Combating Opioid Abuse Through New Formulations and Policy
RELEASE DATE:
March 1, 2018
EXPIRATION DATE:
March 31, 2020
FACULTY:
Kimberly E. Ng, PharmD, BCPS
Assistant Professor
St. John's University
College of Pharmacy and Health Sciences
Department of Clinical Health Professions
Queens, New York
Josh Rickard, PharmD, BCPS, CDE
Assistant Professor
St. John's University
College of Pharmacy and Health Sciences
Department of Clinical Health Professions
Queens, New York
FACULTY DISCLOSURE STATEMENTS:
Drs. Ng and Rickard have no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT:
Pharmacy
 Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-18-007-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE:
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@jobson.com
DISCLAIMER:
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients' conditions and possible contraindications or dangers in use, review of any applicable manufacturer's product information, and comparison with recommendations of other authorities.
GOAL:
To educate pharmacists on the history of opioid and abuse-deterrent formulation development, newly approved abuse-deterrent formulations, and current strategies aimed to combat the opioid epidemic.
OBJECTIVES:
After completing this activity, the participant should be able to:
- Compare and contrast current abuse-deterrent FDA-approved opioids.
- Describe new alternative abuse-deterrent opioids.
- Summarize the strengths and weaknesses of current prescription drug monitoring programs.
- Understand naloxone's role in the opioid epidemic.
- Identify the role of the pharmacist in combating the opioid epidemic.
ABSTRACT: Opioid-related overdose deaths have quadrupled since 1999. To combat this epidemic, pharmaceutical companies are developing various reformulations of commonly used opioid pain medications with abuse-deterring properties. The increase in opioid-overdose deaths has also led to widespread use of prescription drug monitoring programs to identify overprescribing, diversion, and dispensing of naloxone to reverse opioid overdoses without a prescription in pharmacies, thereby outlining a crucial role for the pharmacist in this epidemic.
According to the CDC, since 1999, overdose deaths related to opioids have quadrupled, with a reported 91 Americans dying each day from an opioid overdose.1
When first introduced to the market, opioids were primarily used for acute pain and cancer-related pain management. Through the 1990s, studies indicated poor treatment of chronic noncancer pain, resulting in an increased use of opioids for that purpose. Over the last 20 years, there has been a significant increase in the approval of various opioid formulations, starting with the first formulation, morphine sulfate (MS Contin), approved in 1987, for 12-hour dosing instead of 4 to 6 hours. Shortly thereafter, the first opioid available as a patch, the fentanyl transdermal system, was approved in August 1990.
In 1995, oxycodone controlled-release (OxyContin) was approved for 12-hour dosing, but it would soon become a highly abused medication. At the time of approval, the FDA believed the controlled-release formulation would deter abuse because it would control absorption and avoid the highs desired by drug-seekers. The FDA also updated product labeling to reflect the potential for abuse and warned that crushing and injecting controlled-release products could cause overdose. There was no indication that crushing, followed by oral ingestion and/or snorting, would soon become widespread. In 1998, Actiq (fentanyl) was approved to treat cancer breakthrough pain through a restricted-distribution program to prevent accidental ingestion by children—because the product looks like a lollipop—and to prevent abuse. The product was designed as a transmucosal immediate-release fentanyl product that allows drug delivery through mucous membranes.2
In the early 2000s, there was a marked increase in reported overdoses and deaths from prescription drugs, with opioids like OxyContin being highly abused. In 2001, interagency collaboration between the FDA, the U.S. Substance Abuse and Mental Health Services Administration, the Center for Substance Abuse Treatment, and the National Institute on Drug Abuse allowed for the development of education about prescription-drug abuse. OxyContin-labeled indications were modified to assist prescribers in identifying patients who would benefit from its use, indicating that it was not appropriate for "as needed" pain. A boxed warning was added and a risk-management program implemented to reduce abuse. In 2002, a patient package insert was approved for OxyContin. Actiq developed a Medication Guide in 2006, requiring that it be given to patients when they fill a prescription. Prior to the Medication Guide, Actiq had a patient package insert but decided to convert to the Medication Guide to assure that patients were aware of the risks associated with the medication.2
