Cannabis Use in Pain Patients
RELEASE DATE:
March 1, 2019
EXPIRATION DATE:
March 31, 2021
FACULTY:
Katelyn Boatwright, PharmD
Retail Pharmacist
St. Joseph Medical Center Outpatient Pharmacy
Kansas City, Missouri
FACULTY DISCLOSURE STATEMENTS:
Dr. Boatwright has no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT:
Pharmacy
 Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-19-016-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE:
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER:
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients' conditions and possible contraindications or dangers in use, review of any applicable manufacturer's product information, and comparison with recommendations of other authorities.
GOAL:
To give pharmacists an overview of cannabis and cannabis-related medications available in the United States, with a focus on medical cannabis use for certain pain indications.
OBJECTIVES:
After completing this activity, the participant should be able to:
- Describe the current laws for medical cannabis use in the U.S.
- List the cannabis medications currently on the market.
- Evaluate the evidence for using medical cannabis for various pain indications.
- Discuss potential drug-drug interactions with medical cannabis.
ABSTRACT: In recent years, the medical use of cannabis has garnered attention not only from patients, but from the general public as well. This increased attention makes it necessary for pharmacists to learn more about the potential risks and benefits of medical cannabis for their patients. As more states legalize the use of medical cannabis, pharmacists will need to become increasingly knowledgeable, especially in those states in which a pharmacist is required for the legal dispensing of medical cannabis. Although preliminary evidence suggests that cannabis may be useful for pain, only a few cannabis-based medications are approved by the FDA, and none are currently approved for treating any pain indication in the United States.
Cannabis (marijuana) contains approximately 60 pharmacologically active compounds commonly referred to as cannabinoids.1 Phytocannabinoids are natural plant-derived compounds that interact directly with cannabinoid receptors, share chemical similarities with cannabinoids, or both.2 The most common phytocannabinoids used medically are delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD).3 Two of the most frequently utilized cannabinoid receptors are CB1 (primarily in the brain and peripheral tissue) and CB2 (primarily in the immune and hematopoietic systems).3 Because CBD acts on the CB2 receptors, it does not cause the significant psychoactive effects that THC does (THC acts primarily on the CB1 receptors).4
As part of the endocannabinoid system, endocannabinoids (endogenous neurotransmitters) and their receptors are located throughout the body and are actively involved in the functioning of the nervous system, internal organs, glands, connective tissues, and immune cells.5 Because endocannabinoids help regulate various biological processes, endocannabinoid deficiency could theoretically have a big impact on these processes.5,6 The endocannabinoid system plays a largely homeostatic role that is generally characterized as "eat, sleep, relax, forget, and protect."5,7 It has been proposed that clinical endocannabinoid deficiency syndromes (CEDS) are involved in migraine, fibromyalgia, irritable bowel syndrome, and other related conditions.6 In human studies, CEDS have been implicated in schizophrenia, multiple sclerosis (MS), Huntington disease, Parkinson's disease, anorexia, chronic motion sickness, and infants' failure to thrive.6
IMPORTANCE OF CANNABIS KNOWLEDGE FOR PHARMACISTS
In recent years, the use of cannabis for medical reasons has garnered increased attention from patients, as well as the general public. The rising interest in medical cannabis is thought to be related to heightened awareness of the opioid-abuse epidemic.8 Some preliminary evidence suggests that patients who use cannabis may decrease their opioid use.9,10 In the cannabis debate, one side sees cannabis as relatively safe, with very few reported deaths from its use; the opposing side argues that cannabis lacks high-quality clinical trials confirming its risks and benefits.
Chronic pain has been cited as the most common reason that adults in the United States seek medical care, and in 2016 an estimated 20.4% of U.S. adults had chronic pain.10 The spotlight on opioid abuse is leading patients and physicians to search for alternative ways to effectively reduce pain. Preliminary evidence suggests that cannabis may be useful for reducing opioid use.9 One survey indicates that patients are turning more frequently to medical cannabis for opioid replacement than to other psychoactive medications and substances (e.g., antidepressants and alcohol).9 High-quality randomized, controlled trials need to be performed in order to determine whether there is truly a causal relationship between cannabis and reduced opioid use.
