COVID-19 Infection in Children
RELEASE DATE
August 1, 2022
EXPIRATION DATE
August 31, 2024
FACULTY
Micheline A. Goldwire, PharmD, MS, MA, BCPS
Professor and Director, Drug Information Services
Regis University School of Pharmacy Denver, Colorado
Megan K. Leeds, PharmD, MBA, BCACP
Associate Professor and Assistant Dean of Student Affairs
Regis University School of Pharmacy
Denver, Colorado
FACULTY DISCLOSURE STATEMENTS
Drs. Goldwire and Leeds have no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT
 Pharmacy
Pharmacy
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-22-074-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients’ conditions and possible contraindications or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
GOAL
To provide pharmacists with an overview of COVID-19 infection in children as of July 7, 2022.
OBJECTIVES
After completing this activity, the participant should be able to:
- Describe severe acute respiratory syndrome coronavirus–2 and its variants.
- Describe COVID-19 illness in children.
- Discuss the safety and efficacy of COVID-19 vaccines in children.
- Describe the role of pharmacist in administra- tion of COVID-19 vaccines to children.
ABSTRACT: The severe acute respiratory syndrome coronavirus–2 (SARS-CoV-2), and subsequent disease (COVID-19), has established itself as the first worldwide pandemic of the 21st century. Researchers’ efforts have turned to unraveling the mysteries of this new disease. Despite these endeavors, the effect of SARS-CoV-2 in children has been elusive. Initially, it was thought that children experience asymptomatic or mild disease; however, severe complications of COVID-19 (multisystem inflammatory syndrome in children) and associated complications with the COVID-19 vaccine, albeit rare (myocarditis), have emerged. Emergency use authorization for non–FDA-approved therapies specific to children for prevention and/or treatment of COVID-19 has been forthcoming.
The severe acute respiratory syndrome coronavirus–2 (SARS-CoV-2) virus enters hosts cells through the angiotensin-converting enzyme 2 (ACE2) receptor via the spike protein located on the outer surface of the virus. Several epithelial cells express the ACE2 receptor, including the upper and lower respiratory tract, gastrointestinal tract, and vasculature.1 The expression of ACE2 in the upper respiratory tract increases as age increases, which may account for the milder disease in children compared with adults. Some researchers conclude that the decreased antibody response seen in children infected with SARS-CoV-2 may be secondary to innate or T-cell immune responses.2
The CDC monitors circulation of SARS-CoV-2 and its variants within the United States. Currently, Omicron is considered a variant of concern (VOC) and has seven lineages based on the genetic sequence of the spike protein.3 The prior variant, Delta, was a VOC from June 2021 to April 2022 and is now considered a variant being monitored (VBM).3
EPIDEMIOLOGY
COVID-19 infects persons of all ages. The CDC analyzed a convenience sample of blood specimens submitted for clinical testing for SARS-CoV-2 antibodies and found that seroprevalence increased from 34% in December 2021 to 58% in February 2022 among all age groups, with an increase of 44% to 75% in children aged 0 to 11 years and 46% to 74% in adolescents aged 12 to 17 years.4 At the same time, the Omicron variant was widely circulating. These values are most likely underreported, as many persons with COVID symptoms may have opted for an at-home test rather than a testing center. In addition, those with asymptomatic disease may not have been tested. With the increased seroprevalence from samples taken at testing centers, the increased number of at-home tests used, and the potential for asymptomatic carriers, the infectious nature of the Omicron variant in circulation during 2022 warrants appropriate response.
Clinical Spectrum
The clinical spectrum of COVID-19 in children is much more diverse than in adults. Asymptomatic disease may occur in up to 50% of children.5 Since the start of the pandemic, it was noted that children experience milder COVID-19 disease with fewer complications than adults. Several hypotheses for this include reduced ACE2 expression on epithelial cells, potential immunity from other coronavirus infections such as the common cold, coinfection with other viruses and the subsequent increased immune response, and maternal immunity.1,2 In addition, children seldom have the same comorbidities as adults, e.g., hypertension and diabetes, thereby predisposing them to fewer complications from COVID-19. However, some children who experience severe disease have significant underlying comorbidities.
SIGNS/SYMPTOMS
The most common symptoms in children are cough and fever, although a variety of symptoms occurring less often include shortness of breath, sore throat, headache, myalgia, fatigue, and rhinorrhea.6,7 In addition, gastrointestinal symptoms such as nausea, vomiting, diarrhea, and poor appetite may occur, with or without respiratory symptoms.6,7
SEVERE DISEASE
The following groups may be at increased risk for severe disease: infants younger than age 1 year, older adolescents, and children with significant medical comorbidities, e.g., severe immunosuppression, obesity, cardiopulmonary disease, chronic kidney disease, sickle cell disease, and diabetes. Hospitalizations for COVID-19 during March 2020 to February 2022 showed that the rate of hospitalization per 100,000 infants and children aged 0 to 4 years was significantly higher with Omicron compared with Delta: 14.5 versus 2.9. Infants aged younger than age 6 months accounted for 44% of hospitalizations during Omicron.8 Of note, approximately one-third of hospitalized infants and children had comorbidities.
