Care and Treatment of Dementia
RELEASE DATE:
November 1, 2016
EXPIRATION DATE:
November 30, 2018
FACULTY:
Lori H. Syed, PharmD
Clinical Assistant Professor
of Pharmacy Practice
Director of Experiential Education
Mercer University College of Pharmacy,
Atlanta, Georgia
Diane Nykamp, PharmD
Professor of Pharmacy Practice
Mercer University College of Pharmacy
Clinical Pharmacy Specialist
Emory St. Joseph's Hospital
Atlanta, Georgia
FACULTY DISCLOSURE STATEMENTS:
Drs. Syed and Nykamp have no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT:
Pharmacy
 Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-16-092-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE:
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@jobson.com
DISCLAIMER:
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients' conditions and possible contraindications or dangers in use, review of any applicable manufacturer's product information, and comparison with recommendations of other authorities.
GOAL:
To provide participants with a review of reversible and irreversible dementias and their treatment, as well as the pharmacist's role in screening for dementia and addressing caregiver needs.
OBJECTIVES:
After completing this activity, the participant should be able to:
- Discuss the types of reversible and irreversible dementias.
- Identify the medications that can lead to cognitive impairment, as well as medications used to treat dementia.
- Describe simple assessment tools that can be administered to screen for dementia.
- Understand the role of the pharmacist in patient and caregiver education.
ABSTRACT: Dementia is a decline in cognition severe enough to interfere with functions of daily living. Dementias may be reversible, and associated with adverse effects or toxicities associated with drugs; or irreversible, with the most common type being Alzheimer's disease. Standardized screening tools are available to differentiate between memory lapses or behaviors associated with Alzheimer's disease and those changes that are age-related. If a patient is diagnosed with dementia, drug treatment may be initiated. Pharmacists have a role in screening for dementia and monitoring drug treatment, and can be a resource for caretakers regarding referrals and patient care.
Dementia is a general term used to describe a decline in mental ability severe enough to interfere with functions of daily living.1 Typically, this is manifested in memory losses, the inability to learn new information, behavioral changes, and emotional apathy.1 The terminology of diagnosis for dementia changed with the fifth revision of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) in 2013. The earlier edition, DSM-IV, required memory impairment and at least one of the following: aphasia, apraxia, agnosia, or other disturbances in executive functioning in order to assign a diagnosis of dementia.2 The DSM-5 updated the term for dementia to major neurocognitive disorder. The DSM-5 now lists six cognitive domains to assist in the diagnosis of cognitive disorders: complex attention, executive function, learning and memory, language, perceptual-motor function, and social cognition. Major neurocognitive disorder requires a severe deterioration in one or more of these areas, to the extent of interference with activities of daily living. DSM-5 also includes a diagnosis of mild neurocognitive disorder, which is identified by a modest decline from previous performance in one or more of the domains listed above.3
TYPES OF DEMENTIAS
While more than 60% of dementia cases are caused by Alzheimer’s disease (AD), there are many causes of dementia.1 Dementias may be reversible or irreversible.
