Biological Medication Use in Crohn's Disease
RELEASE DATE:
December 1, 2017
EXPIRATION DATE:
December 31, 2019
FACULTY:
Heather Shiflett, PharmD
PGY-1 Community Pharmacy Resident
Christ Health Center
Samford University
Tiajana Gonzales, PharmD
PGY-1 Community Pharmacy Resident
CVS Health
Samford University
Matthew Holland, PharmD
PGY-1 Community Pharmacy Resident
Chad's Payless Pharmacy
Samford University
Lindsey DeLoach, PharmD
Clinical Pharmacist, Gastrointestinal
Specialty Pharmacy Services
UAB Health System
John A. Galdo, PharmD, BCPS, BCGP
Assistant Professor
Community Pharmacy Residency Director
Samford University,
Birmingham, Alabama
FACULTY DISCLOSURE STATEMENTS:
Drs. Shiflett, Gonzales, Holland, DeLoach, and Galdo have no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT:
Pharmacy
 Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-17-097-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE:
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@jobson.com
DISCLAIMER:
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients' conditions and possible contraindications or dangers in use, review of any applicable manufacturer's product information, and comparison with recommendations of other authorities.
GOAL:
To empower pharmacists to understand the role of biological medications in the treatment of Crohn's disease.
OBJECTIVES:
After completing this activity, the participant should be able to:
- Describe risk factors for the development of Crohn's disease.
- Apply diagnostic criteria for patients with Crohn's disease.
- List biological agents based on mechanism of action.
- Understand the route of administration for commonly used biological medications.
ABSTRACT: Crohn's disease is a medical condition that impacts the entire gastrointestinal tract. Crohn's disease affects approximately 1.3% of adults in the United States and is an inflammatory disorder that is often treated with biological medications. Unfortunately, the use of biologics is not well defined for the treatment of Crohn's disease. Various agents with a primary mechanism of action of tumor necrosis factor inhibition, such as certolizumab and adalimumab, may be used. The pharmacist is able to play a vital role in the treatment of Crohn's disease through insurance navigation, reduction of adverse drug reactions, administration counseling, and adherence assistance.
Crohn’s disease (CD) is a chronic inflammatory bowel disease (IBD) characterized by mucosal ulceration and irritation anywhere along the gastrointestinal tract. It differs from ulcerative colitis in that ulcerative colitis primarily affects the colon. Although the etiology of CD is complex, it is characterized as an immune-mediated condition susceptible to many triggers. The inflammation in CD can lead to thickening of the bowel wall, as well as to lymphoid granulomas.1 In 2015, the CDC reported diagnosis rates of IBD to be an estimated 3.1 million, or 1.3% of adults in the United States.2 The median onset of the disease is age 30 years, and most patients are diagnosed between the ages of 20 and 30 years.3
Risk Factors
The cause of CD is still unknown; however, there are several factors that seem to increase the risk of the disease. Environmental factors, which include disruptions in the gut microbiome and smoking, may increase risk.3 For example, smoking doubles the risk of CD in both current and former smokers.3 The role of diet in the development of CD is still uncertain; further studies are needed.3 Subgroup analyses have shown increased prevalence in adults aged older than 45 years; Hispanics and non-Hispanic whites; those born in the U.S.; those living in suburban areas; and those living in poverty.2 Although family history has not been shown to directly increase risk of the disease, 10% to 25% of patients with IBD have a first-degree relative with the disease.3
Diagnosis
As with all IBDs, there are many variations in the clinical presentation of CD, and the signs and symptoms may mimic many other etiologies, making the differential diagnosis of CD somewhat challenging.3 Typical presentation will include persistent diarrhea, abdominal pain, cramping, nausea, and vomiting.4 Skip lesions, where inflammation skips sections of the gastrointestinal tract, are most characteristic of CD.3,5
Diagnosing CD requires a thorough approach, including conducting a physical examination and taking an accurate patient history to inquire about factors such as nighttime awakenings, weight loss, cigarette smoking, family history of CD, and the use of certain medications, including antibiotics and nonsteroidal anti-inflammatory drugs, which may be linked to CD.3,6 Tests including blood and stool sample evaluation, biopsies, and endoscopies and imaging studies (i.e., MRI or CT scans) may be performed to aid in ruling out other causes and/ or to confirm the diagnosis of CD. Imaging studies and endoscopies are most robust; there are currently no specific serologic markers that alone can rule out or confirm a diagnosis of CD. Bacterial, viral, and parasitic infections should also be ruled out, and testing for Clostridium difficile is encouraged, even in the absence of recent antibiotic use.4 While CD can and usually does have an insidious onset, the appearance of any clinical symptoms like the ones listed above should prompt further investigation to rule out other causes or to confirm a diagnosis of CD.