In 2007, the passage of the Food and Drug Administration Amendments Act allowed for enhanced drug safety, which included Risk Evaluation and Mitigation Strategies (REMS) to ensure that benefits outweigh risks. This law helped pave the way for the future REMS program with Extended-Release/Long-Acting (ER/LA) opioid products. In 2009, the FDA launched a program called the Safe Use Initiative to reduce harm from medications, including opioids. In 2010, a Joint Advisory Committee meeting was held to propose REMS for all ER/LA opioids; it went into effect in 2012.2
In 2012, the FDA initiated discussions on making naloxone available in the community to prevent opioid-overdose deaths. In 2013, to help control the epidemic, the FDA took multiple steps toward decreasing the nation's opioid use. As a means of encouraging development of safe opioid products, the FDA began to provide guidance for pharmaceutical companies seeking to create abuse-deterrent formulations. In 2014, multiple abuse-deterrent formulations were approved. In April 2014, the FDA approved Evzio, the first autoinjector designed to deliver naloxone outside of a healthcare setting. Naloxone nasal spray was approved in 2015 as an alternative formulation to help combat opioid overdoses. In 2016, the FDA announced it would also require labeling changes for immediate-release opioids to further ensure safety, and 2017 saw the approval of additional abuse-deterrent formulations.2
These formulations have been developed to target known or potential routes of abuse, such as crushing for snorting or dissolving for injection. Although abuse-deterrent formulations are not abuse-proof and cannot prevent overdose or death, they are a step toward reducing abuse and making a product more difficult and less rewarding to abuse. The FDA has issued two guidance documents to help the industry understand how the products are evaluated. Guidance for Industry: Abuse-Deterrent Opioids—Evaluation and Labeling, published in 2013, provides recommendations on how studies should be performed and evaluated and discusses appropriate labeling based upon studies. General Principles for Evaluating the Abuse Deterrence of Generic Solid Oral Opioid Drug Products was released in 2017 and gives recommendations on studies that should be completed to demonstrate that a generic opioid is no less abuse-deterrent than the brand product.3
ABUSE-DETERRENT OPIOID FORMULATIONS
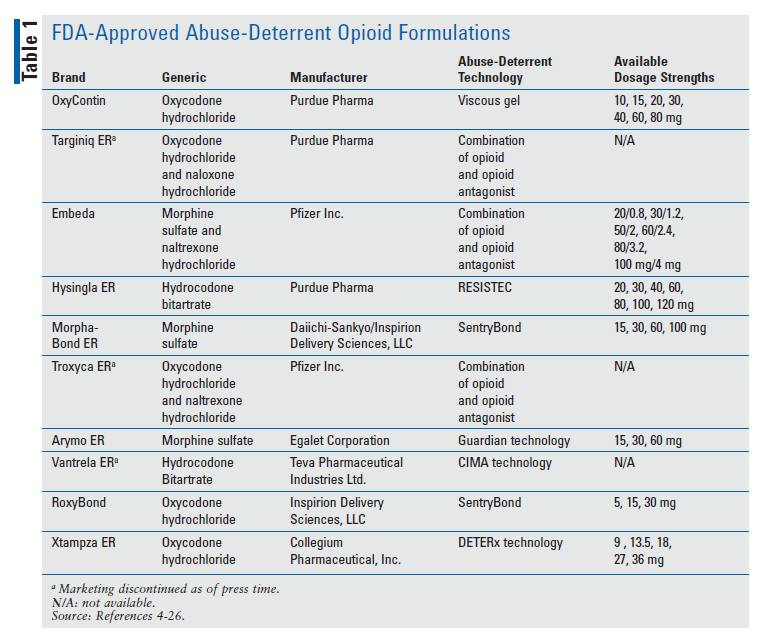
Pharmaceutical companies are developing alternative formulations to decrease abuse potential caused by crushing, inhaling, or injecting the prescription product. A total of 10 opioids with abuse-deterrent properties that employ different technology to minimize abuse potential have been approved by the FDA (TABLE 1).3 The marketing of several of these approved abuse-deterrent formulations—Targeniq ER, Troxyca ER, and Vantrela ER—had been discontinued as of press time.4-6
OxyContin
OxyContin was reformulated and in 2013 became the first opioid with abuse-deterrent characteristics to have FDA-approved labeling. In vitro data demonstrated that injection and abuse via the intranasal route would be difficult; when dissolved, the drug forms a viscous gel that is difficult to inject.7,8
Targiniq ER
Targiniq ER is a combination of oxycodone hydrochloride and naloxone hydrochloride developed by Purdue Pharma and approved in July 2014. The combination product is designed so that when used appropriately, oxycodone hydrochloride is released as an extended-release product. However, if the product is manipulated, the naloxone is released and blocks the opioid effects.8,9
Embeda
Embeda, morphine sulfate and naltrexone hydrochloride extended-release capsules from Pfizer Inc., received FDA updated-label approval in October 2014 to indicate that it can reduce both oral and intranasal abuse when crushed. The capsules are composed of four layers: a rate-controlling membrane, pellets of extended-release morphine sulfate, a sequestering membrane, and a sequestered naltrexone core. When taken as directed, the naltrexone has no effect, but when the capsule is crushed or chewed, the naltrexone will be released. The naltrexone is bioequivalent to an immediate-release naltrexone oral solution and will bind with mu-opioid receptors to reverse the effects of morphine.10,11
Hysingla ER
Hysingla ER, hydrocodone bitartrate extended-release, was approved in November 2014 and uses Purdue Pharma's proprietary extended-release solid oral platform, RESISTEC. The approved labeling indicates that the product has been redesigned to reduce chewing, snorting, and IV forms of abuse. RESISTEC is a combination of polymer and processing that confers tablet hardness and imparts viscosity when dissolved in aqueous solutions. Tablets contain polyethylene oxide in combination with the active ingredient and other excipients for tablet manufacture so that the hydrocodone bitartrate is dispersed within a polyethylene oxide polymer matrix. The matrix has long molecular chains, so the tablet does not dissolve in water. By undergoing a manufacturing process with pressure, heat, and time, the molecular chains transform into a firm, interlocked matrix to make tablets very hard and resistant to tampering.12