Reversible Dementias
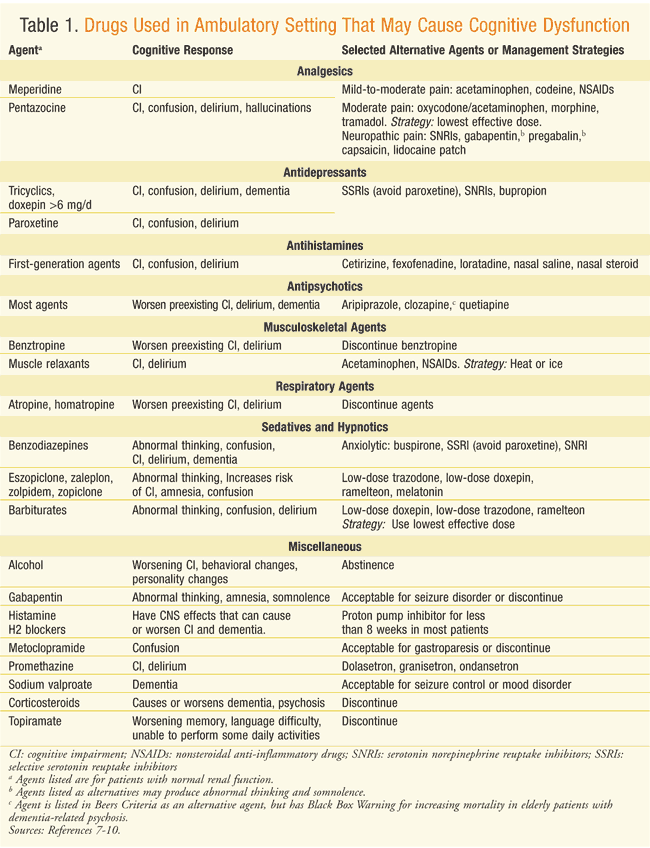
Reversible dementias can be caused by the adverse effects or toxicities of drugs or chemicals.4 These cognitive adverse effects associated with aging may be due to increasing dysfunction in either hepatic or renal metabolism, causing an accumulation of drug.5 Polypharmacy only complicates these issues in metabolism, and increases side effects.4 The American Geriatric Association’s Beers Criteria include many drugs that are associated with cognitive and central nervous system (CNS) effects. The 2015 update to the Beers Criteria reiterates that although potentially inappropriate medications should be carefully considered, they are not wholly unacceptable for all patients.6 Drugs that are recognized to commonly cause cognitive impairment include alcohol, anticholinergics, benzodiazepines, and narcotics. Other drugs that are often used in ambulatory elderly patients and are linked to confusion, impaired memory, or dementia are listed in TABLE 1, as are agents considered to be alternatives.7-10 Using the lowest effective dose and monitoring for drug-drug interactions will likely reduce the duration of cognitive impairment from medications.
Delirium is a commonly encountered reversible dementia. It can be differentiated by its quick onset (hours or days) rather than the slower (weeks, months, or years) onset seen in other dementias. Delirium is also characterized by fluctuating levels of consciousness.11 Delirium should be considered a medical emergency, as it can be the result of life-threatening conditions such as acute illnesses, as well as alcohol or benzodiazepine withdrawal.11
Subdural hematomas, or blood clots and bruising in the subdural area, are typically caused by severe head trauma (violence, falls, or car accidents); in the elderly, however, even minor head injuries can cause hematomas. Depending on the location of the hematoma, symptoms can mimic those of Alzheimer’s disease, causing confusion and cognitive difficulties. These hematomas can be life-threatening, but the cognitive changes may be reversed if the clot is identified early and removed within weeks of the injury. Therefore, acute behavior and memory changes should be assessed quickly.4
Depression that occurs in elderly patients who also exhibit signs of dementia is known as pseudodementia. The depressed mood manifests initially, then progresses to forgetfulness, disorientation, inattentiveness, and slowed responses. Treatment of the underlying depression in these patients will cause an improvement in the symptoms of dementia. However, patients with Alzheimer’s disease may develop a related depression after the initial mental decline, and treatment of depression in these patients will not improve cognitive abilities.4
Other reversible conditions that can cause a range of symptoms from mild cognitive impairment to dementia include acute infections such as urinary tract infections, subdural hematomas, malignant or benign tumors, normal pressure hydrocephalus, vitamin B12 deficiency, and depression.4,12 Both hypothyroidism and hyperthyroidism can be associated with dementia-like symptoms, and treatment will improve cognitive function.13 Alcoholism may affect memory, orientation, and attention. If a chronic thiamine deficiency associated with long-term malnutrition in alcoholism develops and continues, then a permanent state of confusion and memory loss occurs, resulting from Wernicke-Korsakoff syndrome.4
Irreversible Dementias
The most devastating diagnoses are the non-reversible diagnoses. Among these is vascular dementia (VaD), caused by insufficient blood flow to portions of the brain due to atherosclerosis. This causes small infarcts or transient ischemic attacks (TIAs). Individually, these small attacks may not cause much damage, but over time the cumulative damage can cover larger areas of the brain, resulting in dead tissue. The cardinal signs include abrupt mental changes, including paralysis or slurred speech, followed by a leveling period, and then another event or infarct leading to more damage, followed again by a leveling period. These patients will typically have one or more of the following conditions: hypertension, cardiovascular disease, diabetes, or a history of a stroke.4