Classifying and Categorizing CD
CD is classified using the Montreal Classification of Crohn’s Disease. This system incorporates the age at diagnosis and the location of the disease, as well the differentiation between nonstricturing (inflammatory), stricturing (narrowing of the intestinal tract due to scarring), and penetrating disease. Severe disease may be considered penetrating when fistulas develop between the bowel and adjacent organs.3
Another tool used in clinical trials, and sometimes in clinical practice, is the Crohn’s Disease Activity Index (CDAI).3,4 The CDAI helps practitioners categorize the disease to determine disease severity and the effectiveness of treatment. One of the major downsides to the CDAI is timing—it may take up to 2 weeks to obtain all the needed information for this index. Other options may include the Harvey-Bradshaw index, or endoscopic tests like the Rutgeerts score.3,4
Guidelines
The American Gastroenterological Association (AGA) and the World Gastroenterology Organization (WGO) are two of the entities that publish guidelines to steer clinicians in the proper treatment of CD.4,7 The goals of treating CD are to maintain remission and prevent or delay the need for surgical interventions; however, the treatment of CD is variable depending on its severity and location. CD does not follow a clear step-by-step treatment algorithm compared with some other medical conditions, such as diabetes. Treatments are initiated to treat active disease and exacerbations, achieve remission, and maintain remission once it is reached.
Diagnosing CD requires a thorough approach, including conducting a physical examination and taking an accurate patient history to inquire about factors such as nighttime awakenings, weight loss, and smoking.
Nonpharmacologic Treatment
Several nonpharmacologic interventions may be considered to augment medication use. The role of dietary management is being researched; no definite claims can be made.4,6 Eating smaller and more frequent meals may be preferable, and meals that are nondairy and low in fat may prove useful as well. Drinking plenty of water is another standard recommendation for CD patients, as is supplementing with vitamins to help prevent dehydration and deficiencies caused by persistent diarrhea and malabsorption.4
Nonbiological Medications
Corticosteroids are frequently used as first-line therapy for CD. Oral prednisone, rectal or oral budesonide, or IV methylprednisolone is used to induce remission.4,7 The use of corticosteroids is short-term because of side effects with long-term use and complications associated with chronic steroid use.
Corticosteroids are often used with immunosuppressants such as a thiopurine (azathioprine or mercaptapurine) or methotrexate plus folic acid. The AGA and WGO guidelines recommend against using a thiopurine as monotherapy for the induction of remission because of the delay in the onset of action, but these medications have been shown to be effective for maintenance therapy. Using anti–tumor necrosis factor (antiTNF) agents with or without thiopurines is a strong recommendation made by both the AGA and the WGO for maintaining remission.8 It is not recommended to use methotrexate as monotherapy because there is conflicting evidence as to methotrexate’s effectiveness in inducing remission.
The use of 5-aminosalicylates (5-ASAs) in CD is controversial, although 5-ASAs are the mainstay of maintenance therapy for ulcerative colitis. Most studies show that mesalamine and other 5-ASA derivatives are not useful in the treatment of CD, although there are some data that suggest the use of sulfasalazine for inducing remission of colonic CD.9,10 However, this use of sulfasalazine is still controversial, and it should not be recommended. In some patients with mild disease, 5-ASA agents may have a modest role when disease involvement is only in the colon and when it is used as adjunctive therapy.