Of the 3,106 children admitted to the hospital from March 2020 to May 2021 with laboratory-confirmed SARS-CoV-2 identified by the COVID-19 Associated Hospitalization Surveillance Network (across 14 states), 2,293 (73.8%) were admitted primarily for COVID-19 disease.9 Severe disease (requiring ICU admission, invasive mechanical ventilation, or death) occurred in 30%. Risk factors for severe disease included age younger than 6 months and one or more comorbidity. Comorbidities for children younger than age 2 years included chronic lung disease, neurologic disorder, cardiovascular disease, prematurity, airway abnormality, and feeding-tube dependency. For children aged 2 to 17 years, diabetes mellitus, obesity (age 12 years and older), blood disorders, liver or renal disease, and rheumatologic, autoimmune, and inflammatory conditions were identified as significant risk factors.
Multisystem Inflammatory Syndrome in Children
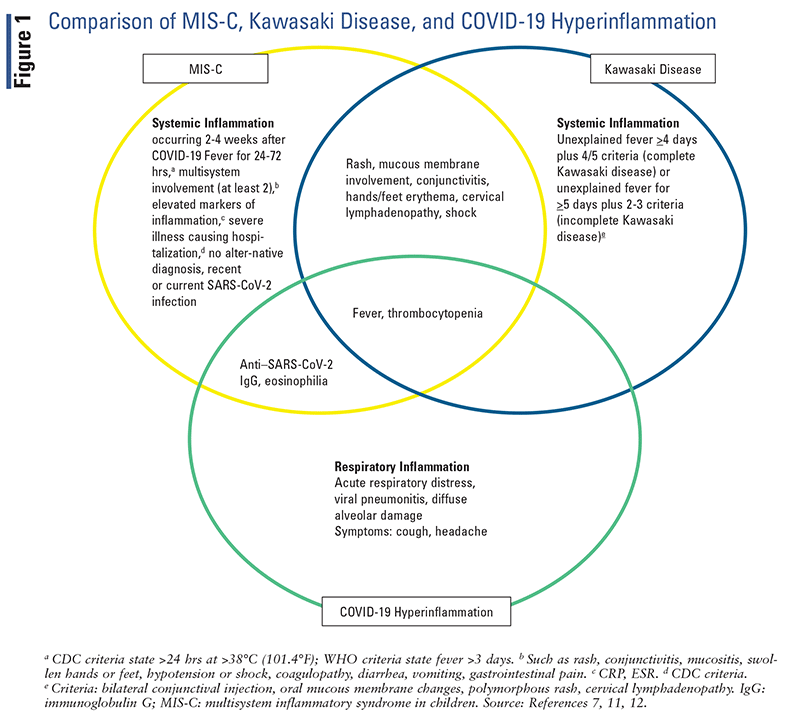
Early in the course of COVID-19 disease, it was thought that children experience only mild or asymptomatic disease. However, case reports began to show that a very small number of children who recovered from COVID-19 experienced a severe hyperinflammatory process that mimics Kawasaki disease and toxic shock syndrome.1,3,10,11 Researchers postulate through in vitro studies that antibody-mediated SARS-CoV-2 uptake by monocytes and macrophages triggers inflammatory cell death, aborting production of the virus while causing systemic inflammation.12 Multisystem inflammatory syndrome in children (MIS-C) is characterized by fever, elevated inflammatory biomarkers, and multisystem organ dysfunction.3,10,11 Symptoms of persistent fever, abdominal pain, vomiting, diarrhea, skin rash, mucocutaneous lesions, and in severe cases hypotension and shock generally appear 2 to 4 weeks after an acute COVID-19 illness.13 As of May 24, 2022, the CDC reports 8,210 patients with MIS-C, of which 46.4% are between the ages of 5 and 11 years, 21.1% between ages 1 and 4 years,
19.2% between ages 12 and 15 years, 10% between ages 16 and 20 years, and 3.3% younger than age 1 year.14 The diagnostic criteria for MIS-C published by the CDC and the World Health Organization differ. FIGURE 1 highlights the overlap in symptoms and differences between MIS-C, Kawasaki disease, and COVID-19 hyperinflammation.