Other sources of irreversible dementias are infectious agents, such as neurosyphilis; historically, Lyme disease or, rarely, Creutzfeldt-Jakob disease, and AIDS-related dementia. The latter conditions may develop and progress rapidly.4,11
There are several degenerative diseases that cause irreversible dementias. These include AD, which accounts for the most cases, but about 25% of these degenerative conditions are caused by frontal temporal lobar degeneration, dementia associated with Parkinson’s disease, and Huntington’s disease.4
Frontal temporal lobar degeneration, formerly called Pick’s disease, is caused by an atrophy of the frontal and temporal lobes. Characterized by loss of language, loss of inhibitions, and compulsively placing objects in the mouth, it may run in families. It can easily be confused with AD.4
Parkinson’s disease is most often thought of as a movement disorder marked by tremors, rigid limbs, or gait difficulties, and caused by dopamine deficiencies. While patients may experience mild cognitive problems early in disease progression, approximately 30% to 80% will ultimately develop dementia in the late stages. Some patients may experience hallucinations and develop dementia much earlier in their disease. This variant is called Lewy body dementia.4
Huntington’s disease is a rare, genetic disorder of chromosome four. Symptoms such as involuntary writhing movements, irritability, aggressiveness, and erratic behaviors can begin to manifest by 20 to 40 years of age, and progress to severe cognitive impairment.4
AD is the condition most often associated with dementia. Its diagnosis is confirmed only on autopsy and is characterized by the presence of beta-amyloid plaques on the outer portions of neurons, causing fibrillary tangles within the neurons. The amyloid plaque formation from the beta-amyloid proteins causes glutamate release, leading to calcium influx into the cells and thus causing neuronal death.14 Tissue loss and damage is most notable in the hippocampus.14 The cardinal signs are memory loss, declines in cognition accompanied by personality changes, and flat affect.14 AD has been the subject of considerable research, owing to its prevalence, especially in developed countries. Experts forecast that by 2050, AD may triple from 5.2 million cases to a projected 13.8 million cases. 11,15
Dementia Risk
After 65 years of age, the risk of developing dementia is 17% to 20%.11 Seventy percent of those patients diagnosed with dementia have AD, while about 17% have vascular dementia, leaving approximately 13% of dementias caused by Lewy body dementia, Parkinson-related dementia, alcoholic dementia, or frontal lobe dementia. Each year, 10% to 15% of patients with mild cognitive impairment will go on to develop AD.11
Currently, AD affects 5.3 million Americans and is the 6th leading cause of death in the United States. Risk factors for progressing to develop AD include age, family history of dementia, having the apolipoprotein E4 type, exhibiting cardiovascular comorbidities, and having a lower educational level. Of all of these risk factors, age is the most significant.11
SCREENING AND EVALUATION
Patients and caregivers will often agonize over whether memory lapses are normal or indicative of a greater problem. They often postpone seeking help out of fear of a devastating diagnosis. However, it is important to communicate the need for early diagnosis, especially in patients who may be experiencing a reversible condition, where early diagnosis and treatment are essential for recovery of near-normal cognition. While age-related memory lapses can be disconcerting, there is no evidence that these patients will benefit from a full work-up.1,11 Therefore, general community screening is not recommended.
