Drug-Induced Severe Cutaneous Adverse Reactions
Potential Signals of Serious Risks and New Safety Information From FAERS
RELEASE DATE:
June 1, 2018
EXPIRATION DATE:
June 30, 2020
FACULTY:
Donna M. Lisi, PharmD, BCPS, BCPP, BCGP
Adjunct Faculty, Union County College
Medical Writer/Educator
Somerset, New Jersey
FACULTY DISCLOSURE STATEMENTS:
Dr. Lisi has no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT:
Pharmacy
 Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-18-028-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE:
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@jobson.com
DISCLAIMER:
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients' conditions and possible contraindications or dangers in use, review of any applicable manufacturer's product information, and comparison with recommendations of other authorities.
GOAL:
To educate pharmacists about potential signals and new safety information involving severe cutaneous adverse reactions that have been identified through the FDA's Adverse Event Reporting System and about pharmacists' role in identifying and minimizing potential harm.
OBJECTIVES:
After completing this activity, the participant should be able to:
- Describe the role of the FDA Adverse Event Reporting System in identifying potential drug safety-risk signals related to severe cutaneous adverse reactions (SCARs).
- Differentiate between the following SCARs: Stevens- Johnson syndrome, toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms, acute generalized exanthematous pustulosis, and erythema multiforme.
- List drugs and drug classes that are associated with potential SCARs.
- Identify the pharmacist's role in recognizing potential SCARs in order to minimize patient harm.
ABSTRACT: Drug-induced severe cutaneous adverse reactions (SCARs) are potentially lifethreatening skin reactions that result from the administration of a medicinal agent. The term SCAR encompasses Stevens-Johnson syndrome, toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms, acute generalized exanthematous pustulosis, and erythema multiforme. A review was conducted of the FDA's Adverse Event Reporting System (FAERS) for potential signals of drug-induced SCARs as well as for new safety information about serious skin reactions. Nineteen drugs or drug classes were identified as being associated with SCARs signals. This article will further examine the drugs/drug classes involved. Pharmacists need to be aware of the potential risk associated with drugs that can cause severe, sometimes fatal, cutaneous adverse reactions.
In 1960, the United States established an adverse drug event spontaneous reporting system.1 Since that time, the reporting system has gone through several evolutions, the latest being in September 2012 when data from the AERS (Adverse Event Reporting System) were moved to FAERS (FDA Adverse Event Reporting System).2 Between 1969 and August 2017, FAERS received more than 14 million reports of adverse drug events (ADEs) for drugs and biologics.3 The FDA evaluates these reports for potential safety signals. Quarterly, the agency publishes lists of drugs and biologics that it has evaluated or is currently evaluating. The appearance of a drug on these lists does not mean that the FDA has identified a causal relationship between the drug and the listed risk.2
In the late 1990s, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use developed MedDRA, the Medical Dictionary for Regulatory Activities. MedDRA refers to a clinically validated international medical terminology used by regulatory authorities and the biopharmaceutical industry throughout the entire regulatory process to help standardize medical terminology and facilitate the sharing of information internationally.4 The MedDRA Standardised MedDRA Queries on Severe Cutaneous Adverse Reactions (SCARs) are tools developed to facilitate identification of cases of severe, sometimes life-threatening skin reactions that are often drug-induced. The term SCARs refers to the following severe dermatologic conditions: StevensJohnson syndrome (SJS), toxic epidermal necrolysis (TENS), drug reaction with eosinophilia and systemic symptoms (DRESS), acute generalized exanthematous pustulosis (AGEP), and erythema multiforme (EM).5
RegiSCAR, the European Registry of Severe Cutaneous Adverse Reactions to Drugs and Collection of Biological Samples also monitors SCARs. The objective of RegiSCAR is to reduce the medical and economic burden of SCARs on public health and to improve the safety of medication use. It is conducting surveillance of new drugs; organizing a centralized collection of biological samples to allow for high quality studies on the pharmacogenetics of SCARs; and examining outcomes, prognostic factors, sequelae, and the impact on quality of life of SCARs.6
This article will examine the FAERS potential signals for SCARs and new safety information identified by FAERS from 2009 through September 2017, which reflects the period of all of the currently active (i.e., nonarchived) quarterly reports on the FAERS website (as of February 2018). The list of drugs/drug classes reviewed was generated based on the information found at www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm082196.htm. These reports were supplemented with the published medical literature, when available. It is important to note that these medicines may have additional dermatologic adverse events that are beyond the scope of this article and will not be addressed. Furthermore, it is important to keep in mind that it is not always possible to reliably estimate the frequency or causal relationship of the drug exposure to adverse events owing to the voluntary nature of the postmarketing reporting system.
DRUG-INDUCED SCARs
SJS and TEN
SJS is characterized by widespread skin lesions that are either target-shaped or consist of erythematous macules with epidermal detachment. This presentation is also accompanied by severe mucosal erosions, which may involve the nasopharynx, oropharynx, eyes, genitalia, or anus.7,8
In the MedDRA system, the severity of SJS is divided into three grades9:
- Grade 3: Skin sloughing covering <10% of the body surface area (BSA) with associated signs (e.g., erythema, purpura, epidermal detachment, and mucous membrane detachment)
- Grade 4: Skin sloughing covering 10% to 30% of the BSA with associated signs (e.g., erythema, purpura, epidermal detachment, and mucous membrane detachment)
- Grade 5: Death
Grades 1 and 2 do not apply to SCARs because these grades refer to asymptomatic or mild disease.