MorphaBond ER
MorphaBond ER, an extended-release morphine formulation that is bioequivalent to MS Contin, was approved by the FDA in October 2015. MorphaBond ER uses SentryBond technology to combine inactive excipients with active pharmaceutical ingredients in a tablet. If the tablet is subjected to physical manipulation or chemical extraction, SentryBond maintains the intended release profile of extended-release products and delays the release of immediate-release products. MorphaBond ER does not incorporate aversive agents or opioid antagonists in its formulation and is expected to deter intranasal and IV abuse.13,14
Troxyca ER
In August 2016, the FDA approved Troxyca ER, which is a combination of oxycodone hydrochloride and naltrexone hydrochloride extended-release capsules. The formulation contains pellets of oxycodone hydrochloride that surround sequestered naltrexone hydrochloride, the opioid antagonist. If taken as directed, the naltrexone will remain sequestered, and the patient will receive the oxycodone in an extended-release manner. If crushed or chewed, up to 100% of the naltrexone could be released. In vitro testing showed that manipulation of the capsule through manual breakdown or mixing with solvents caused both the oxycodone hydrochloride and naltrexone hydrochloride to be extracted, thereby negating abuse potential.15,16
Arymo ER
Arymo ER is an extended-release formulation of morphine sulfate from Egalet Corporation, approved in January 2017, that deters abuse by persons seeking to dissolve and inject it. As described by Egalet, Guardian Technology is a polymer matrix tablet technology that uses a manufacturing process of injection molding to create tablets that are hard and difficult to manipulate. With this technology, tablets that are controlled-release and have increased resistance to physical and chemical manipulation will form a hydrogel when in contact with liquid. However, Arymo ER was not approved for the claim that it deters abuse in those who seek to snort or chew it.17,18
Vantrela ER
Vantrela ER is a formulation of hydrocodone bitartrate extended-release tablets approved in January 2017. The package insert highlights the physiochemical properties intended to make manipulation for misuse and abuse difficult. Vantrela ER uses Teva's proprietary CIMA technology for oral tablets. CIMA technology uses a multilayer gel-forming polymer coating that regulates drug release and combines three physical and chemical barriers (gelling, barrier, and matrix). These barriers help deter crushing for snorting, IV extraction, and dose-dumping in alcohol. In vitro physical and chemical tablet-manipulation studies evaluated potential extraction methods and supported that Vantrela ER was able to resist crushing, breaking, and dissolution and was even able to maintain some extended-release properties despite manipulation. When extraction was attempted, it formed a viscous material that resisted passage through a hypodermic needle.19-21
RoxyBond
RoxyBond, developed by Inspirion Delivery Sciences, LLC, is an abuse-deterrent form of oxycodone hydrochloride. It was approved in April 2017 and uses physical and chemical barriers to deter abuse without the addition of aversive agents or opioid antagonists. RoxyBond uses SentryBond technology, like MorphaBond ER, to combine inactive excipients with active pharmaceutical ingredients in a tablet. Roxybond is said to have increased resistance to cutting, crushing, grinding, or breaking. It was able to resist extraction with selected household and laboratory solvents. RoxyBond forms a viscous material that resists passage through a needle and was more difficult to prepare into solutions for IV injection relative to oxycodone immediate-release tablets. Unfortunately, intranasal, oral, and IV methods of abuse are still possible.13,22,23
Xtampza ER
In November 2017, Collegium Pharmaceutical, Inc. received FDA approval for its Supplemental New Drug Application to enhance its labeling of Xtampza ER, which is a form of oxycodone hydrochloride extended-release tablets. The labeling of Xtampza ER states that it can deter oral, intranasal, and IV abuse. Xtampza ER uses DETERx technology, which combines an oxycodone base with inactive ingredients to form a lipophilic salt. The lipophilic salt allows for homogenous distribution of the active drug in a waxy microsphere. The formulation is designed to avoid a rapid increase in plasma concentrations from any type of manipulation. The microspheres will solidify in a needle so that it cannot be expelled, and mixing with solvents is resisted by the microspheres.24-26