The Alzheimer’s Association provides a useful tool on their website for individuals and caregivers to help differentiate between typical age-related memory lapses or behaviors that may indicate the need for further investigation.16 In addition, the Medicare Annual Wellness Visit (AWV) initiated in January 2011 as a part of the Affordable Care Act allows for annual screenings for AD and dementia in older patients.17
As healthcare providers, pharmacists can provide memory-care screenings.18 In a 2014 study, pharmacists in a dozen community pharmacies were trained to administer an easy-to-use screening tool to a total of 161 patients. Forty-four percent of those screened had at least one cognitive issue that prompted referral to a specialist for further evaluation; patients reported a high level of satisfaction with this clinical service.18
Assessment Instruments
Once a patient has sought the help of a healthcare provider, there are several easily administered screening tools that are allowed by Medicare during the AWV. A variety of cognitive assessments for this purpose were reviewed by Cordell et al, who noted that the General Practitioner Assessment of Cognition (GPCOG), the Memory Impairment Screen (MIS), and the Mini-Cog were most suited to use in primary care settings because they (1) required less than 5 minutes to administer; (2) were validated in primary care or community settings; (3) were easily administered by non-physician medical staff members; (4) included good-to-excellent psychometric properties; (5) were relatively free from biases such as education and language; and (6) could be used in clinic settings without paying fees for copyright privileges.17 A free Cognitive Assessment Toolkit containing these instruments can be downloaded from the Alzheimer’s Association website.19 Cordell et al caution that there is no “best” initial screening tool, and practitioners may choose from other assessments such as the Mini Mental State Examination (MMSE), St. Louis University Mental Status Examination (SLUMS) or Montreal Cognitive Assessment. MoCA.17 Several assessment tools are discussed below.
MiniCog: In this simple assessment tool, the patient is given three unrelated words (such as cat, trailer, and bedroom) and asked to repeat them. The patient is then asked to draw a clock face and indicate a specified time, such as 10 minutes past 11:00 o’clock. Upon completion of this activity, the patient is asked to recall the three unrelated words. It is important for the clock-drawing activity to be included in any cognitive examination because it is a simple tool that assesses organization and planning, and has been validated.11 Patients with an abnormal screening outcome should then be referred for further cognitive testing.
MMSE: The most widely used and studied mental status screening tool worldwide, the MMSE is often used as a comparison for evaluation of newer assessments. Significant barriers with the MMSE include an educational and language bias, as well as a ceiling effect, as very highly educated impaired subjects may pass.17 The tool is proprietary and must be purchased for use.17
SLUMS: Another brief screening tool is the SLUMS examination, primarily studied and utilized at Veterans Administration (VA) clinics.17 It was developed to be more sensitive to mild neurocognitive disorder than the MMSE.20 A 2014 study of the SLUMS tool among a nonveteran community population of adults >60 years of age found that the SLUMS examination and the MMSE were strongly correlated, and that the SLUMS examination may be appropriate for use in cognitive screening of older adults, with fewer ceiling effects.21 The SLUMS examination is a one-page test consisting of 12 questions, including a clock-face drawing question. There are two scoring charts, one for patients who have completed high school and one for those who have not, thus eliminating educational bias. The SLUMS tool is available free online.17, 22
Medical Evaluation
During the initial evaluation, a complete medical history should be obtained. Often patients with moderate-to-severe dementia are not able to evaluate or identify the onset, severity, or progression of their symptoms, so this interview should include both the patient and the caregiver. Assessment of activities of daily living (ADLs) as well as any history of accidents or head injuries should be included. Patients should be evaluated for any current or previous visual hallucinations or inappropriate disinhibition. Patients should also be asked about any history of psychoactive drug use or alcohol, as well as any symptoms of hypothyroidism. A full medication history should be obtained to help identify adverse effects of chronic medications, especially those that have been associated with symptoms of dementia.