TEN, which is also known as Lyell syndrome, is a rare dermatologic reaction that is characterized by apoptosis of keratinocytes resulting in erythema, mucous membrane erosion, extensive epidermal detachment (>30%), and systemic symptoms. TEN is associated with widespread erythematous areas that are marked with epithelial necrosis. There are often small erythematous or purpuric lesions (although purpura may also be seen in SJS), which may or may not be associated with blisters. Extensive mucosal erosions are frequently present. TEN is often accompanied by high fever, malaise, and pain.7
Although drugs are a frequent culprit, TEN occurs more commonly in patients with HIV infection, systemic lupus erythematosus, graft-versus-host disease, and malignancy.10
SCORTEN is a severity-of-illness score for TEN that can be used to predict the risk of death from the condition. Predictive factors include age more than 40 years; malignancy; tachycardia with heart rate >120 beats per minute; initial percentage of epidermal detachment >10%; serum urea >10 mmol/L; serum glucose >14 mmol/L; and bicarbonate <20 mmol/L. For each TEN-specific severity-of-illness score point, the odds ratio is 3.45 for death.11
The severity of TEN is divided into grades9:
- Grade 4: Skin sloughing covering ≥30% of the BSA with associated symptoms (e.g., erythema, purpura, or epidermal detachment)
- Grade 5: Death
SJS and TEN are usually considered together because they represent different extremes of the same spectrum of epidermal necrolysis. Common presenting symptoms include fever, flulike symptoms, ocular symptoms, and ear/nose/throat involvement and pain, which may occur before the rash. Skin involvement may initially present as Nikolsky’s sign, which is the superficial detachment of the top layers of the epidermis. The distinguishing feature between SJS and TEN is the degree of the BSA with skin detachment. In SJS, <10% of skin is detached whereas in TEN, this percentage is >30% reflecting a more life-threatening condition. For skin detachment that involves 10% to 30% of the BSA, the abbreviation SJS-TEN is used.8
The incidence of SJS and TEN is estimated to be two per 1 million people. Evidence of disease typically presents 4 to 28 days after exposure to the offending drug.8
DRESS
DRESS, which is also known as hypersensitivity syndrome or drug-induced hypersensitivity syndrome, is characterized by extensive rash; fever and systemic symptoms such as lymphadenopathy; hematologic abnormalities (i.e., eosinophilia, thrombocytopenia, atypical lymphocytosis); hepatitis; and kidney, lung, heart, and pancreatic involvement.7,8
There is no universal consensus regarding the definition of DRESS. Two sets of diagnostic criteria—the RegiSCAR criteria and the Japanese consensus group criteria—are available.12,13 Diagnosis of DRESS is often difficult because of its complex natural course and heterogeneous presentation. Prodromal symptoms, which may include fever, lymphadenopathy, flulike symptoms, burning pain, or pruritus may precede the rash by 2 weeks. Eighty percent of patients have liver involvement, whereas in 15% of patients the lungs may be affected. The kidneys are also affected and present with interstitial nephritis. Myocarditis or pericarditis can occur. DRESS is also associated with the reactivation of human herpesvirus 6.8
The onset of symptoms is often delayed, occurring 2 to 6 weeks after drug initiation.7 Analysis of 10-year postmarketing surveillance data from the largest national case series of DRESS, which involved 91 patients, found that drugs associated with the SCARs all contained an aromatic ring. The drugs most frequently associated with DRESS include allopurinol, carbamazepine, phenobarbital, sulfasalazine, lamotrigine, vancomycin, amoxicillin, acetaminophen, and strontium ranelate.14
AGEP
AGEP, which has also been called toxic pustuloderma, pustular drug rash or pustular psoriasiform eruption with leukocytosis, is an uncommon, acute pustular dermatologic reaction that is most often triggered by medications, namely ampicillin, amoxicillin, sulfonamides, fluoroquinolones, hydroxychloroquine, terbinafine, diltiazem, and, to a lesser extent, corticosteroids, macrolides, the oxicam family of nonsteroidal anti-inflammatory drugs, and antiepileptics (except valproic acid). It is characterized by an acute erythematous rash that is accompanied by numerous pinhead-sized, nonfollicular sterile pustules. AGEP is associated with fever, leukocytosis, elevated C-reactive protein, and neutrophilia. Nikolsky’s sign can occur. AGEP primarily affects the body folds (e.g., armpits and groin) and upper trunk, but it may also involve the face. It is an initial feature of DRESS. The skin eruptions occur within hours to days (usually 2-11 days) of exposure to the offending drug. It is selflimiting, resolving spontaneously in 1 to 2 weeks following drug discontinuation.7,8 It is considered less severe than the other forms of SCARs described above.
EM
EM is an acute disease characterized by symmetrically distributed papular lesions affecting mostly the extremities. Mucosal erosions are often present. As in SJS, the clearly demarcated lesions are target-shaped and are divided into three different-colored zones, typically with a blister in the center. EM usually presents with constitutional symptoms of fever and malaise.7
The severity of EM is divided into grades9:
- Grade 1: Target lesions covering <10% of the BSA, not associated with skin tenderness
- Grade 2: Target lesions covering 10% to 30% of the BSA, associated with skin tenderness
- Grade 3: Target lesions covering >30% of the BSA, associated with oral or genital erosions
- Grade 4: Target lesions covering >30% of the BSA, associated with fluid or electrolyte abnormalities; ICU care or burn unit indicated
- Grade 5: Death
FAERS-IDENTIFIED SCARs
DRUGS/DRUG CLASSES
Drugs or drug classes that were identified as potentially being associated with SCARs based on the FAERS website are listed in TABLE 1. The table is arranged in the order in which the FDA released new safety information.
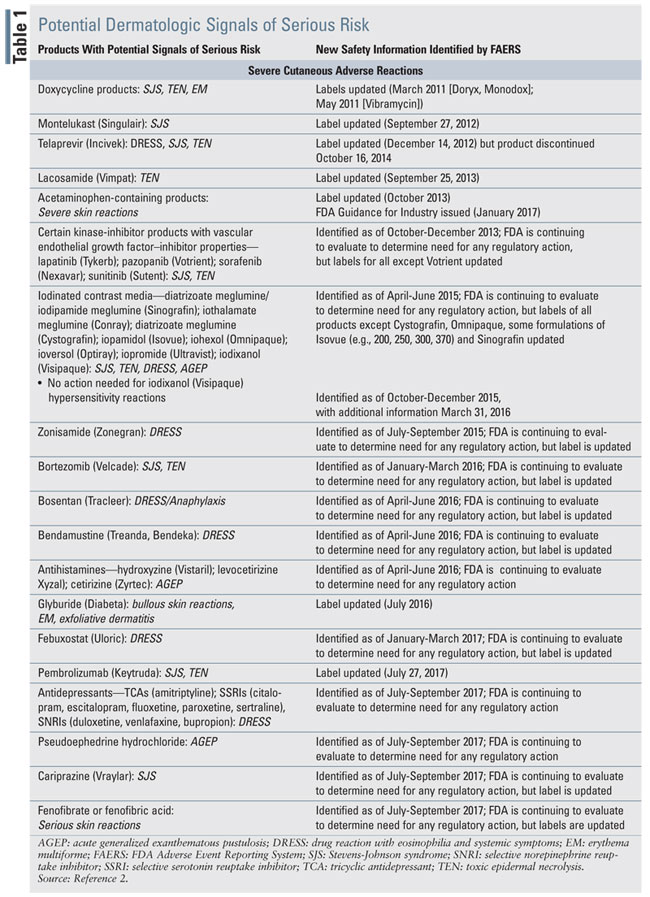
Doxycycline
Doxcycline’s prescribing information was updated to include that the drug has been associated with the development of maculopapular and erythematous rashes, SJS, TENS, and EM. Exfoliative dermatitis is uncommon. If severe skin reactions occur, doxycycline should be discontinued immediately and appropriate therapy instituted.15 Cases of SJS16-18 as well as other bullous or pustular drug eruptions19-21 have been reported in the literature. Doxycycline often causes a fixed drug eruption. Rarely does it cause a generalized bullous fixed drug eruption, which has been documented within 3 hours of a single oral dose of 100 mg of doxycycline.21
Montelukast
Montelukast is a leukotriene receptor antagonist used for asthma and allergic rhinitis. Based on postmarketing experience, the product labeling information of montelukast was updated to include the occurrence of SJS/TEN.22 Published reports in the PubMed database are lacking regarding montelukast-induced TEN, although a case of neutrophilic dermatosis has been identified.23
Telaprevir
Telaprevir (Incivek) is a hepatitis C virus NS3/4A protease inhibitor that was indicated for the treatment of genotype 1 chronic hepatitis C (in combination with peginterferon alfa and ribavirin).24 The sale and distribution of Incivek was halted by Vertex Pharmaceuticals on October 16, 2014. owing to the availability of alternative treatment options for hepatitis C as well as declining sales.25 The drug was associated with serious skin reactions, including DRESS and SJS in <1% of treated patients. If these were to occur, the drug was to be discontinued immediately. All patients who experienced these severe AEs required hospitalization, but fully recovered. Additionally, mild-tomoderate rashes were also observed with telaprevir. In clinical trials, 56% of study subjects who received telaprevir developed rash events compared with 34% of patients who only received peginterferon alfa and ribavirin. The rash typically began in the first 4 weeks of treatment.24 The severe skin reactions necessitated the placement of a boxed warning in the product labeling cautioning about fatal and nonfatal serious skin reactions.26
Lacosamide
Lacosamide is indicated for the treatment of partial-onset seizures in patients aged >4 years. TEN was observed during postmarketing surveillance of lacosamide, although this is a very rare AE with only one case listed in PubMed as of April 2018.27,28
Acetaminophen-Containing Products
Acetaminophen-containing product labeling now contains a warning that the drug may be associated with the risk of serious skin reactions. On August 1, 2013, the FDA disseminated a drug safety communication discussing the risk of SJS, TEN, and AGEP from the use of acetaminophen. These reactions can be fatal. The FDA warned that these reactions may occur even with the firsttime use of acetaminophen. Causality was established based on three published cases that involved positive rechallenge upon reexposure (one in a girl aged 7 years29; a boy aged 11 years30; and an 83-year old man31), reports made to FAERS, and other documented cases of SJS, TEN, and AGEP in which the only drug administered prior to the reaction was an acetaminophen-containing product. A review of FAERS data from 1969 to 2012 identified 91 cases of SJS/TEN (six probable and 85 possible cases) and 16 cases of AGEP (one probable and 15 possible cases), which resulted in 12 deaths. In most cases, single-ingredient acetaminophen was involved. Among the seven probable cases, which occurred 24 hours to 8 days postingestion, there was one fatality. Case-control studies have been conducted examining the risk of SJS/TEN and TEN. One problem with conducting research is that a common reason for the use of acetaminophen is fever, which may be a prodromal symptom of SJS or TEN.32
Since this communication from the FDA, additional reports have been published.33-35 Preliminary evidence indicates a possible association with acetaminophen use and HLA-A*02:06 and HLAB*44:03 in those who developed SJS with severe ocular complications.36
The FDA recommended that manufacturers of both prescription and OTC acetaminophen-containing products include a consumer warning about the risk of severe skin reactions.