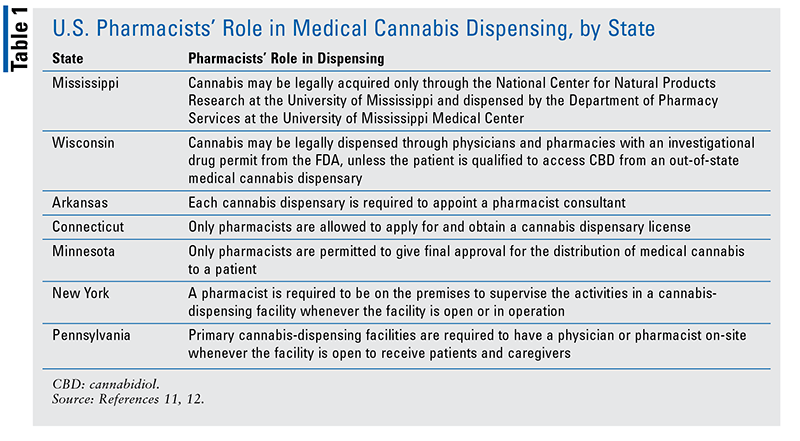
Additionally, pharmacists should become knowledgeable about cannabis because some states require pharmacist involvement in the legal distribution of medical cannabis. This role is described, according to state, in TABLE 1.11,12
FEDERAL LEGALITY OF CANNABIS USE
As of November 2018, 33 states have legalized comprehensive public medical-cannabis programs and 13 states allow the use of low-THC, high-CBD products for medical reasons in limited situations or as a legal defense.11
According to the Drug Enforcement Administration, cannabis is classified as a Schedule I substance (a drug, substance, or chemical with no currently accepted medical use and a high potential for abuse).13,14 In October 2009, the Obama Administration encouraged federal prosecutors not to prosecute for distribution of cannabis for medical purposes in accordance with state law. In January 2018, then–Attorney General Jeff Sessions directed U.S. attorneys to "weigh all relevant considerations, including federal law enforcement priorities set by the Attorney General, the seriousness of the crime, the deterrent effect of criminal prosecution, and the cumulative impact of particular crimes on the community."15
Although pharmacist involvement is legally required in some states, a pharmacist could be prosecuted for his or her participation in cannabis distribution because cannabis is a Schedule I substance; therefore, pharmacists should be diligent in ensuring the provision of evidence-based treatment of all patients.12 On account of the legal implications, pharmacists involved in cannabis dispensing may not be able to get traditional malpractice insurance.
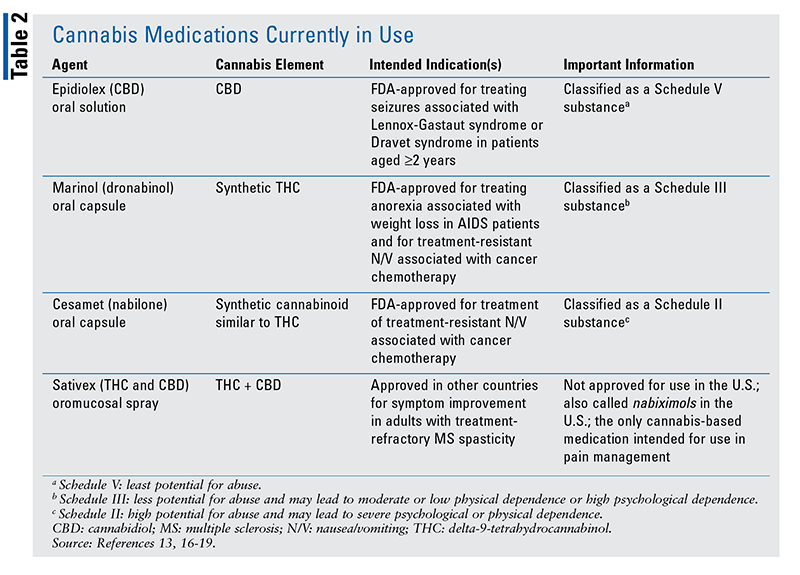
TABLE 2 summarizes the cannabis-related medications that have been formulated for human use.16-19 Some of these products have received FDA approval.