Infants and Neonates
Most neonates born to mothers who are polymerase chain reaction (PCR) SARS-CoV-2–positive do not have COVID-19 when they are born, and infants who test positive shortly after birth have mild or no symptoms.15 However, some neonates with PCR confirmed SARS-CoV-2 present with feeding difficulty and unexplained fever (temperature >100.4°F).16 In a systematic review of clinical studies, case report and case series of neonates with confirmed positive reverse transcriptase–PCR initial symptoms varied.17 Of the 72 included studies describing 236 neonates, the most common symptoms (occurring more than 10%) were fever (43.2%), poor feeding (28.4%), tachypnea (20.3%), respiratory distress (19.5%), lethargy (16.5%), cough (15.2%), rhinorrhea (14.8%), and desaturation (12.3%).17 Another study showed that the most common symptoms (occurring more than 10%) at admission in 432 infants hospitalized for laboratory-confirmed COVID-19 were fever/chills (58%), cough (55%), congestion/runny nose (54%), respiratory distress (37%), poor feeding (32%), nausea/ vomiting (23%), fatigue (11%), and stridor (11%).8
Testing
Infants and children should be evaluated for the presence of SARS-CoV-2 if they are symptomatic or have come in close contact (within 6 feet or less for cumulative time of 15 minutes or more within a 24-hour period) with someone who is positive.18 The American Academy of Pediatrics (AAP) recommends testing for COVID-19 with a single swab for well newborns as close to discharge as possible.19 If the test comes back positive, a confirmatory test is required. If the confirmatory test is positive and the newborn has no symptoms, an outpatient visit within 14 days after birth is recommended.
PREVENTION
Nonpharmacologic
Wearing masks, social distancing, avoiding crowds, isolation, quarantine, and proper hand hygiene help to control spread of COVID-19. The CDC provides recommendations for masking dependent upon COVID-19 levels circulating in the community. Levels are defined as high, medium, or low. For high-risk areas, the CDC recommends well-fitting masks covering the nose and mouth for ages 2 years and older when in public areas, especially indoors, regardless of vaccination status. For medium- and low-risk areas, masks are needed for those who are immunocompromised or at high risk for severe disease. The COVID Data Tracker provides information on COVID in the community.14 Children should cover coughs and sneezes with a tissue, or use the inside of an elbow, and immediately wash hands. They should also handwash with soap and water for at least 20 seconds, especially before eating or when in a public space. If handwashing is not available, a hand sanitizer that contains at least 60% alcohol should be used.
Vaccines
The BNT162b2 Pfizer-BioNTech COVID-19 mRNA vaccine (Pfizer COVID-19 vaccine; Comirnaty), FDA approved those aged 16 years and older, is formulated using the original SARS-CoV-2 variants identified in Wuhan, China, as is the Moderna mRNA1273 vaccine. Since that time, the Delta and Omicron variants emerged. Researchers showed that the original vaccine neutralized the initial variant and the Delta variant after two doses, but three doses were required to neutralize the Omicron variant.20 As a result, the FDA recommends the addition of an Omicron spike protein component to the vaccine composition beginning in fall 2022.20