NEW OPTIONS FOR OPIOID-USE DISORDER TREATMENT
RBP-6000
RBP-6000, developed by Indivior, is an extendedrelease formulation of buprenorphine that is indicated for the treatment of moderate-to-severe opioid-use disorder in patients who have undergone induction with a transmucosal buprenorphine-containing product to suppress opioid-withdrawal signs and symptoms. RBP-6000 is recommended as part of a treatment plan and is intended to help manage patients recovering from opioid-use disorder regardless of whether they have tried medication-assisted treatment in the past.27,28
RBP-6000 is an SC injection that is administered once monthly into the abdominal region and is able to provide adequate plasma levels of buprenorphine over the dosing interval. This medication uses buprenorphine and the ATRIGEL Delivery System, which is made up of a biodegradable polymer, poly(D,L-lactide-co-glycolide), dissolved in a biocompatible solvent. The same delivery system is used in seven other FDA-approved drugs. Although the drug is injected as a solution, the precipitation of the polymer creates a depot containing the buprenorphine, which is released through diffusion and degradation of the depot.27,28
The recommended dosing regimen is 300 mg monthly for the first 2 months, followed by maintenance treatment with 100 mg or 300 mg monthly based on the patient's clinical condition. RBP-6000 is available as a prefilled syringe for administration by a healthcare professional in a healthcare setting.24,25
Indivior has also proposed REMS to ensure safe and appropriate use. The proposed REMS are consistent with the REMS for Suboxone, with the addition of restricted distribution to prevent diversion. The goals of the REMS include mitigating the risks of diversion, misuse, abuse, and accidental pediatric exposure, and informing prescribers, pharmacists, and patients about risks and the long-acting nature of the formulation.27,28
On October 31, 2017, the Psychopharmacologic Drugs Advisory Committee and Drug Safety and Risk Management Advisory Committee of the FDA voted to recommend the approval of RBP-6000. On November 30, 2017, the FDA approved Sublocade, the brand name for RBP-6000, as the first once-monthly injectable buprenorphine product.27,28
CAM2038
CAM2038, developed by Braeburn Pharmaceuticals, is another extended-release buprenorphine formulation indicated for the initiation, stabilization, and maintenance treatment of opioid-use disorder. CAM2038 utilizes a different technology, known as FluidCrystal technology, that is composed of lipid components, a solvent, and buprenorphine. When injected, CAM2038 absorbs interstitial aqueous body fluid and turns the liquid into a viscous, liquid crystalline gel that encapsulates buprenorphine. It is then able to release it as the depot, made up of the formulations' lipid components.29,30
CAM2038 is an extended-release solution, available as a prefilled syringe for once-weekly or once-monthly injection. Weekly formulations are available as 8, 16, 24, and 32 mg delivered in 0.16, 0.32, 0.48, and 0.64 mL, respectively. Monthly formulation dosing is available as 64, 96, 128, and 160 mg delivered in 0.18, 0.27, 0.36, and 0.45 mL, respectively. The formulation is injected SC into the buttock, thigh, abdomen, or upper arm and must be administered as a single injection by a healthcare professional. Injection sites should be rotated, and the product should never be administered intravascularly, intramuscularly, or intradermally. Patients can transition from daily doses of sublingual buprenorphine to weekly and then monthly dosing. The weekly and monthly dosing allows physicians to individualize dosing based on patient needs.29,30
Like RBP-6000, CAM2038 is also considering a REMS program to reduce risks of potential abuse, misuse, and accidental pediatric exposure. Only healthcare professionals will be able to directly administer CAM2038 to patients. The pharmaceutical company has also developed a limited-distribution model to control distribution and ensure that only healthcare providers will have access to CAM2038.29,30
On November 1, 2017, the Psychopharmacologic Drugs Advisory Committee and Drug Safety and Risk Management Advisory Committee of the FDA voted to recommend the approval of some of the proposed dosages of CAM2038. CAM2038 was accepted under a priority-review designation by the FDA in September 2017 and was granted fast-track designation. The New Drug Application for CAM2038 is currently under FDA review.29,30
PRESCRIPTION DRUG MONITORING PROGRAMS