A new term called SDRIFE (symmetrical drugrelated intertriginous and flexural exanthema, also known as baboon syndrome) has been coined to describe a specific skin eruption associated with acetaminophen that resembles the red gluteal area of baboons. SDRIFE presents with symmetric erythematous areas on the gluteal/perianal area and V-shaped erythema of the inguinal/perigenital area.37
Despite these reports, a recent analysis of the French Pharmacovigilance Database using an algorithm to assess drug causality for TEN did not find any obvious risk of SJS/TEN associated with acetaminophen use.38
Although these reactions are thought to be rare, it is difficult to fully assess risk due to the widespread OTC use of acetaminophen. In January 2017, the FDA issued guidance for industry on the warning for OTC acetaminophen-containing products and labeling statements regarding serious skin reactions. The FDA recommended that manufacturers of both prescription and OTC acetaminophen-containing products include a consumer warning about the risk of severe skin reactions. The allergy alert is to read, “Acetaminophen may cause severe skin reactions. Symptoms may include: skin reddening, blisters, and rash. If a skin reaction occurs, stop use and seek medical help right away.” The agency recommended that this caveat be placed under the “Warnings” section of the Drug Facts Label under a heading titled, “Allergy Alert.”39
Patients should be counseled to be observant for skin reddening, rash, blisters, or signs that the upper surface of the skin is separating from the lower layers. If they notice that this is occurring, they are advised to immediately seek medical attention. This reaction is especially problematic since acetaminophen is often an unrecognized ingredient in many combination products.
Kinase Inhibitors
Kinase inhibitors (e.g., lapatinib, pazopanib, sorafenib, and sunitinib), all of which possess vascular endothelial growth factor (VEGF)-inhibitor properties, are being evaluated for possible association with the development of SJS and TEN. These agents are used to treat a variety of cancers. Product labeling varies regarding the different agents’ potential for causing SJS and TEN. For example, Votrient’s (pazopanib) product labeling does not include information about these potentially life-threatening conditions. For sunitinib, in addition to warning about SJS and TEN, necrotizing fasciitis and EM are also included in the prescribing information. Some of these SCARs have been fatal. Lapatinib’s prescribing information indicates that SJS and TEN were identified during postmarketing surveillance. If any of these SCARs occur, the drug should be discontinued and not restarted, especially if SJS or TEN occurs.40-43
As a class, these agents are most typically associated with the development of hand/foot/skin reaction (i.e., a condition characterized by hyperkeratotic lesions with blistering and callus formation on the flexural surface of the digits that is accompanied by paresthesia, burning, pain, and decreased tolerance to heat44), rash/desquamation, and pruritus. Other skin reactions include alopecia, stomatitis, skin discoloration involving the hair and face, subungual splinter hemorrhage, facial swelling or erythema, genital lesions, cutaneous eruptions, vascular lesions, pigmented lesions, and xerosis. Among the VEGF kinase inhibitors, sorafenib and sunitinib appear to be more often associated with dermatologic AEs.45-50 Interestingly, the presence of cutaneous AEs has been associated with improved overall survival and progressionfree survival in metastatic renal cell carcinoma patients.51
SCARs are uncommon with this class of drugs, although they have been reported with sorafenib.52 There are case reports in the literature of SJS, TEN, DRESS, and AGEP associated with sorafenib.53-59 EM may occur more frequently in Asians than in Caucasians—the incidence of this dermatologic condition was 25% in Japanese patients following the administration of sorafenib,60 and sorafenib-induced EM typically occurs in 0.1% to <1% of patients.61 Other cases of sorafenibinduced EM have also been reported.61-64
Iodinated Contrast Media
Iodinated contrast media (ICM) include iothalamate meglumine, diatrizoate meglumine, iopamidol, iohexol, ioversol, iopromide, and iodixanol. Iothalamate meglumine and diatrizoate meglumine are ionic, monomeric, high-osmolality ICM. Iopamidol, iohexol, ioversol, and iopromide are nonionic, monomeric, low-osmolality ICM. Iodixanol is a nonionic, dimeric, isosmolar ICM.65 SCARs, which are nonimmediate or delayed reactions, may develop within 1 hour of infusion or up to several weeks after ICM administration. These reactions include SJS/TEN, AGEP, and DRESS. With repeat administration of such a contrast medium, patients may experience a more severe reaction and quicker onset of adverse effects. Prophylactic medications may not prevent or mitigate these adverse reactions.66-70 It is recommended to avoid the use of these products should a SCAR occur. Cross-reactivity has been clinically observed with the use of ICM.71 The frequency of nonimmediate ICM reactions is 0.5% to 14%,72 but delayed skin AEs occur in 1% to 3% of patients. It is often difficult to verify such AEs. Fortunately, they usually resolve within 7 days.73,74 Nonionic dimers are more often associated with the development of delayed reactions than nonionic monomers.74,75 The risk of a delayed ICM reaction is similar between ionic and nonionic monomers. Children may be at greater risk of developing a delayed reaction from ICM than adults. Upon re-exposure to the offending agent, reactions can occur within 2 to 48 hours.76
Risk factors for delayed reactions to ICM include previous allergic reaction to ICM; nonionic dimers; acute or chronic kidney failure (serum creatinine >2 mg/dL); renovascular involvement (e.g., diabetes, myeloma, dehydration); cardiopulmonary disease; repeated administration; atopy; female gender; mastocitosis; current viral infections; autoimmune diseases (e.g., lupus); use of hydralazine; being a recipient of bone marrow; being of Japanese descent; seasonal variation due to pollens; and treatment with interleukin-2 (interleukin-2 therapy increases risk two-to-fourfold). Treatment with ACE inhibitors, beta-blockers, or proton pump inhibitors is a risk factors for an immediate reaction to ICM.65,73,75,77,78 Delayed reactions are T-cell mediated, but ICM do not function as a hapten.76,79 Histopathology and lymphocyte transformation testing have confirmed the involvement of T cells in delayed ICM reactions.80
There are numerous reports in the literature of ICM being associated with SCARs including AGEP,81-84 TEN,85-90 DRESS,91,92 and SJS.93
Zonisamide
Zonisamide is an antiseizure medication that is chemically classified as a sulfonamide. The product labeling warns that seven fatalities secondary to SJS and TEN have occurred in the first 11 years of availability of the drug in Japan. These deaths are due to a severe reaction to sulfonamides. Subsequent postmarketing analysis found that a total of 49 cases of SJS or TEN have been reported in Japan for a reporting rate of 46 cases per million patient-years of exposure. The manufacturer acknowledges that this may underestimate the true incidence of the AE occurrences. Data from randomized controlled clinical trials conducted in the U.S. and Europe indicated that 2.2% of treated patients discontinued the drug owing to rash. In the Japanese studies, rash occurred in 90% of patients within the first 2 weeks of treatment, and in the U.S./Europe cohort, rash developed in 85% of those studied within 16 weeks of drug initiation.94 Others place the incidence of zonisamiderelated rashes at 4.5%.95
Cases of DRESS, some of which have been fatal or life-threatening, have been reported with zonisamide. Typically, these cases have presented with fever, rash, lymphadenopathy and/or facial swelling, hepatitis, nephritis, hematologic abnormalities, myocarditis, myositis, and eosinophilia. Signs of hypersensitivity (e.g., fever and lymphadenopathy) may be present without rash because the AE manifestation is variable and may include organ systems in addition to those noted above. If patients develop signs of hypersensitivity, they should be evaluated immediately.94 Symptoms of DRESS typically appear 2 to 6 weeks after the initiation of a drug.96