Antibiotics are often used in mild-to-moderate disease, but only as a bridge to immunosuppresant therapy, and they are generally effective for mild colonic disease.9
The patient’s clinical status should steer the clinician’s choice of agents and the route of administration. For example, more severely afflicted individuals who require hospitalization may require more aggressive treatments such as combination therapies and IV therapies.
Biological Medications
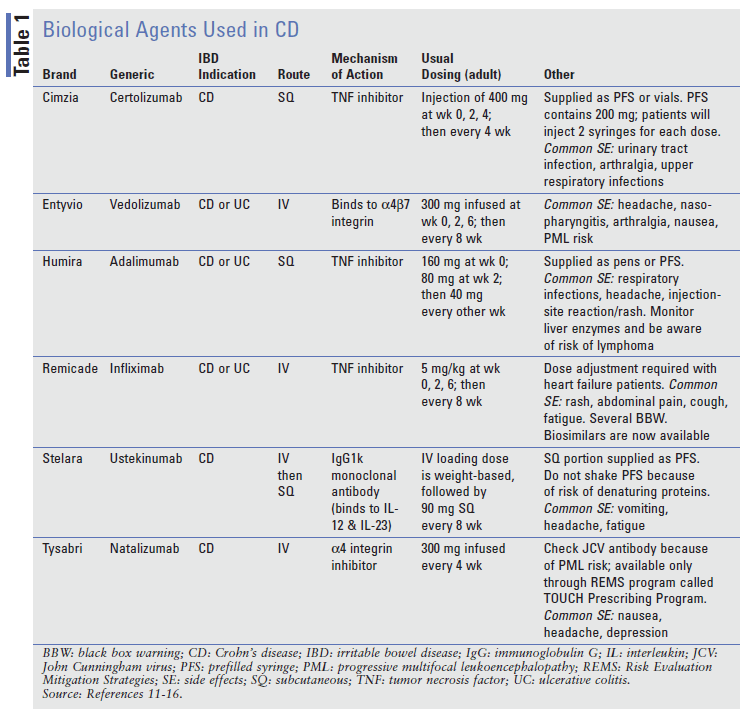
Anti-TNF agents such as adalimumab (Humira), infliximab (Remicade), and certolizumab (Cimzia) are the most effective options for inducing and maintaining remission and are considered the best agents to treat moderate-to-severe CD.3 Anti-TNF agents work by binding a protein called tumor necrosis factor alpha (TNF-α), thus preventing TNF-α from inducing proinflammatory cytokines.7
The AGA and the WGO strongly recommend anti-TNF monotherapy or an anti-TNF with a thiopurine (azathioprine) to induce remission. The use of an anti-TNF as monotherapy or in combination with a thiopurine is the most-recommended approach to maintaining remission, no matter how remission is achieved (i.e., steroid, anti-TNF, or anti-TNF plus thiopurine therapy).4,7 If for some reason a patient is unable to receive anti-TNF therapy, either a thiopurine or methotrexate monotherapy is recommended over no treatment in patients whose remission was induced by corticosteroids.7 Specific agent choice is patient-centered—the patient should be part of the decision-making process. This decision should take into consideration patient preferences, such as route of administration, and restrictions that the patient’s insurance company may have, as well as clinical considerations. Some data suggest that anti-TNF agents may be used in combination with methotrexate or a thiopurine, resulting in higher anti-TNF serum concentrations and reducing the risk that the patient will develop antidrug antibodies against the anti-TNF agents; however, this combination has its drawbacks, including a heightened risk of opportunistic infections.7
Other biological agents include the integrin-receptor antagonists natalizumab (Tysarbi) and vedolizumab (Entyvio), which bind integrin molecules, disabling them from interacting with adhesion molecules and thus preventing the migration of inflammatory cells into the gastrointestinal-tract tissue.11-13 Natalizumab’s use in CD is limited owing to the serious risk of progressive multifocal leukoencephalopathy, and it is usually reserved for patients who have not responded to all other CD treatment options. A similar agent, ustekinumab (Stelara), an interleukin inhibitor, has been available since 2009, but only recently was approved for use in CD.11-13 Ustekinumab is unique because of its IV loading dose, followed by subcutaneous injections