Cannabis Use for Analgesia
Cannabis has been used to treat pain based on its action on both CB1 and CB2 receptors. CB receptor agonists have been shown to cause antinociceptive and antihyperalgesic effects by regulating neuronal and non–nervous system inflammatory activity.20 One theory suggests that activation of CB1 receptors in mast cells elevates cyclic adenosine monophosphate and suppresses degranulation.20 Analgesia may also result from CB1 receptor activation causing negative modulation of the P2X3 receptor in primary afferent neurons.21 Activation of CB2 receptors can inhibit the release of proinflammatory factors, causing suppression of nerve growth factor–induced mast cell degranulation and neutrophil accumulation.20
EVIDENCE FOR CANNABIS USE IN PAIN MANAGEMENT
The next several sections will discuss recent studies that have examined cannabis use for various pain indications. It is important to note that non–FDA-approved formulations of cannabis medications vary in excipients as well as CBD and THC content, so absorption, distribution, and metabolism will differ between products.
General Chronic Pain
Ware and Colleagues22: This prospective, multicenter, placebo-controlled study was conducted over 1 year in 431 patients (cannabis group, n = 215; control group, n = 216) with chronic, treatment-resistant, moderate-to-severe noncancer pain of at least 6 months' duration. Cannabis patients received a standardized potency of 12.5% THC. The risk of having at least one significant adverse event did not differ significantly between the two groups (unadjusted odds ratio, 0.64; 95% CI, 0.38-1.04). Cannabis patients had a significant reduction in average pain intensity over 1 year, but placebo patients did not.
Poli and Colleagues23: This prospective, nonrandomized, single-arm clinical trial at a pain clinic in Italy studied 338 patients with a variety of chronic-pain conditions (including fibromyalgia, radiculopathy, headache, arthritis, and various forms of neuropathic pain, among others). All patients received THC (19%) plus CBD (<1%) in addition to their standard pain regimen. At the end of the study, 124 patients (36.7%) had interrupted their therapy (79 for inefficacy and 33 for side effects [sleepiness in 30% and mental confusion in 25%]). There was a statistically significant improvement in Visual Analogue Scale pain scores from baseline versus 1 month, 3 months, 6 months, and 12 months (P <.01 for all comparisons).
Shah and Colleagues24: Researchers conducted a retrospective study of patients with chronic pain in order to evaluate demographic and clinical characteristics. The study compared 18 THC-positive patients (positive THC screening upon admission to a pain-rehabilitation program) with 22 THC-negative patients who were self-reported prescription opioid users upon admission. A specific cannabis dosage was not studied, since patients were self-dosing. The THC-positive group reported a significantly longer duration of chronic pain compared with the THC-negative group (median, 13.02 years vs. 7.54 years; P = .042). Duration of opioid abuse, daily oral morphine milligram equivalent, and benzodiazepine use upon admission were similar between the THC-positive and THC-negative groups.
Nugent and Colleagues25: This retrospective chart review evaluated patterns and correlates of medical cannabis use in 371 musculoskeletal-pain patients who were prescribed long-term opioid therapy. No specific cannabis dosage was studied, since patients were self-dosing. The researchers found that 18% of patients had used medical cannabis for pain within the past month (of these patients, 60% used cannabis for pain two or more times per week). Self-reported cannabis users had significantly higher risk scores for prescription-opioid misuse (P <.0001), and they were more likely to meet criteria for current hazardous alcohol use (P = .024) and to report using nicotine (P = .03).
Haroutounian and Colleagues26: Researchers conducted a prospective, single-arm, open-label study to examine the long-term effect of medical cannabis on pain and functional outcomes. Included were 206 patients with treatment-resistant chronic pain (most frequently widespread musculoskeletal pain, peripheral neuropathic pain, or radicular low back pain). Patients were given either smoked or oil THC/CBD (titrated to effect) in combination with their current pain regimen. There was a statistically significant reduction in pain from baseline to 6 months (P <.001), and a significant number of participants discontinued opioids during the study. The number of adverse events was small, which the researchers attributed to rigorous exclusion of patients at higher risk for psychiatric adverse events, drug abuse, and noncompliance.