Most patients with zonisamide-related DRESS also have signs of severe drug-induced hypersensitivity syndrome (DIHS), which is associated with an erythematous morbilliform eruption that can progress to exfoliative dermatitis. Spontaneous reports of hypersensitivity syndrome for zonisamide place the incidence at 4.9 per 100,000 exposed patients.95 HLA-A*02:07 may be a biomarker for susceptibility to zonisamide-induced SJS/TEN.97 DIHS/DRESS can also manifest as eosinophilic pneumonia98 or acute kidney injury.99
Bortezomib
Bortezomib is a proteasome inhibitor indicated for multiple myeloma and mantel cell lymphoma. Based on worldwide postmarketing surveillance data, the occurrence of SJS/TEN and acute febrile neutrophlic dermatosis (also known as Sweet syndrome) were included in the prescribing information.100 However, published reports relating bortezomib use with the development of SJS/TEN are lacking.
Bosentan
Bosentan is an endothelin receptor antagonist indicated for the treatment of pulmonary arterial hypertension. Product labeling has been updated to include the information that bosentan is contraindicated in patients with a hypersensitivity to the drug or any of its components. It warns that observed reactions include DRESS, anaphylaxis, rash, and angioedema.101 There are only a few published cases linking bosentan with DRESS or anaphylaxis.102,103
Bendamustine
Bendamustine is an alkylating agent approved for the management of chronic lymphocytic leukemia and indolent B-cell non-Hodgkin lymphoma. “Skin Reactions” have been added to the Warnings and Precautions sections of the prescribing information because fatal and serious dermatologic AEs have been reported in both clinical trials and through postmarketing surveillance. These reactions include SJS, TEN, DRESS, bullous exanthema, and rash. Rashes may be progressive and may increase in severity with subsequent treatments. For rashes that are severe or progressive, therapy should be held or discontinued.104,105 A case series demonstrated the diversity of the severe skin reactions associated with bendamustine and described cases of DRESS, SJS, and bullous pemphigoid.106 A fatal case of SJS has also been reported in a patient receiving bendamustine along with allopurinol and rituximab.107
Antihistamines
Antihistamines (hydroxyzine, levocetirizine, cetirizine) are currently being evaluated for a possible link with AGEP. Several case reports have associated the use of hydroxyzine,108-110 cetirizine, 111,112 and fexofenadine113 with the condition. Symptoms generally appear within 48 hours of drug exposure.110
Glyburide
Glyburide’s product labeling warns that allergic skin reactions, which may be transient and disappear despite continued use, occur in 1.5% of patients receiving the drug. These reactions include pruritus, erythema, urticaria, and morbilliform or maculopapular rashes. However, bullous reactions, EM, and exfoliative dermatitis have also been reported. If these skin reactions persist, the drug should be discontinued.114 The development of cutaneous bullae has been associated with the use of glyburide since at least the early 1980’s.115
Febuxostat
Febuxostat is a xanthine oxidase inhibitor that is indicated for the chronic management of hyperuricemia in patients with gout. Postmarketing reports of serious skin and hypersensitivity reactions, including SJS, DRESS, and TEN have been reported. Many of these patients had a similar reaction to allopurinol. During clinical trials, rash, which was one of the most common adverse reactions, was reported in 0.5% to 1.6% of patients on febuxostat (compared with a 1.6% incidence in allopurinol-treated patients and 0.7% in patients receiving placebo). Febuxostat should be discontinued if a serious skin reaction is suspected.116 There is a strong association between allopurinol-induced SJS or TEN and HLA-B*58:01. In gout patients who test positive for this HLA, febuxostat should be avoided owing to its possible association with DRESS.117 Febuxostat-associated DRESS was first identified in 2015.118 DRESS is thought to occur mainly in patients with chronic kidney disease who had previously demonstrated hypersensitivity to allopurinol.119 Between 9% and 21% of those who experienced a skin ADE to allopurinol also developed a dermatologic reaction with febuxostat; AEs may include SJS.120,121 Possible cross-reactivity between allopurinol and febuxostat may be due to a nonimmunologic mechanism that is related to the drugs’ ability to inhibit xanthine oxidase.122 Febuxostat desensitization has been successful following a hypersensitivity reaction.123 Febuxostat is also associated with the development of a hypersensitivity-type cutaneous leukocytoclastic vasculitis in about 8% of patients who develop a severe reaction to allopurinol.124
Pembrolizumab
Pembrolizumab is a programmed death receptor-1 (PD-1)–blocking antibody (also known as a checkpoint inhibitor) indicated in the treatment of melanoma, non-small cell lung cancer, head and neck squamous cell cancer, classic Hodgkin lymphoma, urothelial carcinoma, microsatellite instability-high cancer, and gastric cancer.125 Dermatologic AEs, most notably a maculopapular rash, are the most common immune-related ADEs associated with this checkpoint inhibitor, occurring in 30% to 40% of pembrolizumab-treated patients.126 Fortunately for the checkpoint inhibitors, <1% to 2% of these skin reactions are considered severe, and <5% discontinue the drug permanently because of these AEs.127,128 Immune-mediated skin adverse reactions associated with the drug include SJS, TEN, exfoliative dermatitis, and bullous pemphigoid. Should these occur, the drug should be withheld for severe reactions and permanently discontinued for lifethreatening reactions.125 Infusion-related reactions occur in 0.2% of those treated, and these can be severe or life-threatening. For severe or life-threatening reactions, the infusion should be stopped and the drug permanently discontinued.125
Skin reactions to pembrolizumab typically occur within the first 3 to 4 weeks of initiation of therapy, although onset may be delayed for several months.126,129 A risk factor for the development of a severe reaction is the use of a combination of checkpoint inhibitors.129 Although the mechanism for skin reactions involving the checkpoint inhibitors is not fully established, it is thought that the blockade of a common antigen coexpressed on a patient’s tumor cell and the dermoepidermal junction (and/or other levels of the skin) may have a role.130
Antidepressants
Antidepressants, including amitriptyline, citalopram, escitalopram, fluoxetine, paroxetine, sertraline, duloxetine, venlafaxine, and bupropion are being evaluated for a possible association with the development of DRESS. An initial signal was identified as of 2017; however, the product labeling for these drugs has not been revised. There is limited published information on hypersensitivity reactions, including drug-induced hypersensitivity syndrome, being associated with the use of bupropion,131,132 fluoxetine,133 clomipramine,134 and amitriptyline.135,136 DRESS symptoms usually manifest within 2 to 8 weeks of drug exposure.136 There may be cross-reactivity between aromatic anticonvulsants (e.g., phenytoin) and tricyclic antidepressants with respect to anticonvulsant hypersensitivity syndrome.137 Therapeutic plasma exchange may be beneficial in cases of life-threating DRESS syndrome associated with bupropion or lamotrigine.132 Two excellent reviews have recently been published—one on severe skin complications associated with antidepressant use138 and the other on the management of psychotropic drug-induced DRESS syndrome.139
Pseudoephedrine
Pseudoephedrine is being evaluated for a possible association with the development of AGEP. One study that analyzed adverse cutaneous drug reactions among hospitalized patients over a 5-year period found that pseudoephedrine or phenylephrine accounted for 6.4% of the dermatologic AEs. Several case reports have been published of pseudoephedrine-associated AGEP.140-144
Cariprazine
Cariprazine is an atypical antipsychotic that is indicated for the treatment of schizophrenia and for the acute treatment of manic or mixed episodes of bipolar 1 disorder in adults. Product information has recently been updated to reflect the occurrence of SJS during postmarketing surveillance.145 However, there are no published cases involving the use of cariprazine and the development of SJS.