In response to the opioid epidemic, state policy makers have implemented a tool called Prescription Drug Monitoring Programs (PDMPs), or Prescription Monitoring Programs (PMPs), to aid in combating this issue.31 In short, PDMPs are electronic databases that allow prescribers and pharmacists in the dispensing roles to sign in and review data regarding controlled substances that have been previously prescribed and dispensed to individual patients.32 Some states have opted to include noncontrolled medications that they deem to have a potential for abuse.32 Forty-nine of the 50 states (excluding Missouri) have implemented a statewide PDMP, and many of these states require the prescriber to review the PDMP prior to prescribing a controlled substance.31 As of July 17, 2017, Missouri's governor signed an executive order for the creation and implementation of a statewide PDMP.33
There are many perceived benefits of PDMPs, including prescriber and pharmacist awareness of patients' previous controlled-substance fill history, the ability to identify "doctor-shopping," the avoidance of polypharmacy, and the potential to change prescribing patterns.31,34 However, the rollouts of PDMPs have identified potential areas for improvement in these systems. First, there are low prescriber PDMP-registration rates.34 It was shown that only about 35% of providers who prescribed controlled substances were registered in their states' affiliated databases.34 This could be due to provider difficulty in registering for log-in credentials. Furthermore, of the 35% of prescribers registered, not all use the system regularly.34 Potential reasons for the limited uptake include the inability to integrate PDMP monitoring into a busy workflow and delays in information being available to view in the database. With optimization of current PDMPs, user uptake may subsequently increase. Some of these improvements include more robust data sharing, real-time reporting, universal required use, integration into electronic medical records, and training for end users.31,35,36
The impact of PDMPs on rates of opioid overdose and opioid overdose–related deaths is mixed. Bao and colleagues found that Schedule II opioid-prescribing rates decreased by 30% in the first year, but there was no effect on Schedule III drugs.37 Also, Moyo and colleagues found a reduction in opioid volume, but not in the number of prescriptions in the Medicare population.38 Nam and colleagues designed a study that analyzed drug-overdose mortality data from the CDC.39 They found that PDMPs did not show a reduction in opioid-overdose mortality rates and that they may increase rates of mortality related to nonprescription opioid and illicit drugs.39 Lastly, a meta-analysis found very mixed results regarding the benefit of PDMPs and little standardization among the outcomes evaluated in each of the studies, and the researchers called for action regarding further examination of PDMPs.40
In support of PDMPs, the CDC has published state-specific success stories. In 2012, New York and Tennessee, for example, required prescribers to check their state's PDMP prior to prescribing an opioid, and as a result, found a 75% and 36% respective decrease in doctor-shopping in 2013.41 Ohio and Kentucky have regulated pain clinics and required prescribers to review the PDMPs. This has led to a decrease in the prescribed morphine-milligram equivalents per capita in 85% and 62% of their counties, respectively. Florida also saw a decrease in opioid prescribing in 80% of its counties after implementing multiple interventions, including mandating that prescribers review their PDMP. Because these data also represent multiple initiatives, it cannot be concluded that the decrease in prescribing by county was related to the mandated PDMP review.41
NALOXONE ACCESS
Because the rate of opioid overdose–related deaths has increased across the United States, access to naloxone in the community has grown significantly.42 Naloxone is a lifesaving drug that temporarily reverses the effects of opioid overdoses (such as respiratory depression and sedation) by displacing the opioid from the receptor.43 The CDC recommends that naloxone access be expanded into multiple arenas, including community pharmacies, distribution by community organizations, use by law-enforcement officials, and training of staff who work in emergency medicine.44 As of July 1, 2017, all 50 states have some sort of expanded naloxone-access laws, including legal immunity to those who administer the drug.45,46 Because every state is different, it is important for the pharmacist to review the laws in the state in which he or she practices.
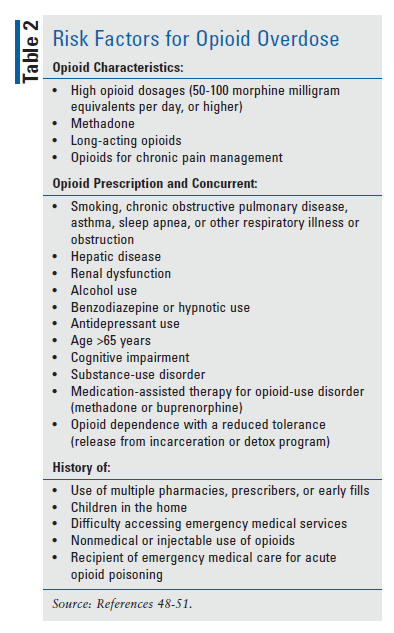
To dispense naloxone from the community pharmacy, many different access models are in place. In some states, pharmacists are allowed to prescribe naloxone directly or through some type of collaborative-practice agreement, whereas others use standing orders.47 According to the College of Psychiatric and Neurologic Pharmacists, naloxone should be available for anyone in contact with opioids, including caregivers.48 Pharmacists should screen patients at high risk for opioid overdose to identify candidates for naloxone treatment. See TABLE 2 for a summary of risk factors for opioid overdose.48-51
ROLE OF THE PHARMACIST