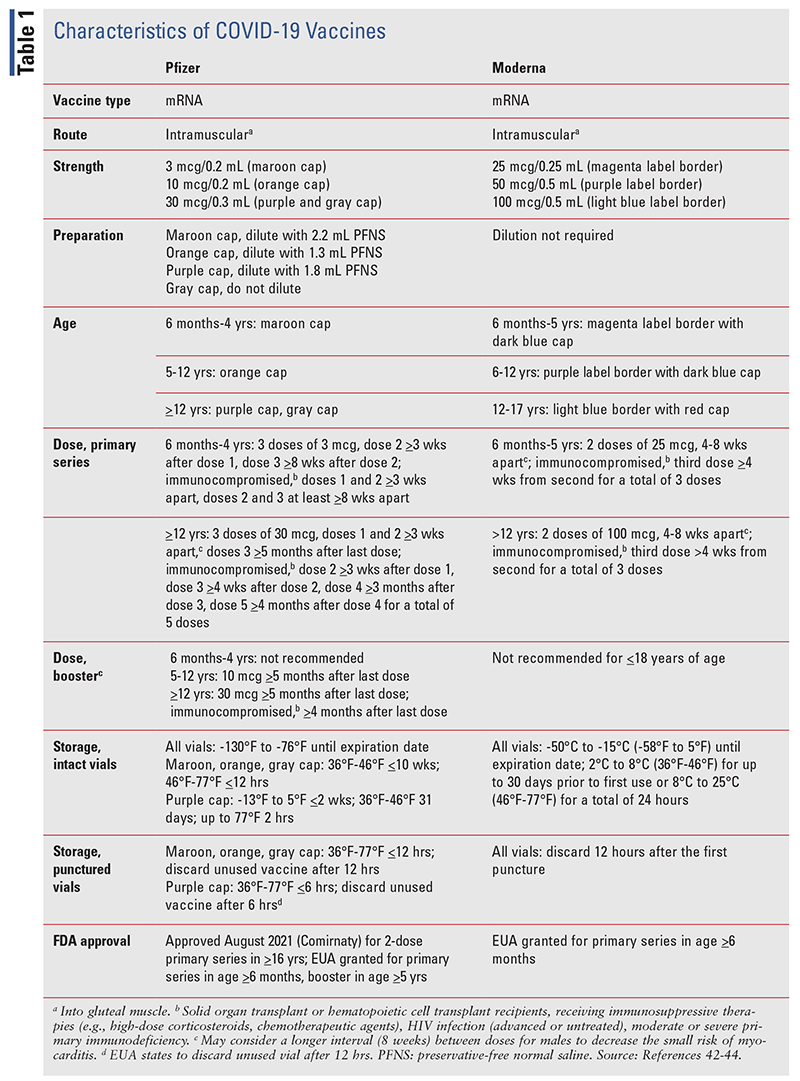
Efficacy
The CDC recommends that persons aged 6 months and older receive the primary COVID-19 vaccine series (TABLE 1). The FDA has granted emergency use authorization (EUA) for the Pfizer and Moderna COVID-19 vaccines for the primary series and one booster dose for the Pfizer vaccine in children aged 5 years and older.21 Data for EUA of the Moderna mRNA-1273 vaccine in children aged 6 months to 2 years and 2 to 6 years were sent to the FDA for review, based on findings of the KidCove study.22
Results of the KidCove study in children aged 6 and 11 years show that two 50-mcg doses of the Moderna mRNA-1273 vaccine 28 days apart produced geometric mean titers (GMT) at 57 days non-inferior to those reported in young adults aged 18 to 25 years.23 Vaccine efficacy of COVID-19 occurring within 14 days of the first dose was 88% (95% CI, 70-95.8). At the time of the study, Delta was the dominant circulating variant.
Children aged 5 to 11 years who received two 10-mcg doses of the Pfizer COVID-19 vaccine 21 days apart showed similar GMT as those aged 16 to 25 years who received the 30-mcg series 1 month after the second dose.24 Confirmed COVID-19 with positive SARS-CoV-2 within 7 days or more after the second dose occurred in three patients receiving vaccine and 16 receiving placebo, representing a vaccine efficacy of 90.7% (95% CI, 67.7-98.3). Therapy was well tolerated within 30 days after series completion, with pain at the injection site occurring most when averaging occurrence after the first and second dose (72.5% vaccine, 30% placebo), following by fatigue (36.5% vaccine, 37.5% placebo), and headache (25% vaccine, 21.5% placebo). It should be noted that the study was conducted between March and September 6, 2021; Delta became a VOC in June 2021.
Adolescents aged 12 to 15 years who received two 30-mcg doses of Pfizer COVID-19 vaccine 21 days apart showed GMT that were non-inferior to those aged 16 to 25 years.25 No cases of confirmed COVID-19 infection occurred with the vaccine, while 16 occurred with placebo, representing a vaccine efficacy of 100%. In children aged 12 to 15 years, the incidence of adverse events within 30 days of series completion averaged from the first and second dose occurring more than 20% included pain at injection site (82.5% vaccine, 20.5% placebo), followed by fatigue (63% vaccine, 33% placebo), headache (60% vaccine, 29.5% placebo), chills (35% vaccine, 8.5% placebo), and muscle pain (28%, 10.5%). It should be noted that the study was conducted between March and September 2021; Delta became a VOC in June 2021.
Results from a prospective cohort study in which authors compared vaccination status of 1,128 children in 6th through 12th grade (ages 11-19 years, August-November 2021), of which 829 (73.5%) were vaccinated and 299 (26.5%) were unvaccinated, showed that symptomatic infection was nine times higher in unvaccinated children compared with vaccinated.26 Rates of infection were low, with seven (0.8%) vaccinated children and 20 (6.7%) unvaccinated testing positive for the SARS-CoV-2 virus.