Laboratory tests in a dementia work-up should include a complete blood count (CBC), a comprehensive metabolic panel (CMP), thyroid function tests, and folate and vitamin B12 levels, as well as urine analysis and culture to rule out any of the conditions known to cause dementia and dementia-like symptoms.4,11 Rarely, where a patient’s sexual history indicates, a rapid plasma regain (RPR) may be useful to rule out neurosyphilis.11
TREATMENT
The cause of a dementia will determine the treatment. Patients with reversible dementias have a better chance of resolving the cognitive impairment the earlier the condition is discovered and treated. Often removing the offending agent is the only treatment necessary.
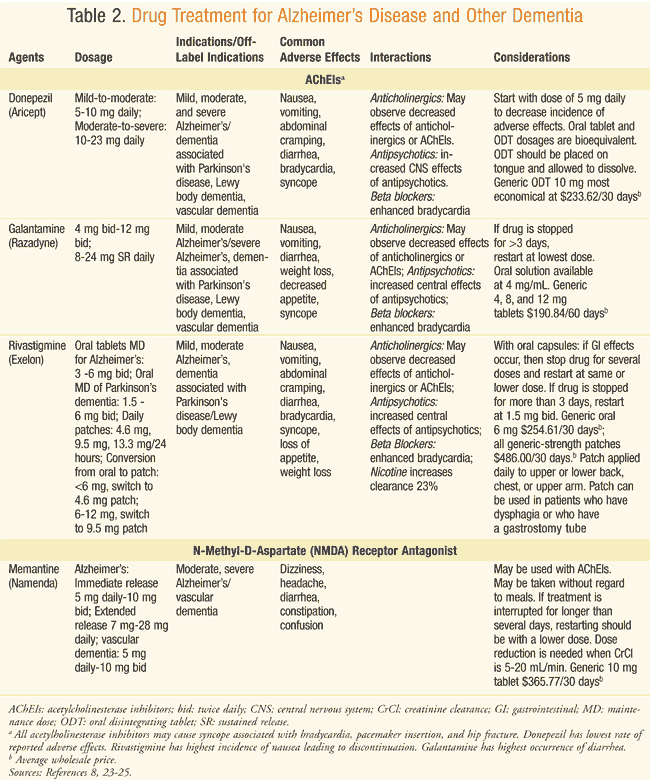
Drug research has generally focused on AD. Most drug therapies for AD treat the symptoms, and perhaps delay the progression of the disease.23 At this time, there are no available drug therapies to stop or reverse the effects of AD.12, 23 These drugs are also used to treat other dementias (TABLE 2), although only rivastigmine is indicated for a dementia (Parkinson’s) other than AD.
Alzheimer’s Disease
Five drugs have been approved for the treatment of AD. The first drug, approved in 1993, was tacrine, (Cognex), which has since been discontinued due to limited efficacy and significant side effects.8 Two classes of drugs are currently used as therapies for AD: acetyl cholinesterase inhibitors (AChEIs), and an N-methyl-D-aspartate (NMDA) receptor antagonist. Dosage information, indications, off-label uses, adverse effects, interactions, and special considerations for these drugs are presented in TABLE 2.8,23-25
AChEIs approved for treatment of symptoms of AD include donepezil, galantamine, and rivastigmine.26 They are used primarily in mild-to-moderate dementia. AD patients have decreased acetylcholine synthesis and impaired cortical cholinergic function; the amount of cholinergic loss correlates with the amount of cognitive impairment.18,26 AChEIs increase cholinergic transmission.26
 A 2014 update to the 2007 American Psychiatric Association (APA) Practice Guidelines for the Treatment of Patients with Alzheimer’s Disease and Other Dementias reviewed and summarized the drug-treatment research that has become available since 2007.27 The authors note that differences in focus and methods made many studies difficult to consider reliable. One well-designed study by McMaster University Evidence Based Practice Center reviewed 59 studies of pharmacologic agents for dementia and found similar effectiveness in all three AChEIs.27 Although the strength of available evidence has changed, the authors concluded that the 2007 Guideline recommendations were supported, and remain current.27
A 2014 update to the 2007 American Psychiatric Association (APA) Practice Guidelines for the Treatment of Patients with Alzheimer’s Disease and Other Dementias reviewed and summarized the drug-treatment research that has become available since 2007.27 The authors note that differences in focus and methods made many studies difficult to consider reliable. One well-designed study by McMaster University Evidence Based Practice Center reviewed 59 studies of pharmacologic agents for dementia and found similar effectiveness in all three AChEIs.27 Although the strength of available evidence has changed, the authors concluded that the 2007 Guideline recommendations were supported, and remain current.27
The Guidelines’ synthesis of new data found a modest benefit from AChEIs for cognitive symptoms in mild-to-severe AD.27 A 2016 review by Press and Alexander supports that conclusion, and also found a beneficial impact on activities of daily living.26