Pharmacists are accessible and trusted healthcare professionals with a unique opportunity to educate patients on how to reduce the risk of overdose, what to do when an overdose occurs, and how to access addiction-management services.52 Pharmacists can be directly involved in fighting the opioid epidemic by dispensing naloxone to those at high risk for opioid overdose.52 Patient and caregiver education is the biggest weapon pharmacists can provide. Some of the topics to discuss with patients or caregivers include (but are not limited to)53:
- Naloxone's use in opioid overdose and training on how to use the devices
- Concomitant disease-state education
- Substance-use disorder treatment
- Safe medication storage and disposal
The management of opioids in pharmacy practice involves identifying potential opioid misuse or abuse.54 The pharmacist should evaluate each patient and the prescriptions that are presented to the pharmacy for any suspected modifications or should review fill history to identify whether patients are filling too frequently.54 Using resources such as the PDMP and the VIGIL system (verification, identification, generalization, interpretation, and legalization) can aid in the evaluation of patients and prescriptions when the pharmacist feels uncomfortable about dispensing a prescription.54 VIGIL is a controlled-substance prescription-screening tool that helps pharmacists classify patients by risk. This risk-stratification tool provides guidance on how to counsel patients based on their risk score.55
Another excellent opportunity for pharmacists is medication therapy management (MTM) visits.51 By completing an MTM visit with the patient, the pharmacist can assess a patient's risk for misuse and risk of overdose.54 This allows the pharmacist to be directly involved with the patient's care, which in turn will optimize treatments and any adverse events that occur.54 When misuse, abuse, or diversion is confirmed, the pharmacist should maintain professionalism when speaking with the patient and have a list of local providers of addiction-treatment services for referral, as well as knowledge of other available resources.54
CONCLUSION
Advances within the pharmaceutical industry and strong public-health efforts are needed to combat the opioid epidemic that has engulfed the U.S. Alternative formulations of opioid medications, more stringent monitoring programs, opioid education, and naloxone distribution are some of the tools that can help. The pharmacist is well positioned to use many of these tools and help save lives.
REFERENCES
- CDC. Understanding the epidemic. Updated August 30, 2017. www.cdc.gov/drugoverdose/epidemic/index.html. Accessed December 27, 2017.
- FDA. Timeline of selected FDA activities and significant events addressing opioid misuse and abuse. Updated December 12, 2017. www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ ucm338566.htm. Accessed December 27, 2017.
- FDA. Opioid medications. Updated December 12, 2017. www. fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm337066. htm. Accessed December 27, 2017.
- FDA. Drugs@FDA. Approved drug products. Targiniq ER. www.accessdata.fda.gov/scripts/cder/daf/index. cfm?event=overview.process&ApplNo=205777. Accessed February 23, 2018.
- FDA. Drugs@FDA. Approved drug products. Troxyca ER. www.accessdata.fda.gov/scripts/cder/daf/index. cfm?event=overview.process&ApplNo=207621. Accessed February 23, 2018.
- FDA. Drugs@FDA. Approved drug products. Vantrela ER. www.accessdata.fda.gov/scripts/cder/daf/index. cfm?event=overview.process&ApplNo=207975. Accessed February 23, 2018.
- Purdue Pharma. OxyContin was reformulated and in 2013 became the first opioid with FDA-approved labeling describing abuse-deterrent characteristics. OxyContin.com. Updated 2017. www.oxycontin.com/abuse-deterrence-studies-summary.html. Accessed December 27, 2017.
- Becker WC, Fiellin DA. Abuse-deterrent opioid formulations—putting the potential benefits into perspective. N Engl J Med. 2017;376(22):2103-2105.
- Targiniq ER (oxycodone and naloxone) package insert. Stamford, CT: Purdue Pharma L.P.; 2014.
- FDA approves abuse deterrent labeling for Embeda (morphine sulfate and naltrexone hydrochloride) extended-release (ER) capsules CII [press release]. New York, NY: Pfizer Inc. October 17, 2014. www.pfizer.com/news/press-release/pressrelease-detail/fda_approves_abuse_deterrent_labeling_for_ embeda_morphine_sulfate_and_naltrexone_hydrochloride_extended_release_er_capsules_cii. Accessed December 27, 2017.
- The abuse-deterrent technology of Embeda. Pfizer for professionals. 2017. www.pfizerpro.com/product/embeda/hcp/technology. Accessed December 27, 2017.
- Hysingla ER. Formulated with RESISTEC technology. Stamford, CT: Purdue Pharma; 2017. https://hysinglaer.com/opioidabuse-deterrence-studies/resistec.html. Accessed December 27, 2017.
- Inspirion Delivery Sciences. Inspirion Delivery Sciences: our technology. www.inspirionds.com/. Accessed December 27, 2017.