Safety
In general, both vaccines are well tolerated, with local injection-site reactions occurring most often followed by fatigue, headache, chills, and muscle pain.24,25 Through the Vaccine Adverse Event Reporting System (VAERS), reports of myocarditis occurring after COVID-19 vaccine administration began to appear shortly after widespread adoption of vaccination in December 2020.27 Researchers noted a rate of myocarditis higher in adolescent and young adult males compared with females or other age groups after the second booster dose.28 Although very rare, the rate of myocarditis for the 7-day risk period after administration of the second booster dose in males aged 12 to 15 years compared with females of the same age is 10 times higher at 39.9 cases (per million doses administered) compared with 3.9 cases, and this rate increases in the 16- to 17-year age group to 69.1 cases (per million doses administered) in males compared with 7.9 in females.28 In a retrospective review of 139 adolescents and young adults (aged <21 years; 126 male) with suspected myocarditis within 30 days of COVID-19 vaccination, the median time to symptom onset was 2 days (interquartile range [IQR], 1-3 days) after vaccination with 128 (91.4%) occurring after the second dose; the most common symptom was chest pain (99.3%).29 All patients had elevated troponin I (n = 111, 8.12 ng/mL [normal < 0.04]; IQR, 3.50-15.90) or T (n = 28, 0.61 ng/mL [normal <0.014]; IQR, 0.25-1.30).29 Most patients had a mild clinical course, with only 26 (18.7%) admitted to the ICU and all patients discharge to home. Possible myocarditis should be considered in adolescents or young adults who have acute chest pain, shortness of breath, or palpitations after COVID-19 vaccination.27
TREATMENT
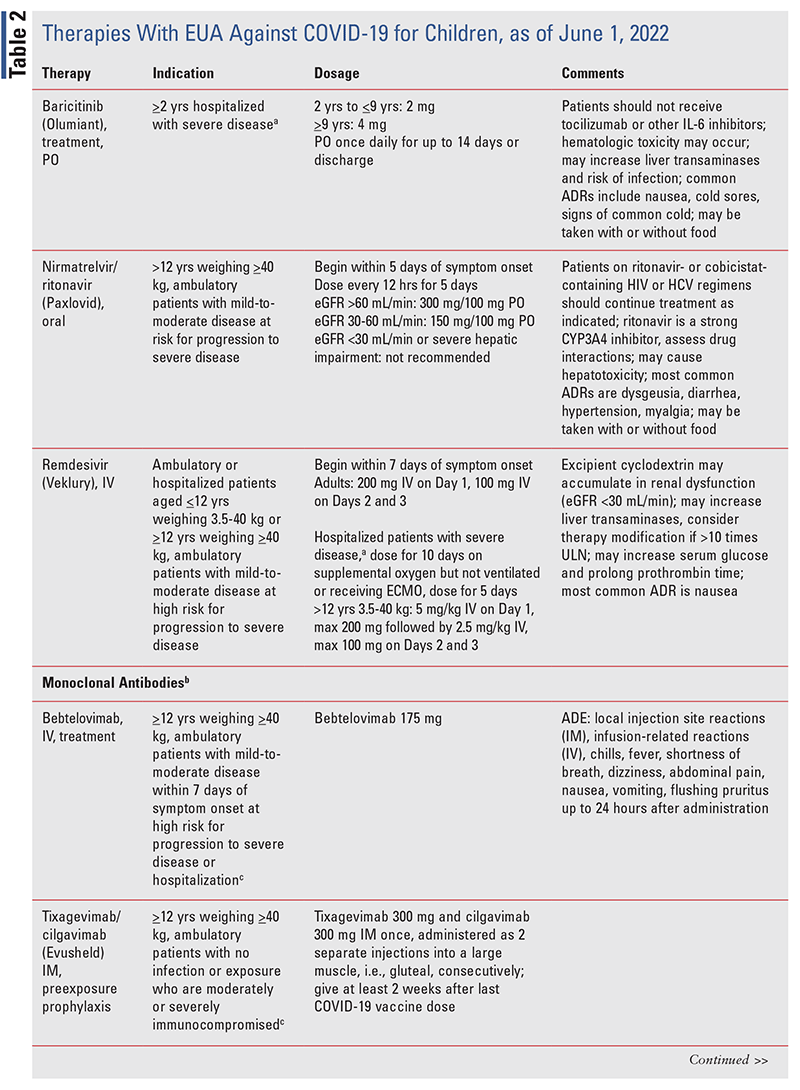
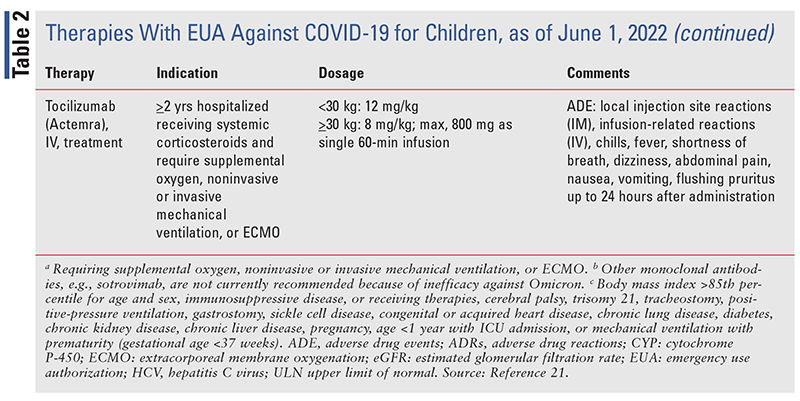
In most cases of asymptomatic, mild, or moderate disease, supportive care provides necessary therapy for recovery. Treatment of hospitalized children with severe disease (those requiring supplemental oxygen, without need for noninvasive or invasive mechanical ventilation or extracorporeal membrane oxygenation [ECMO]) is largely based on results from adults. Infants and children with severe disease that does not respond to supportive care or who have underlying risk factors for worsening disease should include a trial of remdesivir, an EUA-approved therapy (TABLE 2).2 Such infants and children should be enrolled in a clinical trial. Other potential therapies include dexamethasone as an immunomodulator and monoclonal antibodies (TABLE 2).