Fenofibrate and Fenofibric Acid
Fenofibrate and fenofibric acid use can be associated with the development of serious skin reactions. According to the prescribing information, acute hypersensitivity reactions such as SJS and TEN have been reported in individuals treated with fenofibrates.146,147 Urticaria was seen in 1.1% versus 0% and rash in 1.4% versus 0.8% of fenofibrate and placebo patients, respectively, in controlled trials.146 Fenofibrate use has been associated with the development of AGEP148 and delayed-type hypersensitivity reaction.149 Fenofibrate rarely causes cutaneous ADEs.148
CONCLUSION
As drug experts, pharmacists have an important role in recognizing potential ADEs For SCARs, it is imperative that health professionals quickly identify the offending agent and take precautions to prevent negative outcomes. As more medications, especially biologics, come to market, it will become increasingly important to be knowledgeable about potentially severe cutaneous adverse reactions. Monitoring the FAERS website for identification of potential signals of serious risks and for new safety information is imperative.
References
- Edlavitch SA. Adverse drug event reporting—improving the low US reporting rates. Arch Intern Med. 1988;148(7):1499-1503.
- FDA. Potential signals of serious risks/new safety information identified from the FDA Adverse Event Reporting System (FAERS). www.fda.gov/ Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm082196.htm. Accessed February 1, 2018.
- De S. FDA Adverse Event Reporting System (FAERS) public dashboard. www.fda.gov/downloads/AboutFDA/WorkingatFDA/ellowshipInternshipGraduateFacultyPrograms/PharmacyStudentExperientialProgramCDER/ UCM594030.pdf. Accessed February 1, 2018.
- MedDRA. Welcome to the ICH MedDRA website. www.meddra.org/ how-to-use/support-documentation/english. Accessed February 17, 2018.
- MedDRA. Introductory guide for standardised MedDRA queries (SMQs) Version 21.0. March 2018. www.meddra.org/sites/default/files/ guidance/file/smq_intguide_21_0_english.pdf. Accessed March 10, 2018.
- RegiSCAR project. www.regiscar.org/Project.html. Accessed March 1, 2018.
- MedDRA. Introductory guide for Standardised MedDRA Queries (SMQs) Version 21.0. March 2018. www.meddra.org/sites/default/files/ guidance/file/smq_intguide_21_0_english.pdf. Accessed March 10, 2018.
- Duang TA, Valeyrie-Allanore L, Wolkenstein P, et al. Severe cutaneous adverse reactions to drugs. Lancet. 2017;390:1996-2011.
- Medical Dictionary for Regulatory Activities (MedDRA) criteria source: MedDRA Version 12.0 Common Terminology Criteria for Adverse Events v5. https://safetyprofiler-ctep.nci.nih.gov/CTC/CTC.aspx. Accessed March 1, 2018.
- Choi MK, Woo HY, Heo J, et al. Toxic epidermal necrolysis associated with sorafenib and tosufloxacin in a patient with hepatocellular carcinoma. Ann Dermatol. 2011;23(suppl 3):S404-S407.
- Bastuji-Garin S, Fouchard N, Bertocchi M, et al. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115:149-153.
- Kardaun SH, Sidoroff A, Caleyrie-Allanore L, et al. Variability in the clinical pattern of cutaneous side effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br Dermatol. 2007;156:609-611.
- Shiohara T, Iijima M, Ikezawa Z, et al. The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical features and viral reactivation. Br J Dermatol. 2007;156:1083-1084.
- Renda F, Landoni G, Malgarini RB, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): a national analysis of data from 10-year post-marketing surveillance. Drug Saf. 2015;38(12):1211-1218.
- Vibramycin (doxycycline monohydrate, doxycycline calcium, doxycycline) package insert. New York, NY: Pfizer Laboratories; August 2017.
- Lau B, Mutyala D, Dhaliwal D. A case report of doxycycline-induced Stevens-Johnson syndrome. Cornea. 2011;30(5):595-597.
- Cac NN, Messingham MJ, Sniezek PJ, et al. Stevens-Johnson syndrome induced by doxycycline. Cutis. 2007;79:119-122.
- Curley RK, Verbov JL. Stevens-Johnson syndrome due to tetracyclines—a case report (doxycycline) and review of the literature. Clin Exp Dermatol. 1987;12(2):124-125.
- Lazarov A, Livni E, Halevy S. Generalized pustular drug eruptions: confirmation by in vitro tests. J Eur Acad Dermatol Venereol. 1998;10(1):36-41.
- Lewis-Jones MS, Evans S, Thompson CM. Erythema multiforme occurring in association with lupus erythematosus during therapy with doxycycline. Clin Exp Dermatol. 1988;13(4):245-247.
- Podder I, Chandra S, Das A, et al. Doxycycline induced generalized bullous fixed drug eruption. Indian J Dermatol. 2016;61(1):128.
- Singulair (montelukast sodium) package insert. Whitehouse Station, NJ: Merck & Co; March 2018.
- Cetin GY, Sayarlioglu H, Erhan C, et al. A case of neutrophilic dermatosis who develop palpable purpura during the use of montelukast. Eur J Rheumatol. 2014;1(4):170-171.
- Incivek (telaprevir) package insert. Cambridge, MA: Vertex Pharmaceuticals Inc; May 2011.
- MPR. Hepatitis C drug Incivek to be discontinued. www.empr.com/ hepatitis-c-drug-incivek-to-be-discontinued/printarticle/366206/. Accessed February 11, 2018.
- Vertex announces update to U.S. prescribing information for Incivek (telaprevir). http://investors.vrtx.com/releasedetail.cfm?releaseid=727785. Accessed February 11, 2018.
- Vimpat (lacosamide) package insert. Smyrna, GA: UCB Inc; November 2017.
- Kardaun SH, Vos BJ, Chandran NS. Stevens Johnson syndrome/toxic epidermal necrolysis-overlap, induced by lacosamide. Eur J Dermatol. 2016;26(2):185-186.