- Inspirion Delivery Sciences. Inspirion Delivery Technologies receives FDA approval for MorphaBond (morphine sulfate) extended-release tablets CII, an opioid analgesic formulated with abuse-deterrent properties[press release]. www.prnewswire.com/ news-releases/inspirion-delivery-technologies-receives-fdaapproval-for-morphabond-morphine-sulfate-extended-release-tablets-cii-an-opioid-analgesic-formulated-with-abuse-deterrent-properties-300153910.html. Accessed December 27, 2017.
- Pfizer. FDA approves Troxyca ER (oxycodone hydrochloride and naltrexone hydrochloride) extended-release capsules CII with abuse-deterrent properties for the management of pain [press release]. www.pfizer.com/news/press-release/press-release-detail/ fda_approves_troxyca_er_oxycodone_hydrochloride_and_naltrexone_hydrochloride_extended_release_capsules_cii_with_abuse_ deterrent_properties_for_the_management_of_pain. Accessed December 27, 2017.
- Troxyca ER (oxycodone and naltrexone) package insert. New York, NY: Pfizer Inc.; 2016.
- Clarke T. Egalet painkiller wins FDA approval but label disappoints. Reuters. Updated January 9, 2017. www.reuters.com/ article/us-egalet-fda/egalet-painkiller-wins-fda-approval-but-labeldisappoints-idUSKBN14T209. Accessed December 27, 2017.
- Egalet. Applying guardian technology to deter prescription misuse & abuse. Guardian Technology. Updated 2016. http://egalet. com/staging/rd/technology-overview/. Accessed December 27, 2017.
- Vantrela ER (hydrocodone) package insert. North Wales, PA: Teva Pharmaceuticals USA, Inc.; 2017.
- Teva Pharmaceutical Industries Limited, Inc. Easing the challenge of pain. www.tevapharm.com/research_development/rd_ focus/pain/. Accessed December 27, 2017.
- Teva receives FDA approval for Vantrela ER (hydrocodone bitartrate) extended-release tablets [cii] formulated with proprietary abuse deterrence technology [press release]. North Wales, PA: Teva Pharmaceuticals, Inc. January 18, 2017. www.tevapharm.com/news/teva_receives_fda_approval_for_vantrelatm_er_ hydrocodone_bitartrate_extended_release_tablets_cii_formulated_ with_proprietary_abuse_deterrence_technology_01_17.aspx. Accessed December 27, 2017.
- Inspirion Delivery Sciences receives FDA approval for Roxybond (oxycodone hydrochloride) tablets CII, the first and only immediate release opioid analgesic with abuse deterrent label claims [press release]. Valley Cottage, NY: Inspirion Delivery Sciences. April 26, 2017. www.prnewswire.com/news-releases/ inspirion-delivery-sciences-receives-fda-approval-for-roxybondoxycodone-hydrochloride-tablets-cii-the-first-and-only-immediaterelease-opioid-analgesic-with-abuse-deterrent-labelclaims-300445964.html. Accessed December 27, 2017.
- RoxyBond (oxycodone) package insert. Valley Cottage, NY: Inspirion Delivery Sciences LLC; 2017.
- Xtampza ER. DETERx technology makes the formulation more difficult to manipulate. 2016. www.xtampzaer.com/hcp/ deterx-technology.html#tab-2. Accessed December 27, 2017.
- Collegium receives FDA approval for sNDA for Xtampza ER [press release]. Cincinnati, OH: Collegium Pharmaceuticals. November 7, 2017. http://phx.corporate-ir.net/phoenix. zhtml?c=253995&p=irol-newsArticle&ID=2314949. Accessed December 27, 2017.
- Xtampza ER (oxycodone) package insert. Cincinnati, OH: Collegium Pharmaceuticals, 2016.
- Indivior. FDA advisory committees recommend approval of Indivior's RBP-6000 for the treatment of opioid use disorder. October 31, 2017. www.indivior.com/investor-news/fda-advisorycommittees-recommend-approval-indiviors-rbp-6000-treatmentopioid-use-disorder/. Accessed December 27, 2017.
- FDA. FDA briefing document: joint meeting of Psychopharmacologic Drugs Advisory Committee and Drug Safety and Risk Management Advisory Committee October 31, 2017. www.fda. gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/PsychopharmacologicDrugsAdvisoryCommittee/ UCM582447.pdf. Accessed December 27, 2017.
- Braeburn announces FDA advisory committee recommends approval of CAM2038 buprenorphine depot for the treatment of opioid use disorder. Princeton, NJ: Braeburn Pharmaceuticals [press release]. November 1, 2017. www.braeburnpharmaceuticals.com/braeburn-announces-fda-advisory-committee-recommends-approval-of-cam2038-buprenorphine-depot-for-thetreatment-of-opioid-use-disorder/. Accessed December 27, 2017.
- FDA. FDA briefing document: joint meeting of Psychopharmacologic Drugs Advisory Committee and Drug Safety and Risk Management Advisory Committee November 1, 2017. www.fda. gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/PsychopharmacologicDrugsAdvisoryCommittee/ UCM582592.pdf. Accessed December 27, 2017.