Chronic Neuropathic Pain
Abrams and Colleagues27: A prospective, randomized, placebo-controlled trial of smoked cannabis was conducted in 50 patients with HIV-related peripheral neuropathy. Cannabis patients received smoked THC (3.56% THC). Smoked cannabis was found to significantly reduce daily pain compared with placebo (34% vs. 17%; P = .03). A significantly greater number of cannabis patients reported chronic-pain reduction of more than 30% compared with placebo (52% vs. 24%; P <.001), and no serious adverse events were reported.
Rog and Colleagues28: This randomized, double-blind, placebo-controlled parallel-group study investigated cannabis treatment in 66 patients with MS and central neuropathic pain. The 32 cannabis patients, who self-titrated their cannabis dosage up to 48 sprays per 24 hours, received oromucosal THC/CBD spray (each spray containing 2.7 mg THC and 2.5 mg CBD) plus their current pain regimen. The mean daily number of sprays was 9.6 (range, 2-25) in cannabis patients and 19.1 in placebo patients (range, 1-47). Cannabis treatment led to a significantly higher reduction in mean intensity of pain versus placebo (P = .003), and cannabis patients had more adverse effects than placebo patients did.
Svendsen and Colleagues29: A randomized, double-blind, placebo-controlled crossover trial was conducted to assess the effect of dronabinol in 24 MS patients with central neuropathic pain. Cannabis patients received dronabinol titrated to a maximum dosage of 5 mg twice daily. Spontaneous-pain intensity (on a scale of 0 = no pain relief to 10 = best pain relief) was significantly lower with dronabinol compared with placebo (4.0 vs. 5.0; P = .035). The number of adverse events was higher with dronabinol treatment, although functional ability appeared to be unaffected.
Mücke and Colleagues30: Researchers conducted a Cochrane review of 16 randomized, double-blind, controlled trials with a total of 1,750 patients to analyze the efficacy, tolerability, and safety of cannabis-based medications (CBMs) versus placebo or conventional drugs. The studies involved adult patients with chronic neuropathic pain and included both plant-based and synthetic CBMs. CBMs were found to increase the number of patients achieving pain relief of 50% or more compared with placebo (21% vs. 17%, respectively; risk difference [RD], 0.05; 95% CI, 0.00-0.09), based on 1,001 participants, eight studies, and low-quality evidence. Compared with placebo patients, cannabis patients withdrew from the studies more often for adverse events (10% vs. 5%; RD, 0.04; 95% CI, 0.02-0.07), based on 1,848 participants, 13 studies, and moderate-quality evidence. Study limitations were small sample size and the exclusion of participants with substanceabuse history and significant comorbidities.
Lee and Colleagues31: This review examined seven randomized, double-blind, placebo-controlled studies that evaluated the use of cannabis for the treatment of neuropathic pain. Based on these studies, cannabis treatment was generally well tolerated and appeared to provide more pain relief compared with placebo. Limitations of the studies included in this review were varying study durations, cannabis dosing, ratio of cannabinoids used, route of administration, and etiologies of neuropathic pain.
Kahan and Colleagues32: Researchers performed a review of 102 studies (five of which specifically examined severe neuropathic pain) to examine the use of medical cannabis in primary care patients. There were no specific inclusion or exclusion criteria for the studies included in this review. The researchers found that smoked cannabis could be indicated for treatment-resistant patients with severe neuropathic pain (level II evidence; based on five observational single-arm studies).