IMPACT
The hidden impact of COVID-19 as it relates to children has emerged over the past year. Specific to neonates, limiting parental contact due to potential for adult transmission may impact parental attachment, as well as physical and mental well-being.30 Infants and children living with stress and the consequences of the pandemic have the potential for delayed developmental milestones, difficulty with emotional regulation, and social or behavioral limitations.31 Ongoing research is needed, especially on the effect of language and communication skills in young children who may have been affected by daycare or school closures, masking, or other barriers to child development.
The consequences of the pandemic led to excessive screen time, cyberbullying, lack of physical activity, and decreased socialization in children, which may have long-term direct and indirect health effects. A systematic review of 83 studies found that separation and loneliness impacted the mental health of children and adolescents and increased the risk of depression and anxiety.32 Social isolation leads to higher levels of cortisol and worsening cognitive development.33 Mental health disorders and substance-use disorders have also risen substantially during the pandemic.34 Preventative support and early intervention are best when possible, but pharmacists and healthcare professionals should be prepared for an increase in mental health problems as a result of the pandemic.35
Rates of primary care visits, preventive screenings, and services such as speech therapy, physical therapy, and occupational therapy among children in Medicaid and the Child Health Insurance Program have declined steeply. The Centers for Medicare & Medicaid Services reported that compared with the same time in 2019, in 2020 there were 22% fewer (1.7 million) vaccinations received by beneficiaries aged 2 years and younger, and 44% fewer (3.2 million) childhood screening services that can detect physical and cognitive developmental delay.36
ROLE OF THE PHARMACIST
Pharmacists are a key part of the Federal Retail Pharmacy Program (FRPP), which expanded access to free COVID-19 vaccines. As of May 25, 2022, pharmacists have administered more than 252.1 million COVID-19 vaccinations.37 A total of 21 retail pharmacy partners are participating in the FRPP, with more than 41,000 locations nationwide, including long-term care pharmacies.37 The Public Readiness and Emergency Preparedness Act (PREP Act) issued by the Secretary of the Department of Health and Human Services authorizes and provides liability protections to licensed providers to administer COVID19 vaccines authorized by the FDA under an EUA.38 This authorization preempts state requirements that would otherwise prohibit providers from administering these vaccines. This includes expanded availability of vaccination administration options by allowing pharmacists in all states to administer vaccines to children as young as age 3 years. Previously, the minimum age was state dependent, and only 28 states permitted pharmacists to vaccinate children.38
As with other childhood vaccinations, there are some differences from adult vaccine administration including needle size and injection site. To aid in vaccinating children, parents can participate in the process by holding the child on their lap; placing the child’s arm under their armpit and applying gentle pressure with their upper arm for a secure, hug-like hold; using their lower arm and hand to hold the child’s other arm gently but securely; and anchoring the child’s feet firmly between their thighs or holding securely with their other hand.39
The Institute for Safe Medication Practices (ISMP) warns of confusion between pediatric and adult formulations of the Pfizer COVID-19 vaccine. One in three errors involving vaccines reported to the ISMP National Vaccine Errors Reporting Program includes age-related mix-ups.40 Pfizer colored coded the vaccine vials according to age-specific formulations (TABLE 1). Vial confusion may occur once the cap is removed or doses are prepared one at a time rather than all at once, making relying on cap color impossible. Pharmacists should develop a plan for storing vaccines apart from one another, such as in separate labeled plastic bins, and use barcode scanning to verify product when dispensing. Individual-prepared syringes should be labeled for each patient and be differentiated to show adult versus pediatric doses. Only bring the intended syringe into the administration area and involve the patient (or parent) in verifying by reading the label to confirm that the correct vaccine is being administered.
In addition to providing the COVID-19 vaccination, it is critical for the pharmacist to work towards helping children acquire missed healthcare opportunities due to the consequences of the pandemic. Telehealth options can combat the issues around the lack of access to care and increase availability of healthcare options.36 However, some services, such as vaccinations, cannot be provided through telehealth. Decreased vaccinations may increase the risk of transmission of vaccine-preventable illnesses, such as measles, mumps, and influenza, which may result in decreased school attendance and learning and increased illness. The pharmacist can be a key player in the healthcare team to help coordinate well-child visits and monitor that children are up to date on their immunizations.
Vaccine-hesitant parents may fear or distrust the COVID-19 vaccine. However, pharmacists can influence parental decision making by using the C.A.S.E approach: Corroborate, About me, Science, and Explain/advise.41 The approach organizes a response to concerns but keeps the reply brief and to the point. Instead of countering an argument, which may come across as dismissive or challenging, the first step is to respectfully corroborate the parent’s feelings by acknowledging and empathizing with their concern. The next “About me” phase highlights the knowledge, care, and expertise of the healthcare provider to establish a trusting connection. Data and vaccine information (purpose, efficacy, safety) can then be shared with the parent or caregiver in a straightforward and easy-to-understand way. Pharmacists can close the conversation with a strong explanation of why this is important and best for the health of the child. A recommendation from a healthcare professional does increase the likelihood that parents and caregivers will immunize their child, so having these thoughtful and respectful conversations is crucial.