for maintenance doses.11-13 Ustekinumab is effective in both anti-TNF–naïve patients and those who have not responded to anti-TNF agents.11-13 With these and all biological agents, the induction of remission may be seen as early as 1 week, but it can take up to 6 weeks or longer. These agents should be discontinued if there has been no clinical response after a predetermined amount of time, which varies slightly from drug to drug.11-13
Before using any biological agent, the patient must undergo purified protein derivative testing to rule out tuberculosis infections, and hepatitis B titers must be negative.11-16 While a patient is on any immunosuppressive biological agent, he or she should not receive live vaccinations.11-16 Additionally, biological agents should not be administered concomitantly.11-16 The most common adverse effects include injection-site reactions and an increased risk of infections.11-16
Role of Biologics in the Treatment of CD
TABLE 111-16 lists the biological medications used in the treatment of CD. As discussed previously, prior to initiating biologics, patients may first try oral and topical aminosalicylates (i.e., mesalamine products); steroids (i.e., prednisone, budesonide); immunomodulators (i.e., azathioprine, methotrexate, mercaptopurine); or antibiotics (i.e., ciprofloxacin, metronidazole). This is known as “step-up” therapy, in which less aggressive agents may be used first, based on disease severity or patient preference. Additionally, some insurance companies require patients to have been unresponsive to some of the above medications prior to approving specific biologics.
Many gastroenterologists will follow the traditional step-up therapy and, if that fails, move to biological therapy. Alternatively, some gastroenterologists will do “top-down” therapy, starting with biologics to achieve and maintain remission in patients who have more severe disease.17 The top-down treatment is known to sometimes have lower rates of flare-ups and steroid use, although this is controversial; some experts believe that top-down therapy can cause overtreatment.17 No matter which treatment style is chosen, the goal of medication therapy is to aid in induction and maintenance of remission. If a patient does not respond to one biologic, alternative agents may be used. Finally, if there is no response to multiple biologics or there is more severe disease, some patients are managed surgically rather than medically.
Pharmacist Involvement
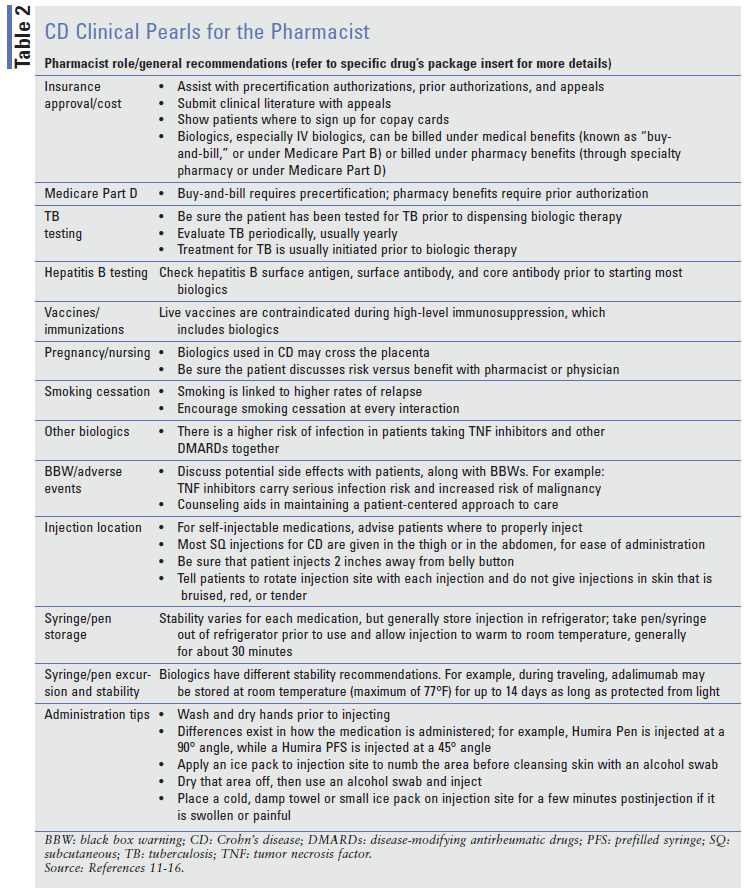
Biological agents used in CD require extensive monitoring and administration counseling, such as that necessitated by the black box warnings that many biological agents carry. These represent two of the many ways in which pharmacists can help both providers and patients. TABLE 2 provides some tips to aid in patient care.