Cancer Pain and Neuropathy
Noyes and Colleagues33,34: Researchers conducted a preliminary prospective, placebo-controlled study in 10 patients with advanced cancer and continuous moderate pain attributable to their cancer.33 Cannabis patients received 5 mg, 10 mg, 15 mg, or 20 mg of oral THC. Patients receiving 15 mg or 20 mg of THC had significantly greater reductions of pain compared with patients given placebo. Patients receiving these THC dosages also experienced substantial sedation and mental clouding.33
Subsequently, Noyes and colleagues conducted a prospective, active-controlled study in 36 patients with advanced cancer and continuous moderate cancer-related pain.34 Cannabis patients received 10 mg or 20 mg of oral THC, and controls received 60 mg or 120 mg of codeine. In this study, 10 mg of THC produced analgesic effects similar to those for 60 mg of codeine, and 20 mg of THC produced analgesic effects similar to those for 120 mg of codeine. This study also found that significant side effects were caused by 20 mg of THC, and patients reported that the higher doses of THC had a greater sedative effect than codeine. Patients reported that THC improved mood and sense of well-being and reduced anxiety.34
Johnson and Colleagues35: This 2-week, multicenter, double-blind, randomized, placebo-controlled, parallel-group trial included 177 patients with cancer pain who had experienced inadequate analgesia despite chronic opioid dosing. There were three treatment groups: THC:CBD extract, THC extract, and placebo. Patients taking THC:CBD extract experienced a significantly higher change in mean pain numerical rating scale compared with placebo (–1.37 vs. –0.67; P = .014); THC extract, however, did not evoke a significant change over placebo. Treatment-related adverse events (somnolence, dizziness, confusion, nausea and vomiting) were similar between all groups, although fewer placebo patients experienced these adverse events.
Portenoy and Colleagues36: A randomized, double-blind, placebo-controlled, graded-dose study was performed in patients with advanced cancer and opioid-refractory pain. Cannabis patients received low-dose nabiximols (1-4 sprays/day), medium-dose nabiximols (6-10 sprays/day), or high-dose nabiximols (11-16 sprays/day). The low- and medium-dose groups had significantly higher reports of analgesia compared with placebo (P = .008 and P = .039, respectively). Adverse events were similar between all groups, and only the high-dose group had more events than placebo patients.
CURRENT GUIDELINE RECOMMENDATIONS
According to the most recently updated American Academy of Neurology (AAN) guidelines regarding complementary and alternative medicine for MS, there is strong evidence supporting the use of oral cannabis extract and moderate evidence supporting the use of THC or Sativex in MS patients to reduce patient-reported spasticity and pain (excluding central neuropathic pain).36 The AAN also states that there is inadequate evidence to support or refute the use of smoked cannabis for spasticity or pain in MS.37
According to the American Cancer Society (ACS), cannabis could potentially be useful for symptoms of cancer, such as neuropathic pain, and could possibly reduce the need for other pain medications.38 The ACS also warns of such harms as less control over movement and feelings of anxiety and paranoia when using cannabis.37 Harms could be increased if the patient smokes the cannabis, as the additional substances the smoke delivers into the body are harmful.38
The College of Family Physicians of Canada (CFPC) published a guideline for prescribing medical cannabinoids in primary care.39 The guideline recommends against the use of medical cannabinoids for acute pain, headache, and rheumatologic pain. Although the guideline advises against using medical cannabinoids as first or second-line therapy for neuropathic pain, palliative cancer pain, and spasticity in MS or spinal cord injury, it does state that cannabinoids may be considered as adjunctive treatment for refractory patients. Overall, the CFPC recommends against using medical cannabis (particularly smoked) as the initial treatment option and advises that patients try a pharmaceutically developed product (such as nabilone or nabiximols) as the initial cannabinoid agent.39