- Griggs CA, Weiner SG, Feldman JA. Prescription drug monitoring programs: examining limitations and future approaches. West J Emerg Med. 2015;16(1):67-70.
- Heagerty KE. Prescription monitoring programs: to use or not to use. Innov Clin Neurosci. 2013;10(11-12):28-30.
- Office of Missouri Governor Eric Greitens. Governor Eric Greitens announces statewide prescription drug monitoring program. July 17, 2017. https://governor.mo.gov/news/archive/governor-eric-greitens-announces-statewide-prescription-drug-monitoring-program. Accessed February 5, 2018.
- Haffajee RL, Jena AB, Weiner SG. Mandatory use of prescription drug monitoring programs. JAMA. 2015;313(9):891-892.
- Rutkow L, Smith KC, Lai AY, et al. Prescription drug monitoring program design and function: a qualitative analysis. Drug Alcohol Depend. 2017;180:395-400.
- CDC. What states need to know about PDMPs. Updated October 3, 2017. www.cdc.gov/drugoverdose/pdmp/states.html. Accessed December 8, 2017.
- Bao Y, Pan Y, Taylor A, et al. Prescription drug monitoring programs are associated with sustained reductions in opioid prescribing by physicians. Health Aff (Millwood). 2016;35(6):1045-1051.
- Moyo P, Simoni-Wastila L, Griffin BA, et al. Impact of prescription drug monitoring programs (PDMPs) on opioid utilization among Medicare beneficiaries in 10 US States. Addiction. 2017;112(10):1784-1796.
- Nam YH, Shea DG, Shi Y, Moran JR. State prescription drug monitoring programs and fatal drug overdoses. Am J Manag Care. 2017;23(5):297-303.
- Finley EP, Garcia A, Rosen K, et al. Evaluating the impact of prescription drug monitoring program implementation: a scoping review. BMC Health Serv Res. 2017;17:420.
- CDC. Opioid overdoses. State successes. Updated October 5, 2017. www.cdc.gov/drugoverdose/policy/successes.html. Accessed December 8, 2017.
- Bulloch M. As naloxone accessibility increases, pharmacist's role expands. Pharmacy Times. October 25, 2016. www.pharmacytimes.com/contributor/marilyn-bulloch-pharmd-bcps/2016/10/ as-naloxone-accessibility-increases-pharmacists-role-expands. Accessed December 8, 2017.
- Narcan Nasal Spray (naloxone) package insert. Radnor, PA: Adapt Pharma, Inc.; 2015.
- CDC. Opioid overdose. Reverse overdose to prevent death. Updated August 29, 2017. www.cdc.gov/drugoverdose/prevention/reverse-od.html. Accessed December 8, 2017.
- The Network for Public Health Law. Legal interventions to reduce overdose mortality: naloxone access and overdose Good Samaritan laws. Updated July 2017. www.networkforphl.org/_ asset/qz5pvn/legal-interventions-to-reduce-overdose.pdf. Accessed December 8, 2017.
- PDAPS: Prescription Drug Abuse Policy System. Naloxone overdose prevention laws. Updated July 1, 2017. http://pdaps. org/datasets/laws-regulating-administration-of-naloxone-1501695139. Accessed December 8, 2017.
- National Alliance of State Pharmacy Associations. Naloxone access in community pharmacies. https://naspa.us/resource/naloxone-access-community-pharmacies/. Published June 1, 2017. Accessed December 8, 2017.
- College of Psychiatric and Neurologic Pharmacists. Naloxone access: a practical guideline for pharmacists. https://cpnp.org/ guideline/naloxone. Accessed December 8, 2017.
- Substance Abuse and Mental Health Services Administration. SAMHSA opioid overdose prevention toolkit. HHS Publication No. (SMA) 16-4742. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2016.
- World Health Organization. Community management of opioid overdose. Geneva, Switzerland: World Health Organization Document Production Services; 2014.
- CDC. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49.
- Gao F. The essential role of pharmacists in the opioid crisis. Can Pharm J (Ott). 2017;150(3):216-217.
- Reynolds V, Causey H, McKee J, et al. The role of pharmacists in the opioid epidemic: an examination of pharmacistfocused initiatives across the United States and North Carolina. NC Med J. 2017;78(3):202-205.
- American Pharmacists Association. Pharmacists' role in addressing opioid abuse, addiction, and diversion. J Am Pharm Assoc (2003). 2014;54(1):e5-e15.
- Tanzi MG. VIGIL helps pharmacists screen control substances. American Pharmacists Association. September 1, 2012. www.pharmacist.com/vigil-helps-pharmacists-screen-controlledsubstances. Accessed February 5, 2018.