Although donepezil is now available in a higher dose, the 2014 Guideline concluded that recent evidence did not show clinical benefit; however, the 2016 review found some evidence of cognitive benefit at the higher dose, but no functional improvement; side effects were burdensome.26 Regarding rivastigmine, the higher-dose patch may confer greater cognitive benefit.26,27
Although the APA Guidelines discerned no benefit from AChEIs for agitation, a 2016 study by Yoon et al found that rivastigmine may benefit some patients with non-aggressive agitation.27,28 The Guidelines’ analysis of ten studies also concluded that AChEIs and memantine were not effective in treating disruptive behaviors. Recent evidence suggests that psychosocial interventions can positively impact adaptive behavior.27
More data are now available on the adverse-effect profiles of AChEIs (TABLE 2), which are similar.8,23-25 The gastrointestinal effects can be significant; therefore, titration is important to achieving compliance. Given their similar effectiveness, factors including adverse-effect profiles, dosage forms, tolerability, and whether the patient is ambulatory need to be considered when selecting one drug over another.22,26
NMDA: Memantine has been approved for use in moderate-to-severe AD. Memory impairment has been linked to excessive glutamate release at the neurons, leading to neuronal death. Memantine is able to block this receptor and is thought to improve both synaptic function as well as memory. Memantine has demonstrated significantly greater improvements than placebo in cognition and global status, but has not shown the same benefits for function and behavior.23 The 2014 APA Guideline concluded that trials that added memantine to an AChEI were of “slight or unclear clinical significance.”27
Antipsychotics, previously used to treat behavioral disturbances in dementia, have been discouraged for a number of reasons, including increased risk of death, especially in the elderly. A Black Box Warning states there is an increased mortality in elderly patients with dementia-related psychosis.27 The 2014 APA Guidelines cite new evidence from 3 trials confirming this warning, noting that antipsychotics demonstrated little efficacy.27 The authors also noted that antipsychotics could be tapered and finally discontinued without risk of significant withdrawal or return of behavioral symptoms.27
PHARMACIST’S ROLE
A pharmacist’s responsibility to offer medication counseling can be challenging when patients have mild cognitive impairment, or an AD or other dementia diagnosis. Initially, in the early stages of cognitive dysfunction, communication can be directed to the patient. Later, family members or caregivers will be involved in matters related to patient care and drug treatments. The caregivers may share their concerns or difficulties in communicating with the AD patient. There are several organizations, such as the Family Caregiver Alliance or the Alzheimer’s Association, which offer practical advice on communication.29,30 Suggestions include maintaining a positive tone; getting the person’s attention by limiting distractions and addressing him or her by name; identifying yourself simply; and stating the message clearly.31
Assisting Caregivers
