify, Abilify Maintena, Aristada) https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-warns-about-new-impulse-control-problems-associated-mental-health
- Abilify Maintena [package insert]. Tokyo, Japan: Otsuka Pharmaceutical Co., Ltd; 2016.
- Abilify MyCite [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc; 2020.
- Aristada Initio [package insert]. Waltham, MA Alkermes, Inc; 2018.
- Hard ML, Wehr AY, Sadler BM, et al. Population pharmacokinetic analysis and model-based simulations of aripiprazole for a 1-day initiation regimen for the long-acting antipsychotic aripiprazole lauroxil. European J Drug Metabolism and Pharmacokinetics. 2018;43(4):461-469.
- What is the Abilify MyCite System? www.abilifymycite.com/about. Accessed April 13, 2020.
- Invega Trinza [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2015.
- Demler TL, Morabito NE, Meyer CE, Opler L. Maximizing clozapine utilization while minimizing blood dyscrasias: evaluation of patient demographics and severity of events. International Clinical Psychopharmacology. 2016;31(2):76-83.
- FDA clozapine REMS Program. www.clozapinerems.com/CpmgClozapineUI/home.u Accessed February 29, 2020.
- Stone JM. Glutamate and dopamine dysregulation in schizophrenia-a synthesis and selective review. J Psychopharmacol. 2007;21;440-452.
- Stone, J. M. Glutamatergic antipsychotic drugs: a new dawn in the treatment of schizophrenia? Therapeutic Advances in Psychopharmacology. 2011;1(1):5-18.
- Leppien E, Blum C, Demler TL, Opler L. Adjunctive sarcosine and N-acetylcysteine use for treatment-resistant schizophrenia. J Neurology and Neurocritical Care. June 2018. http://researchopenworld.com/wp-content/uploads/2018/07/JNNC-18-103.pdf
- Stone JM, Erlandsson K, Arstad E, et al. Relationship between ketamine-induced psychotic symptoms and NMDA receptor occupancy—a [123 I] CNS-1261 SPET study. Psychopharmacology. 2008;197(3):401-408.
- Krystal JH, Perry EB, Gueorguieva, R., et al. Comparative and interactive human psychopharmacologic effects of ketamine and amphetamine: implications for glutamatergic and dopaminergic model psychoses and cognitive function. Archives of General Psychiatry. 2005;62(9):985-995.
- Tsai GE, Lin PY. Strategies to enhance N-methyl-D-aspartate receptor mediated neurotransmission in schizophrenia, a critical review and meta-analysis. Curr Pharm Des. 2010;16(5):522-537.
- Vyas P, Hwang BJ, Brasic JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2019;30:1-7.
- Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
- Kumar B, Kuhad A, Kuhad A. Lumateperone: a new treatment approach for neuropsychiatric disorders. Drugs Today. 201;54(12):713-719.
- Perseris [package insert]. North Chesterfield, VA Indivior Inc; 2018.