In addition, these medications are relatively expensive, and helping patients obtain their medications requires an understanding of health insurance and the ability to communicate with insurers and drug companies regarding patient-assistance programs. Pharmacists are skilled in this area and can provide information to physicians when they are choosing drug therapy for patients with CD. The inclusion of a pharmacist on a healthcare team reduces time spent by physicians on medication issues and frees up more time for other aspects of patient care.18
CD medications are relatively expensive, and helping patients obtain their medications requires an understanding of health insurance and the ability to communicate with insurers and drug companies.
Patient education is very important for these medications; patients need to be properly trained to self-inject their medications, and pharmacists should be able to assist. It is also important to discuss potential risks, side effects, warnings/precautions, product storage and stability, injection locations, and injection technique in detail with each patient.18 Another area where pharmacists can help is the insurance-navigation process for specialty medications.18 Some insurance companies require patients to use certain specialty pharmacies, which can pose a problem when the prescription is sent to their community pharmacy. However, the community pharmacist can help with onsite training and counseling, especially in helping patients understand injection technique. Also, the community pharmacist can counsel the patient regarding drug-drug interactions or OTC interactions associated with multiple medications.18
Community pharmacists can also help patients obtain appropriate supplies for injectable biological agents, such as a sharps container, alcohol swabs, and adhesive bandages. Additionally, pharmacists may discuss with patients how to dispose of used products.
One area of confusion for patients, with some of the biological agents, is packaging. Pharmacists should be mindful of how each product is supplied so they can ensure that the patient has an adequate supply of their medication.18 For example, the adalimumab CD pen starter kit includes six pens, which will last 28 days. However, the standard adalimumab maintenance dosing requires 1 kit (adalimumab pen carton = 2 pens), which will last 28 days. For certolizumab, each pre-filled syringe contains 200 mg. The usual dose is 400 mg; therefore, patients will need to administer two injections to get each 400-mg dose.
Pharmacists may also aid in the identification of allergic reactions.18 Some allergic reactions are less severe, with mild itching that can be resolved with a dose of an antihistamine prior to self-injection. More severe cases of allergic reactions include chest pain or tightness, severe rash or hives, and swelling of the face, tongue, lips, and throat. These reactions should be reported to emergency services and followed up with medical care. Finally, patients also may be allergic to various aspects of the products. For example, the adalimumab PFS needle cap contains latex.
Finally, pharmacists may help triage questions about biosimilar products. Biosimilars are products that are very similar to an FDA-approved product, but are not necessarily interchangeable.19 Biosimilars of drugs used to treat CD are becoming available. For example, infliximab has several biosimilars available: infliximab-dyyb (Inflectra) and infliximab-abda (Renflexis).20 Adalimumab also has biosimilars on the market, such as adalimumab-atta (Amjevita) and adalimumab-adbm (Cyltezo).20 These drugs pose another challenge and potential source of confusion for physicians and pharmacists; however, drug costs may be reduced given that multiple products are available.20,21 The FDA provides additional information on biosimilar products and availability.20
Conclusion
CD is a medical condition that requires extensive healthcare-provider and patient interventions. Guidelines recommend initiation of biological therapy, often with TNF inhibitors. These agents carry many specific monitoring and treatment considerations that give pharmacists ample opportunities to provide high quality care. Nonbiological medications and nonpharmacologic care often play a small role in the overall management of CD. Often, pharmacists will need to aid in providing a variety of services to ensure medication adherence and assist in the mitigation of adverse drug reactions.