Latham and Posner define caregiver syndrome as “a debilitated condition brought on by unrelieved, constant caring for a person with a chronic illness or dementia.”32 A pharmacist may be the first member of the healthcare team to recognize this condition. Pharmacists can listen to the caregiver’s concerns and provide referrals to appropriate community resources for tasks such as grocery shopping and transportation, as well as sources for durable medical equipment, alert systems for patients who wander, and adult day care. Pharmacists may also encourage participation in a caregiver support group. Caregivers may be referred to websites such as the Alzheimer’s Association or the Family Caregivers Alliance, which have caregiver information, links to local chapters, caregiver message boards, and helpline phone numbers.29,30 A standardized program, Resources for Enhancing Alzheimer’s Caregiver Health (REACH) may be available locally, and provide specialized interventions such as one-to-one counseling and education; caregiver recipients self-reported better sleep quality and physical and mental health.33,34
CONCLUSION
Alzheimer’s disease and other dementias are becoming increasingly prevalent with the aging population, and the human and economic costs of these diseases are significant. They provide a challenge to the patients, their families, and other caregivers. Identifying the type of dementia provides some help with treatments, especially if a reversible dementia can be identified, treated, and reversed. An early diagnosis also helps with the treatment of AD. The drugs approved to treat AD show modest improvement in mild-to-moderate disease, possibly delaying disease progression. Pharmacists are on the frontline of access for patients and caregivers; it is important to consider the potential for caregiver fatigue and be knowledgeable about referral resources.
REFERENCES
- Dementia – Signs, symptoms, causes, tests, treatment, care. alz.org. www.alz.org/what-is-dementia.asp. Accessed June 2, 2016.
- DSM-IV criteria for the diagnosis of vascular dementia. Internet Stroke Center. http://www.strokecenter.org/professionals/stroke-diagnosis/stroke-assessment-scales/dsm-iv-criteria-for-the-diagnosis-of-vascular-dementia/. Accessed June 2, 2016.
- Changes from dsm-iv-tr to dsm-5.pdf. http://www.dsm5.org/documents/changes%20from%20dsm-iv-tr%20to%20dsm-5.pdf. Accessed June 2, 2016.
- What’s causing your memory loss? It’s not necessarily Alzheimer’s. http://www.helpguide.org/harvard/whats-causing-your-memory-loss.htm. Accessed May 16, 2016.
- Lund BC, Carnahan RM, Egge JA, Chrischilles EA, Kaboli PJ. Inappropriate prescribing predicts adverse drug events in older adults. Ann Pharmacother. 2010;44:957-963.
- Expanded AGS Beers Criteria offer new guidance, tools for safer medication use among older adults. The American Geriatrics Society. http://www.americangeriatrics.org/press/id:5907. Accessed June 16, 2016.
- American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
- Drug Facts and Comparisons. Facts and Comparisons e-Answers. Hudson, Ohio: Wolters Kluwer. Accessed September 23, 2016.
- Pharmacist’s Letter. Potentially harmful drugs in the elderly: Beers List. Pharm Lett. 2015;31(12):Detail Document #311201.
- Ioannidis P, Karacostas D. How reversible are “reversible dementias”? Eur Neurol Rev. 2011;6(4):230-233.
- Simmons BB, Hartmann B, Dejoseph D. Evaluation of suspected dementia. Am Fam Physician. 2011;84(8):895-902.
- National Institute on Aging. The dementias: hope through research. www.nia.nih.gov/alzheimers/publication/dementias/other-conditions-cause-dementia. Accessed September 29, 2016.
- Samuels MH. Thyroid disease and cognition. Endocrinol Metab Clin North Am. 2014;43(2):529-543. doi:10.1016/j.ecl.2014.02.006.
- Querfurth H. Alzheimer’s Disease. N Engl J Med. 2010;362(19):1844-1845.
- Alzheimer’s Association. 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 2015;12(4):25.
- Know the 10 Signs - checklist_10signs.pdf. Alzheimer’s Association. https://www.alz.org/national/documents/checklist_10signs.pdf. Accessed June 17, 2016.