CONCLUSION
The COVID-19 pandemic continues to evolve. The FDA granted an EUA for both the Pfizer and Moderna COVID-19 vaccines for the primary series in children aged 6 months and older as the vaccines are safe and effective in preventing SARS-CoV-2 infections. Pharmacists play an important role in facilitating childhood vaccinations and engaging parents and caregivers in conversations about vaccine efficacy and safety.
REFERENCES
- Galindo R, Chow H, Rongkavilit C. COVID-19 in children: clinical manifestations and pharmacologic interventions including vaccine trials. Pediatr Clin North Am. 2021;68(5):961-976.
- Howard-Jones AR, Burgner DP, Crawford NW, et al. COVID-19 in children. II:Pathogenesis, disease spectrum and management. J Paediatr Child Health. 2021;58(58):46-53.
- CDC. SARS-CoV-2 Variant Classifications and Definitions. www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html#anchor_1632150752495. Accessed May 24, 2022.
- Clarke KEN, Jones JM, Deng Y, et al. Seroprevalence of infectioninduced SARS-CoV-2 antibodies—United States, September 2021–February 2022. MMWR Morb Mortal Wkly Rep. 2022;71(17):606-608.
- Zachariah P. COVID-19 in children. Infect Dis Clin North Am. 2022;36(1):1-14.
- American Academy of Pediatrics. Coronavirus Disease 2019 (COVID19). Red Book Online. https://publications-aap-org.proxy.hsl.ucdenver.edu/redbook/resources/15186. Accessed May 21, 2022.
- Case SM, Son MB. COVID-19 in pediatrics. Rheum Dis Clin North Am. 2021;47(4):797-811.
- Marks KJ, Whitaker M, Agathis NT, et al. Hospitalization of infants and children aged 0–4 years with laboratory-confirmed COVID-19— COVID-NET, 14 States, March 2020–February 2022. MMWR Morb Mortal Wkly Rep. 2022;71(11):429-436.
- Woodruff RC, Campbell AP, Taylor CA, et al. Risk factors for severe COVID-19 in children. Pediatrics. 2022;149(1):2021053418.
- Henderson LA, Canna SW, Friedman KG, et al. American College of Rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID 19: Version 2. Arthritis Rheumatol. 2021;73(4):e13-e29.
- Zhang QY, Xu BW, Du JB. Similarities and differences between multiple inflammatory syndrome in children associated with COVID-19 and Kawasaki disease: clinical presentations, diagnosis, and treatment. World J Pediatr. 2021;17(4):335-340.
- Junqueira C, Crespo Â, Ranjbar S, et al. FcγR-mediated SARS-CoV-2 infection of monocytes activates inflammation. Nature. 2022;April 6:1-9.
- CDC. Information for healthcare providers about multisystem inflammatory syndrome in children (MIS-C). www.cdc.gov/mis/mis-c/hcp/. Accessed May 25, 2022.
- CDC. CDC COVID data tracker: multisystem inflammatory syndrome in children (MIS-C). https://covid.cdc.gov/covid-data-tracker/#misnational-surveillance. Accessed May 25, 2022.
- CDC. Breastfeeding and Caring for Newborns if You Have COVID19. https://www.cdc.gov/coronavirus/2019-ncov/if-you-are-sick/pregnancybreastfeeding.html. Accessed May 26, 2022.
- Blázquez-Gamero D, Epalza C, Cadenas JAA, et al. Fever without source as the first manifestation of SARS-CoV-2 infection in infants less than 90 days old. Eur J Pediatr. 2021;180(7):2099-2106.
- García H, Allende-López A, Morales-Ruíz P, et al. COVID-19 in neonates with positive RT–PCR test. Systematic review. Arch Med Res. 2022;53(3):252-262.
- AAP. COVID-19 testing guidance. www.aap.org/en/pages/2019-novelcoronavirus-covid-19-infections/clinical-guidance/covid-19-testing-guidance/. Accessed May 29, 2022.
- AAP. FAQs: management of infants born to mothers with suspected or confirmed COVID-19. www.aap.org/en/pages/2019-novel-coronaviruscovid-19-infections/clinical-guidance/faqs-management-of-infants-born-tocovid-19-mothers/. Accessed May 26, 2022.
- Maldonado YA, O’Leary ST, Ardura MI, et al. COVID-19 Vaccines in children and adolescents. Pediatrics. 2022;149(1).
- FDA. Emergency Use Authorization. www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization. Accessed June 1, 2022.