- Halevi A, Ben-Arnitai D, Garty BZ. Toxic epidermal necrolysis associated with acetaminophen ingestion. Ann Pharmacother. 2000;34:32-34.
- Trujillo C, Gago C, Ramos S. Stevens-Johnson syndrome after acetaminophen ingestion, confirmed by challenge test in an eleven-year-old patient. Allergol Immunopathol (Madr). 2010;38:99-100.
- Leger F, Machet L, Jan V, et al. Acute generalized exanthematous pustulosis associated with paracetamol. Acta Derm Venereol. 1998;78:222223.
- FDA. FDA Drug Safety Communication: FDA warns of rare but serious skin reactions with the pain reliever/fever reducer acetaminophen. www.fda.gov/Drugs/DrugSafety/ucm363041.htm. Accessed February 1, 2018.
- Watanabe H, Kamiyama T, Sasaki S, et al. Toxic epidermal necrolysis caused by acetaminophen featuring almost 100% skin detachment: acetaminophen is associated with a risk of severe cutaneous adverse reactions. J Dermatol. 2016;43:321-324.
- Pena MA, Pere S, Zazo MC, et al. A case of toxic epidermal necrolysis secondary to acetaminophen in a child. Curr Drug Saf. 2016;11:99101.
- Umayahara T, Shimauchi T, Fujiyama T, et al. Paediatric acute generalized exanthematous pustulosis induced by paracetamol with high serum levels of interleukin-8 and 022: a case report. Acta Derm Venereol. 2013;93:362-363.
- Ueta M, Kaniwa N, Sotozono C, et al. Independent strong association of HLA-A*02:06 and HLA-B*44:03 with cold medicine-related StevensJohnson syndrome with severe mucosal involvement. Sci Rep. 2014;(4):4862.
- Lugovic-Mihic L, Duvancic T, Vucic M, et al. SDRIFE (baboon syndrome) due to paracetamol: case report. Acta Dermatovenerol Croat. 2013;21:113-117.
- Lebrun-Vignes B, Guy C, Jean-Pastor MJ, et al for the French Network of Regional Centres of Pharmacovigilance and the French Investigators for Adverse Skin Reactions to Drugs. Br J Clin Pharmacol. 2018;84;331-338.
- FDA. Guidance for Industry: recommended warning for over-thecounter acetaminophen-containing drug products and labeling statement regarding serious skin reactions. www.fda.gov/downloads/drugs/guidances/ ucm424898.pdf. Accessed March 1, 2018.
- Votrient (pazopanib) package insert. East Hanover, NJ: Novartis Pharmaceuticals Corp; May 2017.
- Sutent (sunitinib malate package insert. New York, NY: Pfizer Laboratories; November 2017.
- Nexavar (sorafenib) package insert. Whippany, NJ: Bayer HealthCare Pharmaceuticals, Inc.; July 2015.
- Tykerb (lapatinib) package insert. East Hanover, NJ: Novartis Pharmaceuticals, Inc; April 2017.
- McLellan B, Kerr H. Cutaneous toxicities of the multikinase inhibitors sorafenib and sunitinib. Dermatologic Ther. 2011;24:396-400.
- Massey PR, Okman JS, Wilkerson J, et al. Tyrosine kinase inhibitors directed against the vascular endothelial growth factor receptor (VEGFR) have distinct cutaneous toxicity profiles: a meta-analysis and review of the literature. Support Care Cancer. 2015;23(6):1827-1835.
- Vignand-Courtin C, Martin C, Le Beller C, et al. Cutaneous side effects associated with sunitinib: an analysis of 8 cases. Int J Clin Pharm. 2012;34:286-289.
- Lee WJ, Lee JL, Change SE, et al. Cutaneous adverse effects in patients treated with the multitargeted kinase inhibitors sorafenib and sunitinib. Br J Dermatol. 2009;161(5):1045-1051.
- Abdel-Rahman O, Fouad M. Risk of mucocutaneous toxicities in patients with solid tumors treated with sorafenib: an updated systematic review and meta-analysis. Expert Rev Anticancer Ther. 2014;14(6):751760.
- Zhang L, Zhou Q, Ma L, et al. Meta-analysis of dermatological toxicities associated with sorafenib. Clin Exp Dermatol. 2011;36(4):344-350.
- Zhu Y, Zhang X, Lou X, et al. Vascular endothelial growth factor (VEGF) antibody significantly increases the risk of hand-foot skin reaction to multikinase inhibitors (MKIs): a systematic literature review and metaanalysis. Clin Exp Pharmacol Physiol. March 15, 2018 [E-pub ahead of print].
- Poprach A, Pavlik T, Melichar B, et al, on behalf of the Czech Renal Cancer Cooperative Group. Skin toxicity and efficacy of sunitinib and sorafenib in metastatic renal cell carcinoma: a national registry-based study. Ann Oncol. 2012;23:3137-3143.
- Bryce J, Boers-Doets CB. Non-rash dermatologic adverse events related to targeted therapies. Semin Onc Nurs. 2014;30(3)155-168.
- Ikeda M, Fujita T, Amoh Y, et al. Stevens-Johnson syndrome induced by sorafenib for metastatic renal cell carcinoma. Urol Int. 2013;91(4):482483.
- Chakiri R, Douhi Z, Meziane M, et al. Stevens-Johnson syndrome induced by sorafenib for hepatocellular carcinoma. Int J Clin Dermatol Res. 2016;4(1):78-80.
- Lee JH, Lee JH, Lee JH, et al. Case of sunitinib-induced Stevens-Johnson syndrome. J Dermatol. 2013;40(9):753-754.
- Choi MK, Woo HY, Heo J, et al. Toxic epidermal necrolysis associated with sorafenib and tosufloxacin in a patient with hepatocellular carcinoma. Ann Dermatol. 2011;23 (suppl 3):S404-407.
- Sohn K, Oh S, Lim KW, et al. Sorafenib induces delayed-onset cutaneous hypersensitivity: a case series. Allergy Asthma Immunol Res. 2015;7:304-307.
- Kim DK, Lee SW, Nam HS, et al. A case of sorafenib-induced DRESS syndrome in hepatocellular carcinoma. Korean J Gastroenterol. 2016;67(6):337-340.
- Pretel M, Inarrairaegui M, Lera JM, et al. Acute generalized exanthematous pustulosis induced by sorafenib. JAMA Dermatol. 2014;150:664-666.
- Ikeda M, Fujita T, Mili S, et al. Erythema multiforme induced by sorafenib and metastatic renal cell carcinoma. Jpn J Clin Oncol. 2012;42:820-824.
- MacGregor JL, Silvers DN, Grossman ME, et al. Sorafenib-induced erythema multiforme. J Am Acad Dermatol. 2007;56(3):527-528.
- Feltes RA, Feito Rodriguez M, Gonzalez-Beato MJ. Erythema multiforme induced by sorafenib. Clin Exp Dermatol. 2009;34:e368-e369.
- Bilac C, Muezzinoglu T, Ermertcan AT, et al. Sorafenib-induced erythema mutliforme in metastatic renal cell carcinoma. Cutan Ocul Toxicol. 2009;28:90-92.
- Kodaira M, Takahashi S, Takeuchi K, et al. Sorafenib-induced erythema multiforme for metastatic renal cell carcinoma. Ann Oncol. 2010;21(7):1563-1565.
- Rosado Ingelmo A, Dona Diaz I, Cabanas Moreno R, et al. Clinical practice guidelines for diagnosis and management of hypersensitivity reactions to contrast media. J Investig Allergol Clin Immunol. 2016;26(3):144155.
- Vispaque (iodixanol) package insert. Marlborough, MA: GE Healthcare; April 2017.