REFERENCES
- Boyapati R, Satsangi J, Ho G-T. Pathogenesis of Crohn’s disease. F1000Prime Rep. 2015;7(44):Epub.
- Dahlhamer JM, Zammitti EP, Ward BW, et al. Prevalence of inflammatory bowel disease among adults aged ≥18 years — United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(42):11661169.
- Feuerstein JD, Cheifetz AS. Crohn disease: epidemiology, diagnosis, and management. Mayo Clin Proc. 2017;92(7):1088-1103.
- Berstein C, Eliakin A, Fedail S, et al. World Gastroenterology Organization global guidelines. Inflammatory bowel disease. Updated August 2015. www.worldgastroenterology.org/UserFiles/file/guidelines/inflammatory-bowel-disease-english-2015-update.pdf. Accessed August 26, 2017.
- Sandborn WJ. Crohn’s disease evaluation and treatment: clinical decision tool. Gastroenterology. 2014;147:702-705.
- Crohn’s and Colitis Foundation of America. The facts about inflammatory bowel diseases. www.crohnscolitisfoundation.org/ assets/pdfs/updatedibdfactbook.pdf. Accessed August 25, 2017.
- Terdiman JP, Gruss CB, Heidelbaugh JJ, et al. American Gastroenterological Association Institute guideline on the use of thiopurines, methotrexate, and anti-TNF-alpha biologic drugs for the induction and maintenance of remission in inflammatory Crohn’s disease. Gastroenterology. 2013;145:1459-1463.
- Dassopoulos T, Sultan S, Falck-Ytter YT, et al. American Gastroenterological Association Institute technical review on the use of thiopurines, methotrexate, and anti-TNF-α biologic drugs for the induction and maintenance of remission in inflammatory Crohn’s disease. Gastroenterology. 2013;145(6):1464-1478:e1-e5.
- Lichtenstein GR, Hanauer SB, Sandborn WJ. Practice Parameters Committee of American College of Gastroenterology. Management of Crohn’s disease in adults. Am J Gastroenterol. 2009;104(2):465483:quiz 464, 484.
- Steinhart AH, Hemphill D, Greenberg GR. Sulfasalazine and mesalazine for the maintenance therapy of Crohn’s disease: a meta-analysis. Am J Gastroenterol. 1994;89(12):2116-2124.
- Entyvio (vedolizumab) package insert. Deerfield, IL: Takeda Pharmaceuticals America, Inc; May 2014.
- Stelara (ustekinumab) package insert. Horsham, PA: Janssen Biotech, Inc; 2012; revised September 2016.
- Tysabri (natalizumab) package insert. Cambridge, MA: Biogen Inc; 2015; revised August 2017.
- Remicade (infliximab) package insert. Horsham, PA: Janssen Biotech, Inc; 2013; revised October 2015.
- Humira (adalimumab) package insert. North Chicago, IL: AbbVie Inc; 2002; revised May 2017.
- Cimzia (certolizumab) package insert. Smyrna, GA: UCB, Inc; 2008; revised January 2017.
- Antunes O, Filippi J, Hébuterne X, Peyrin-Biroulet L. Treatment algorithms in Crohn’s—up, down or something else? Best Pract Res Clin Gastroenterol. 2014;28(3):473-483.
- Bhat S, Nehrin K, Sherif A, et al. The pharmacist’s role in biologic management for IBD in a health system—integrated practice model. Am J Pharm Benefits. Epub October 23, 2015.
- Paramsothy S, Cleveland NK, Zmeter N, Rubin DT. The role of biosimilars in inflammatory bowel disease. Gastroenterol Hepatol. 2016;12(12):741-751.
- FDA. Biosimilar product information. Updated October 23, 2017. www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/Biosimilars/ucm580432.htm. Accessed November 16, 2017.