- Moderna files for authorization of its COVID-19 vaccine in young children six months to under six years of age. https://investors.modernatx.com/news/news-details/2022/Moderna-Files-for-Authorization-of-ItsCOVID-19-Vaccine-in-Young-Children-Six-Months-to-Under-Six-Years-ofAge/default.aspx. Accessed May 26, 2022.
- Creech CB, Anderson E, Berthaud V, et al. Evaluation of mRNA-1273 Covid-19 vaccine in children 6 to 11 years of age. N Engl J Med. 2022;386(21).
- Walter EB, Talaat KR, Sabharwal C, et al. Evaluation of the BNT162b2 Covid-19 vaccine in children 5 to 11 Years of Age. N Engl J Med. 2022;386(1):35-46.
- Frenck RW, Klein NP, Kitchin N, et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med. 2021;385(3):239-250.
- Thakkar P V., Zimmerman KO, Brookhart MA, et al. COVID-19 incidence among sixth through twelfth grade students by vaccination status. Pediatrics. 2022;149(5).
- Oster ME, Shay DK, Su JR, etal. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020-August 2021. JAMA. 2022;327(4):331-340.
- FDA. Vaccines and Related Biological Products Advisory Committee October 26, 2021 Meeting Presentation-Vaccine Associated Myocarditis. www.fda.gov/media/153514/download. Accessed May 26, 2022.
- Truong DT, Dionne A, Muniz JC, et al. Clinically suspected myocarditis temporally related to COVID-19 vaccination in adolescents and young adults: suspected myocarditis after COVID-19 vaccination. Circulation. 2022;145(5):345-356.
- Ryan L, Plötz FB, Van Den Hoogen A, et al. Neonates and COVID19: state of the art Neonatal Sepsis series. Pediatr Res. 2022;91(2):432439.
- Green J, Staff L, Bromley P, et al. The implications of face masks for babies and families during the COVID-19 pandemic: a discussion paper. J Neonatal Nurs. 2021;27(1):21-25.
- Imboden A, Sobczak BK, Griffin V. The impact of the COVID-19 pandemic on infant and toddler development. J Am Assoc Nurse Pract. 2022;34(3):509-519.
- Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid systematic review: the impact of social isolation adolescents in the context of COVID-19. J Am Child Adolesc Psychiatry. 2020;59(11):1218-1239.
- Almeida IL, Rego JF, Teixeira ACG, et al. Social isolation and its impact on child and adolescent development: a systematic review. Rev. Paul. Pediatr. 2022; https://doi.org/10.1590/1984-0462/2022/40/2020385. 35. Gadermann AC, Thomson KC, Richardson CG, et al. Examining the impacts of the COVID-19 pandemic on family mental health in Canada: findings from a national cross-sectional study. BMJ Open. 2021;11(1):e042871.
- CMS. CMS issues urgent call to action following drastic decline in care for children in Medicaid and Children’s Health Insurance Program due to COVID-19 pandemic.www.cms.gov/newsroom/press-releases/cmsissues-urgent-call-action-following-drastic-decline-care-children-medicaidand-childrens-health. Accessed May 31, 2022.
- CDC. COVID-19 Vaccination Federal Retail Pharmacy Partnership Program. www.cdc.gov/vaccines/covid-19/retail-pharmacy-program/index.html. Accessed May 31, 2022.
- HHS. COVID-19 PREP Act Declarations. Public Readiness and Emergency Preparedness Act. Accessed https://aspr.hhs.gov/legal/PREPact/Pages/default.aspx. May 29, 2022.
- CDC. You call the shots–vaccine administration: intramuscular (IM) injection children 1 through 2 years of age. www.cdc.gov/vaccines/hcp/acip-recs/general-recs/administration.html. Accessed May 31, 2022.
- ISMP. ISMP urges steps be taken to prevent mix-ups between pediatric and age 12 and up COVID-19 vaccines. www.ismp.org/news/ismp-urgessteps-be-taken-prevent-mix-ups-between-pediatric-and-age-12-and-covid19-vaccines. Accessed May 31, 2022.
- Jacobson RM, van Etta L. Applying the C.A.S.E. approach to COVID19 mRNA vaccine hesitancy. Minnesota Med. 2021;104(2):16-19.
- FDA. FDA expands eligibility for Pfizer-BioNTech COVID-19 vaccine booster dose to children 5 through 11 years. www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-expands-eligibilitypfizer-biontech-covid-19-vaccine-booster-dose. Accessed May 22, 2022.
- CDC. US COVID-19 vaccine product information. www.cdc.gov/vaccines/covid-19/info-by-product/index.html. Accessed July 5, 2022.
- CDC. COVID-19 vaccine interim COVID-19 immunization schedule for 6 months of age and older. www.cdc.gov/vaccines/covid-19/downloads/COVID-19-immunization-schedule-ages-6months-older.pdf. Accessed July 5, 2022.