- Cordell CB, Borson S, Boustani M, et al. Alzheimer’s Association recom-mendations for operationalizing the detection of cognitive impairment during the Medicare Annual Wellness Visit in a primary care setting. Alzheimers Dement. 2013;9(2):141-150.
- Rickles NM, Skelton JB, Davis J, Hopson J. Cognitive memory screening and referral program in community pharmacies in the United States. Int J Clin Pharm. 2014;36(2):360-367.
- Alzheimer’s Association. Cognitive assessment toolkit. www.alz.org/hcps. Accessed October 19, 2016.
- Tariq SH, Tumosa N, Chibnall JT, Perry MH 3rd, Morley JE. Comparison of the Saint Louis University mental status examination and the mini-mental state examination for detecting dementia and mild neurocognitive disorder--a pilot study. Am J Geriatr Psychiatry. 2006;14(11):900-910.
- Feliciano L, Horning SM, Klee KJ, et al. Utility of the SLUMS as a cognitive screening tool among a nonveteran sample of older adults. Am J Geriatr Psychiatry. 2013;21(7):623-630.
- St. Louis University Medical School. Division of Geriatric Medicine. VAMC SLUMS Examination. http://aging.slu.edu/index.php?page=saint-louis-university-mental-status-slums-exam. Accessed October 17, 2016.
- Herrmann N, Chau SA, Kircanski I, Lanctôt KL. Current and emerging drug treatment options for Alzheimer’s disease: a systematic review. Drugs. 2011;71(15):2031-2065.
- Howard R, McShane R, Lindesay J, et al. Donepezil and memantine for moderate-to-severe Alzheimer’s disease. N Engl J Med. 2012;366:893-903.
- Lexicomp Online. Adult and Pediatric Lexi-Drugs. Hudson, Ohio; Lexi-Comp, Inc. Accessed June 16, 2016.
- Press D, Alexander M. Cholinesterase inhibitors in the treatment of dementia. August 16, 2016. Up-toDate.com. Accessed September 26, 2016.
- Rabins PV, Rovner BW, Rummans T, Schneider LS, Tariot PN. Guideline Watch (October 2014): Practice guideline for the treatment of patients with alzheimer’s disease and other dementias. October 2014.
- Yoon SJ, Choi SH, Na HR, et al. Effects on agitation with rivastigmine patch monotherapy and combination therapy with memantine in mild to moderate Alzheimer’s disease: a multicenter 24-week prospective randomized open-label study (the Korean EXelon Patch and combination with memantine: rivastigmine and memantine on agitation). Geriatr Gerontol Int. 2016.
- Alzheimers Association. Alzheimer’s and Dementia Caregiver Center. www.alz.org/care/overview.asp.
- Family Caregiver Alliance. https://www.caregiver.org/.
- National Institutes of Health. National Institute on Aging. Caring for a person with Alzheimer’s disease. May 2009. www.nia.nih.gov/alzheimers/publication/caring-person-alzheimers-disease/about-guide. Accessed October 4, 2016.
- Latham P, Posner J. Caring for persons with dementia.pdf. American Stroke Association. Published 2006. http://www.strokeassociation.org/idc/groups/stroke-public/@wcm/@hcm/@sta/documents/downloadable/ucm_310595.pdf. Accessed June 17, 2016.
- Elliott AF, Burgio LD, DeCoster J. Enhancing caregiver health: findings from the resources for enhancing Alzheimer’s caregiver health II intervention: J Am Geriatr Soc. 2010;58(1):30-37.
- Burgio LD, Collins IB, Schmid B, et al. Translating the REACH caregiver intervention for use by area agency on aging personnel: the REACH OUT program. Gerontologist. 2009;49(1):103-116.
- Qaseem A, Snow V, Cross JT Jr, et al. Current pharmacologic treatment of dementia: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2008;148(5):370-378.
- Aricept and Aricept ODT (donepezil) [prescribing information]. January 2015.