- Conray (iothalamate meglumine) package insert. Raleigh, NC: LiebelFlarsheim Co; March 2017.
- Isovue-250, 300, 370 (iopamidol) package insert. Monroe Township, NJ: Bracco Diagnostics, Inc; March 2017.
- Optiray (ioversol) package insert. Raleigh, NC: Liebel-Flarsheim Co; April 2017.
- Ultravist (iopromide) package insert. Wayne, NJ: Bayer HealthCare Pharamceuticals, Inc; July 2017.
- Lerch M, Keller M, Britschgi M, et al. Cross-reactivity patterns of T-cells specific for iodinated contrast media. J Allergy Clin Immunol. 2007;119(6):1529-1536.
- ACR Manual on Contrast Media version 10.3, May 31, 2017. www. acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf. Accessed March 1, 2018.
- Bumbacea RS, Petrutescu B, Bumbacea D, Strambu. Immediate and delayed hypersensitivity reactions to intravascular iodine based radiocontrast media—an update. Pneumologia. 2013;62(1):47-51.
- Gomez E, Ariza A, Blanca-Lopez N, et al. Nonimmediate hypersensitivity reactions to iodinated contrast media. Curr Opin Allergy Clin Immunol. 2013;13:345-353.
- Stacul F, Nellin MF. Late adverse reactions to iodine-based contrast media. In: Thomsen HS, Webb JAW, ed. Contrast media safety issues and ESUR guidelines. 3rd ed. Berlin Heidelberg, Germany: Springer-Verlag; 2014; pp. 141-146.
- Dewachter P, Laroche D, Mouton-Faivre C, et al. Immediate and late adverse reactions to iodinate contrast media: a pharmacological point of view. Antiinflamm Antiallergy Agents Med Chem. 2006;5(2):105-117.
- Brockow K, Christiansen C, Kanny G, et al for the ENDA and EAACI interest group on drug hypersensitivity. Management of hypersensitivity reactions to iodinated contrast media. Allergy. 2003;60:150-158.
- Wu YW, Leow KS, Zhu Y, et al. Prevention and management of adverse reactions induced by iodinated contrast media. Ann Acad Med Singapore. 2016;45:157-164.
- Kanny G, Pichler W, Morisset M, et al. T-cell mediated reactions to iodinated contrast media: evaluation by skin and lymphocyte activation tests. J Allergy Clin Immunol. 2005;115:179-185.
- Brockow K. Immediate and delayed cutaneous reactions to radiocontrast media. Chem Immunol Allergy. 2012;97:180-190.
- Ozturk U, Sungur MA, Karakas T, et al. Acute generalized exanthematous pustulosis induced by iodixanol (Visipaque): a serious reaction to a commonly used drug. Cutan Ocul Toxicol. 2015;34(4):344-346.
- Peterson A, Katzberg RW, Fung MA, et al. Acute generalized exanthematous pustulosis as a delayed dermatotoxic reaction to IV-administered nonionic contrast media. AJR Am J Roentgenol. 2006;187:W198-W201.
- Hammerbeck AA, Daniels NH, Callen JP. Ioversol-induced acute generalized exanthematous pustulosis: a case report. Arch Dermatol. 2009;145(6):683-687.
- Atasoy M, Erdem T, Sari RA. A case of acute generalized exanthematous pustulosis (AGEP) possibly induced by iohexol. J Dermatol. 2003;30(10):723-726.
- Brown M, Yowler C, Brandt C. Recurrent toxic epidermal necrolysis secondary to iopromide contrast. J Burn Care Res. 2013;34(1):e53-e56.
- Lee ML, Chiu IS. Toxic epidermal necrolysis incriminating iopamidol in a child after cardiac catheterization. Int J Cardio. 2002;82(1):95-97.
- Rosado A, Canto G, Veleiro B, et al. Toxic epidermal necrolysis after repeated injections of iohexol. AJR Am J Roentgenol. 2001;176(1);262263.
- Schmidt BJ, Foley WD, Bohorfoush AG. Toxic epidermal necrolysis related to oral administration of diluted diatrizoate meglumine and diatrizoate sodium. AJR Am J Roentgenol. 1998;171(5):1215-1126.
- Kaftori JK, Abraham Z, Gilhar A. Toxic epidermal necrolysis after excretory pyelography. Immunologic-mediated contrast medium reaction? Int J Dermatol. 1988;27(5):346-347.
- Yang SY, Chandran NS. Severe cutaneous adverse reactions following intravenous contrast: a report of 2 cases. Ann Acad Med. 2015;44:561564.
- Belhadjali H, Bouzgarrou L, Youssef M, et al. DRESS syndrome induced by sodium meglumine ioxitalamate. Allergy. 2008;63(6):786-787.
- Macias EM, Munoz-Bellido FJ, Velasco A, et al. DRESS syndrome involving 2 unrelated substances: imipenem and iodinated contrast media. J Investig Allergol Clin Immunol. 2013;23(1):56-57.
- Savill JS, Barrie R, Ghosh S, et al. Fatal Stevens-Johnson syndrome following urography with iopamidol in systemic lupus erythematosus. Postgrad Med J. 1988;64:392-394.
- Zonegran (zonisamide) prescribing information. St. Michael, Barbados: Concordia Pharmaceuticals, Inc; April 2016.
- Blaszczyk B, Lason W, Czuczwar SJ. Antiepileptic drugs and adverse skin reactions: an update. Pharmacol Rep. 2015;67(3):426-434.
- Fujita Y, Hasegawa M, Nabeshima K, et al. Acute kidney injury caused by zonisamide-induced hypersensitivity syndrome. Inter Med. 2010;49:409-413.
- Vivar KL, Mancl K, Seminario‐Vidal L. Stevens–Johnson syndrome/ toxic epidermal necrolysis associated with zonisamide. Clin Case Reports. 2018;6(2):258-261.
- Shibuya R, Tanizaki H, Nakajima S, et al. DIHS/DRESS with remarkable eosinophilic pneumonia caused by zonisamide. Acta Derm Venereol. 2015;95:229-230.
- Fujita Y, Hasegawa M, Nabeshima K, et al. Acute kidney injury caused by zonisdamide-induced hypersensitivity syndrome. Inter Med. 2010;49:409-413.
- Velcade (bortezomib) package insert. Cambridge, MA: Millennium Pharmaceuticals Inc; June 2017.
- Tracleer (bosentan) tablets. South San Francisco, CA: Actelion Pharmaceuticals, Inc; September 2017.
- Allanore Y, Moachon L, Maury E, et al. Bosentan-induced drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome. J Rheumatol. 2010;37:1077-1078.
- Romano A, Giovannetti A, Caruso C, et al. Delayed hypersensitivity to bosentan. Allergy. 2009;64:496-502.
- Treanda (bendamustine hydrochloride) package insert. North Wales, PA: Teva Oncology; December 2017.
- Bendeka (bendamustine hydrochloride) package insert. North Wales, PA: Teva Oncology; September 2017.
- Carilli A, Favis G, Sundharkrishnan L, et al. Severe dermatologic reactions with bendamustine: a case series. Case Rep Oncol. 2014;7:465470.
- Fallon M, Heck JN. Fatal Stevens-Johnson syndrome/toxic epidermal necrolysis induced by allopurinol-rituximab-bendamustine therapy. J Oncol Pharm Pract. 2015;21:388-392.
- Tsai YS, Tu ME, Wu YH, et al. Hydroxyzine-induced acute generalized exanthematous pustulosis. Br J Dermatol. 2007;157:1296-1297.