OTHER CONSIDERATIONS FOR MEDICAL CANNABIS
The aforementioned review by Kahan and colleagues included 102 articles deemed to be relevant to prescribing cannabis in primary care.32 The authors found that patients aged younger than 25 years are potentially at greater risk for cannabis-related psychosocial harms, including crime, suicidal thoughts, illicit drug use, cannabis-use disorder, and long-term cognitive impairment (level II evidence: well-conducted observational studies). The authors also found that smoked cannabis has the potential for an association with physiological effects on the heart, such as increased blood pressure and heart rate; therefore, it is recommended that patients with cardiovascular disease not be prescribed smoked cannabis (level III evidence: expert opinion).32
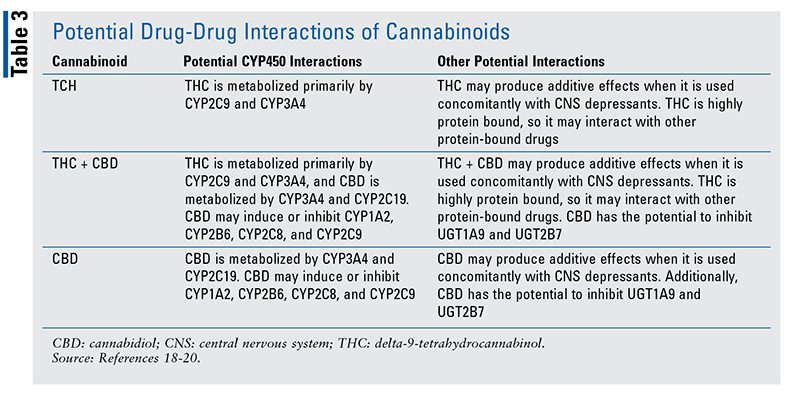
Based on in vitro data and studies, cannabinoids have the potential for various drug-drug interactions. These interactions (TABLE 3) should be considered.18-20
THE PHARMACIST'S ROLE
Because people are increasingly using medical cannabis to treat pain conditions, pharmacists should be aware of the risks and benefits of medical cannabis for their patients. Although very little high-quality evidence and information currently exist, pharmacists are responsible for keeping up-to-date with the literature and for educating patients and providers. Pharmacists should be willing to instruct patients on the benefits of medical cannabis, as well as on the health hazards of inhaling smoke, the delayed onset of action, and the enhanced effects after ingestion.40
REFERENCES
- Koppel BS, Brust JCM, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: a report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82(17):1556-1563.
- Gertsch J, Pertwee RG, Di Marzo V. Phytocannabinoids beyond the Cannabis plant—do they exist? Br J Pharmacol. 2010;160(3): 523-529.
- Jensen B, Chen J, Furnish T, Wallace M. Medical marijuana and chronic pain: a review of basic science and clinical evidence. Curr Pain Headache Rep. 2015;19(10):50.
- Pertwee RG, Howlett AC, Abood ME, et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. 2010;62(4):588-631.
- McParland JM, Duncan M, Di Marzo V, Pertwee RG. Are cannabidiol and Δ9-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br J Pharmacol. 2015;172(3):737-753.
- Kaur R, Ambwani SR, Singh S. Endocannabinoid system: a multi-facet therapeutic target. Curr Clin Pharmacol. 2016;11(2): 110-117.
- Bridgeman MB, Abazia DT. Medical cannabis: history, pharmacology, and implications for the acute care setting. P T. 2017; 42(3):180-188.
- Vigil JM, Stith SS, Adams IM, Reeve AP. Associations between medical cannabis and prescription opioid use in chronic pain patients: a preliminary cohort study. PLoS One. 2017;12(11): e0187795.
- Piper BJ, DeKeuster RM, Beals ML, et al. Substitution of medical cannabis for pharmaceutical agents for pain, anxiety, and sleep. J Psychopharmacol. 2017;31(5):569-575.
- Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001-1006.
- National Conference of State Legislatures. State medical marijuana laws. www.ncsl.org/research/health/state-medical-marijuanalaws.aspx. Accessed November 26, 2018.
- National Community Pharmacists Association. State medical marijuana legislation and the pharmacist's role. www.ncpanet.org/ advocacy/state-advocacy/medical-marijuana. Accessed November 26, 2018.
- Drug Enforcement Administration. Controlled substances: alphabetical order [Orange Book]. www.deadiversion.usdoj.gov/schedules/ orangebook/c_cs_alpha.pdf. Accessed November 26, 2018.
- Drug Enforcement Administration. Drug schedules. www.dea. gov/drug-scheduling. Accessed November 26, 2018.
- United States Department of Justice. Justice Department issues memo on marijuana enforcement. www.justice.gov/opa/pr/justicedepartment-issues-memo-marijuana-enforcement. Accessed November 26, 2018.
- Epidiolex (cannabidiol) package insert. Carlsbad, CA: Greenwich Biosciences, Inc; June 2018.
- Marinol (dronabinol) package insert. North Chicago, IL: AbbVie Inc; August 2017.