- O'Toole A, Lacroix J, Pratt M, et al. Acute generalized exanthematous pustulosis associated with 2 common medications: hydroxyzine and benzocaine. J Am Acad Dermatol. 2014;71:e147-e149.
- Kumar SL, Rai R. Hydroxyzine-induced acute generalized exanthematous pustulosis: an uncommon side effect of a common drug. Indian J Dermatol. 2011;56:447-448.
- Badawi AH, Tefft K, Fraga GR. Cetirizine-induced acute generalized exanthematous pustulosis: a serious reaction to a commonly used drug. Dermatology Online J. 2014;20(5):7.
- Pancar GS, Kalkan G, Durer PG, et al. Acute generalized exanthematous pustulosis (AGEP) induced by cetirizine in a child: a case report. J Clin Anal Med. 2016;7(suppl 2):116-117.
- Gupta T, Garg VK, Sarkar R, Maden A. Acute generalized exanthematus pustulosis induced by fexofenadine. Indian J Dermatol. 2016;61(2):235.
- Diabeta (glyburide) package insert. Bridgewater, NJ: Sanofi-Aventis US; January 2017.
- Wongpaitoon V, Mills PR, Russell RI, et al. Intraheptic cholestasis and cutaneous bullae associated with glibenclamide therapy. Postgrad Med J. 1981;57:244-246.
- Uloric (febuxostat) package insert. Deerfield, IL: Takeda Pharmaceuticals America, Inc; February 2018.
- Chong HY, Lim YH, Prawjaeng J, et al. Cost-effectiveness analysis of HLA-B*58:01 genetic testing before initiation of allopurinol therapy to prevent allopurinol-induced Stevens Johnson syndrome/toxic epidermal necrolysis in a Malaysian population. Pharmacogenet Genomics. 2018;28(2):56-67.
- Chou HY, Chen CB, Cheng CY, et al. Febuxostat-associated drug reaction with eosinophilia and systemic symptoms (DRESS). J Clin Pharm Ther. 2015;40:689-692.
- Paschou E, Gavriilaki E, Papaioannnou G, et al. Febuxostat hypersensitivity: another cause of DRESS syndrome in chronic kidney disease? Eur Ann Allergy Clin Immunol. 2016;48(6):251-255.
- Quilis N, Andrés M, Vela P, et al. Skin events with febuxostat in gout patients with previous skin reactions to allopurinol. A retrospective review. Ann Rheum Dis. 2016;75:370-371.
- Bardin T, Charles G, Pascart T, et al. Risk of cutaneous adverse events with febuxostat treatment in patients with skin reaction to allopurinol. A retrospective, hospital-based study of 101 patients with consecutive allopurinol and febuxostat treatment. Joint Bone Spine. 2016;83:314-317.
- Lien YHH, Logan JL. Cross-reactions between allopurinol and febuxostat. Am J Med. 2017;130(2):e67-68. Epub September 22, 2016.
- Sulaiman N, Zaidi Othman A, Shuhaila Shahril N, et al. Successful febuxostat desensitization in a patient with febuxostat hypersensitivity: a Malaysian experience. SAGE Open Med Case Reports. 2017;5:1-3.
- Choban S. Safety and efficacy of febuxostat treatment in subjects with gout and severe allopurinol adverse reactions. J Rheumatol. 2011;38:1957-1959.
- Keytruda (pembrolizumab) package insert. Whitehouse Station, NJ: Merck & Co; November 2017.
- Postow M, Wolchok J. Toxicities associated with checkpoint inhibitor immunotherapy. UpToDate. January 8, 2018. www.uptodate.com/contents/toxicities-associated-with-checkpoint-inhibitor-immunotherapy. Accessed February 2, 2018.
- Fay AP, Moreira RB, Nunes PRS, et al. The management of immunerelated adverse events associated with immune checkpoint blockade. Expert Rev Quality Life Cancer Care. 2016;1(1):89-97.
- Demlova R, Valik D, Obermannova R, et al. The safety of therapeutic monoclonal antibodies: implications for cancer therapy including immune-checkpoint inhibitors. Physiol Res. 2016;65(suppl 4):S455-S462.
- Puzanov I, Diab A, Abdallah K, et al, for the Society for Immunotherapy of Cancer Toxicity Management Working Group. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):95.
- Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and antiPD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26:2375-2391.
- Bagshaw SM, Cload B, Gilmour J, et al. Drug-induced rash with eosinophilia and systemic symptoms syndrome with bupropion administration. Ann Allergy Asthma Immunol. 2003;90:572-575.
- Alexander T, Iglesia E, Park Y, et al. Severe DRESS syndrome managed with therapeutic plasma exchange. Pediatrics. 2013;131;e945-e949.
- Cignesh P, Kishore J, Kumar A, et al. A young child with eosinophilia, rash, and multisystem illness: drug rash, eosinophilia, and systemic symptoms syndrome after receipt of fluoxetine. Pediatr Dermatol. 2017;34(3):e120-e125.
- Gallego J, Hauss PA, Salaun M, et al. Clomipramine hypersensitivity with predominantly pulmonary involvement. Rev Mal Respir. 2012;29:430-434.
- Milionis HJ, Skopelitou A, Elisaf MS. Hypersensitivity syndrome caused by amitriptyline administration. Postgrad Med J. 2000;76:361-363.
- van Westerloo DJ, Juffermans NP. Red and wet. Netherlands J Med. 2010;68:230-231.
- Seitz CS, Pfeuffer P, Raith P, et al. Anticonvulsant hypersensitivity syndrome: cross-reactivity with tricyclic antidepressant agents. Ann Allergy Asthma Immunol. 2006;97:698-702.
- Herstowska M, Komorowska O, Cubala WJ, et al. Severe skin complications in patients treated with antidepressants: a literature review. Postop Derm Alergol. 2014;2:92-97.
- Bommersbach TJ, Lapid MI, Leung JG, et al. Management of psychotropic drug-induced DRESS syndrome: a systematic review. Mayo Clin Proc. 2016;91(6):787-801.
- Mayo-Pampin E, Florez A, Feal C, et al. Acute generalized exanthematous pustulosis due to pseudoephedrine with positive patch test. Acta Derm Venereol. 2006;86:542-543.
- Padial MA, Alvarez-Ferreira J, Tapia B, et al. Acute generalized exanthematous pustulosis associated with pseudoephedrine. Br J Dermatol. 2004;150:139-142.
- Assier-Bonnet H, Viguier M, Dubertret L, et al. Severe adverse drug reactions due to pseudoephedrine from over-the-counter medications. Contact Dermatitis. 2002;47:165-182.
- Ben Salem C, Slim R, Denguezil M, et al. Pseudoephedrine-induced acute generalized exanthematous pustulosis. Int J Dermatol. 2008;47:418419.
- Fukuda R, Ouchi T, Hirai I, et al. Non-pigmenting fixed drug eruption with mixed features of acute generalized exanthematous pustulosis induced by pseudoephedrine: a case report. Contact Dermatitis. 2017;77:123.
- Vraylar (cariprazine) package insert. Irvine, CA: Allergan USA; November 2017.
- Fenofibrate package insert. Morganville, WV: Mylan Pharmaceuticals, Inc; January 2018.
- Fenofibric acid package insert. Mylan Pharmaceuticals, Inc; December 2016.
- Morais P, Barros AM, Cunha AP, et al. Fenofibrate-induced acute generalized exanthematous pustulosis. Egypt Derm Online J. 2009;4(2):1.
- Pecora V, Nucera B, Aruanno A, et al. Delayed-type hypersensitivity to fenofibrate. J Investig Allergol Clin Immunol. 2012;22:286-312.