- Cesamet (nabilone) package insert. Somerset, NJ: Meda Pharmaceuticals Inc; September 2013.
- Sativex (delta-9-tetrahydrocannabinol and cannabidiol) package insert. Reading, Berkshire, UK: GW Pharma Ltd; July 2017.
- Manzanares J, Julian MD, Carrascosa A. Role of the cannabinoid system in pain control and therapeutic implications for the management of acute and chronic pain episodes. Curr Neuropharmacol. 2006;4(3):239-257.
- Oliveriera-Fusaro MCG, Zanoni CIS, Dos Santos GG, et al. Antihyperalgesic effect of CB1 receptor activation involves modulation of P2X3 receptor in the primary afferent neuron. Eur J Pharmacol. 2017;798:113-121.
- Ware MA, Wang T, Shapiro S, et al. Cannabis for the management of pain: assessment of safety study (COMPASS). J Pain. 2015;16(12):1233-1242.
- Poli P, Crestani F, Salvadori C, et al. Medical cannabis in patients with chronic pain: effect on pain relief, pain disability, and psychological aspects. A prospective non randomized single arm clinical trial. Clin Ter. 2018;169(3):e102-e107.
- Shah A, Craner J, Cunningham JL. Medical cannabis use among patients with chronic pain in an interdisciplinary pain rehabilitation program: characterization and treatment outcomes. J Subst Abuse Treat. 2017;77:95-100.
- Nugent SM, Yarborough BJ, Smith NX, et al. Patterns and correlates of medical cannabis use for pain among patients prescribed long-term opioid therapy. Gen Hosp Psychiatry. 2018;50:104-110.
- Haroutounian S, Ratz Y, Ginosar Y, et al. The effect of medicinal cannabis on pain and quality-of-life outcomes in chronic pain: a prospective open-label study. Clin J Pain. 2016;32(12): 1036-1043.
- Abrams DI, Jay CA, Shade SB, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68(7):515-521.
- Rog DJ, Nurmikko TJ, Friede T, Young CA. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology. 2005;65(6):812-819.
- Svendsen KB, Jensen TS, Bach FW. Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trial. BMJ. 2004; 329(7460):253-260.
- Mücke M, Phillips T, Radbruch L, et al. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2018;(3):CD012182.
- Lee G, Grovey B, Furnish T, Wallace M. Medical cannabis for neuropathic pain. Curr Pain Headache Rep. 2018;22(1):8.
- Kahan M, Srivastava A, Spithoff S, Bromley L. Prescribing smoked cannabis for chronic noncancer pain: preliminary recommendations. Can Fam Physician. 2014;60(12):1083-1090.
- Noyes R Jr, Brunk SF, Baram DA, Canter A. Analgesic effect of delta-9-tetrahydrocannabinol. J Clin Pharmacol. 1975;15(2-3):139-143.
- Noyes R Jr, Brunk SF, Avery DA, Canter AC. The analgesic properties of delta-9-tetrahydrocannabinol and codeine. Clin Pharmacol Ther. 1975;18(1):84-89.
- Johnson JR, Burnell-Nugent M, Lossignol D, et al. Multicenter, double-blind, randomized, placebo-controlled, parallelgroup study of the efficacy, safety and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage. 2010;39(2):167-178.
- Portenoy RK, Ganae-Motan ED, Allende S, et al. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J Pain. 2012;13(5):438-449.
- Yadav V, Bever C Jr, Bowen J, et al. Summary of evidencebased guideline: complementary and alternative medicine in multiple sclerosis: a report of the guideline development subcommittee of the American Academy of Neurology. Neurology. 2014;82(12): 1083-1092.
- American Cancer Society. Marijuana and cancer. www.cancer. org/treatment/treatments-and-side-effects/complementary-and-alternative-medicine/marijuana-and-cancer.html. Accessed November 26, 2018.
- Allan GM, Ramji J, Perry D, et al. Simplified guideline for prescribing medical cannabinoids in primary care. Canadian Fam Physician. 2018;64(2):111-120.
- Wallace M, Furnish T. What steps should be taken to integrate marijuana into pain regimens? Pain Manag. 2015;5(4):225-227.