Evolving Therapies for Metabolic Liver Disease
RELEASE DATE
October 1, 2024
EXPIRATION DATE
October 31, 2026
FACULTY
Meg Conger Ason, PharmD, BCACP, CDCES, CPP
Clinical Pharmacist
Atrium Health
Huntersville, North Carolina
Julia Freudenberg Clements, PharmD, BCACP, CDCES, CPP
Clinical Pharmacist
Atrium Health
Concord, North Carolina
Jennifer LaPreze, PharmD, BCACP, CDCES, CPP
Clinical Pharmacist
Atrium Health
Fort Mill, South Carolina
DISCLOSURE STATEMENTS:
Faculty Disclosures: Dr. Ason, Dr. Clements, and Dr. LaPreze have no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT
 Pharmacy
Pharmacy
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-24-107-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients’ conditions and possible contraindications or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
GOAL
To provide an update to nomenclature and treatment for metabolic liver disease.
OBJECTIVES
After completing this activity, the participant should be able to:
- Distinguish between old and new nomenclature.
- Understand the pathophysiology for metabolic liver disease.
- Review the risk factors and diagnostic tools for metabolic liver disease.
- Recognize the pharmacologic and nonpharmacologic treatment options for metabolic liver disease.
ABSTRACT: Nonalcoholic fatty liver disease is growing in prevalence in Western countries, due in part to the increase in obesity. In recent years, liver experts have pushed to revise how liver disease is classified to stress the importance of the links between the condition and metabolism. That connection led to treatment recommendations that stress diet and lifestyle, but recently those guidelines have included medications that have been shown to have some benefit on steatosis but not on fibrosis. In 2024, resmetirom became the first drug of its kind engineered to treat advanced metabolic liver disease.
Metabolic diseases have become more widespread in the United States in the past decades. Diabetes and hyperlipidemia have always been linked to metabolism, but certain types of liver diseases, such as steatosis and steatohepatitis, have had exclusionary and stigmatizing criteria that have made diagnosis confusing and less specific.
Recently, liver specialists from the American Association for the Study of Liver Disease and the European Association for the Study of the Liver have stressed the connection between metabolism and those liver diseases in the renaming of nonalcoholic fatty liver disease (NAFLD) to metabolicassociated fatty liver disease (MAFLD) and nonalcoholic steatohepatitis (NASH) to metabolic dysfunction–associated steatohepatitis (MASH). The push was designed to decrease the stigma and was done in the hope that it would provide context to the complexity of the disease, improve diagnosis, and expand awareness, research, and treatment options.1 (Because this nomenclature change is new and has not been fully adopted across professional societies, this article will use the old nomenclature to maintain clarity, not as a statement about the nomenclature.)
NAFLD can be divided into two categories. The first category has a correlation with metabolic syndrome in which insulin resistance is the principal underlying pathophysiological process. The second category is linked to infections and other pathologies that can contribute to the advancement of the disease to liver steatosis. Hepatotropic viruses such as hepatitis B, hepatitis C, and HIV can play a role, as can some inherited or acquired disorders (lipodystrophy, cachexia, or intestinal bypass surgery). Medications (e.g., total parenteral nutrition, glucocorticoids, tamoxifen, tetracycline, amiodarone, methotrexate, valproic acid, and vinyl chloride), as well as specific toxins, can also contribute to the development of liver steatosis. The pathogenesis of NAFLD can also be multifactorial.2
EPIDEMIOLOGY
Overall, the prevalence of NAFLD is estimated at 25% to 30% of adults worldwide and is expected to increase with the rise in obesity and metabolicrelated diseases (hypertension, hyperlipidemia, central obesity, and insulin resistance).3 As many as 80 million Americans are estimated to currently have NAFLD, and as many as 100 million people are expected to have the disease by 2030.1 The prevalence can vary among clinical settings and populations, but it is often undiagnosed.3 It also increases with the age of the population.4
The prevalence of NAFLD in early stages appears to be higher in men, but fibrosis and cirrhosis seem to occur more in women, particularly among postmenopausal women. This is likely due to the decreasing levels of estrogen, which provides liver tissue some protection from fibrogenesis.1
The incidence of NASH is more difficult to report due to the potentially invasive testing that is required to diagnose it, but experts estimate that 25% of patients with NAFLD in the U.S. have NASH.17 About 16.5 million people had NASH in 2015, and as many as 3.3 million people had advanced fibrosis in that year.1
As of 2020, NAFLD was the second leading cause of liver transplantation, just behind alcohol-related liver disease.1 NASH was also noted to be linked to about 16% of hepatocellular carcinoma cases in the U.S.1
RISK FACTORS AND COMORBID CONDITIONS
NAFLD is associated with other metabolic conditions, such as obesity, type 2 diabetes mellitus (T2DM), obstructive sleep apnea, cardiovascular disease, and chronic kidney disease.3 Higher rates of NAFLD have been associated with hypothyroidism, hypogonadism, growth hormone deficiency, and polycystic ovarian syndrome.3 While it has not been concluded that these conditions are direct risk factors for NAFLD, a strong correlation between these conditions and NAFLD has been noted.
PATHOPHYSIOLOGY
Inflammation is the liver’s first response to injury or toxicity. Acute inflammation will resolve when the injury or toxicity is no longer present; however, when the injury or toxicity persists, so does the inflammation. Chronic inflammation can cause hyperactivity that will eventually result in scarring or fibrosis. Liver fibrosis results from deposits of extracellular matrix and collagen associated with degradation and remodeling of liver tissue.5
NAFLD is the accumulation of fat without inflammation and hepatic injury. This accumulation can progress to NASH, which involves inflammation and hepatic injury that can lead to fibrosis and cirrhosis.6
The progression of NAFLD is not completely understood; however, it is thought that underlying fatty infiltration in the liver contributes to NAFLD progression. The reasons for fatty liver infiltration have been attributed to T2DM or obesity. The initial injury is thought to be from acquired insulin resistance and hepatic steatosis caused by excess fatty acids. Subsequently, NAFLD can progress from oxidative stress, lipid peroxidation, inflammation, and fibrosis, which leads to pathological changes in hepatocytes. NAFLD can lead to cirrhosis and hepatocellular carcinoma, contributing to mortality from liver-related diseases.2
SCREENING AND DIAGNOSIS
NAFLD often is asymptomatic, which can lead to patients being undiagnosed or diagnosed as an incidental finding on abdominal imaging.6 Because early identification allows for interventions that may prevent future hepatic complications, screening is vital.3
Recent guidelines suggest screening patients at increased risk for advanced liver disease to identify and manage those with clinically significant fibrosis (stage 2 or greater), including those with T2DM and obesity with metabolic complications.7
Patients who are suspected to have NAFLD based on metabolic risk factors or incidental findings of fatty liver by imaging in the absence of other etiologies of hepatic steatosis should undergo primary risk assessment using the Fibrosis-4 Index (FIB-4). Patients who are low risk (FIB-4 <1.3) should be reassessed periodically. Low-risk patients with ≥2 metabolic risk factors, prediabetes mellitus (pre- DM), or T2DM should undergo repeat risk assessment with FIB-4 every 1 to 2 years. Low-risk patients without pre-DM or T2DM and <2 metabolic risk factors should be reassessed every 2 to 3 years (TABLE 1).3,8
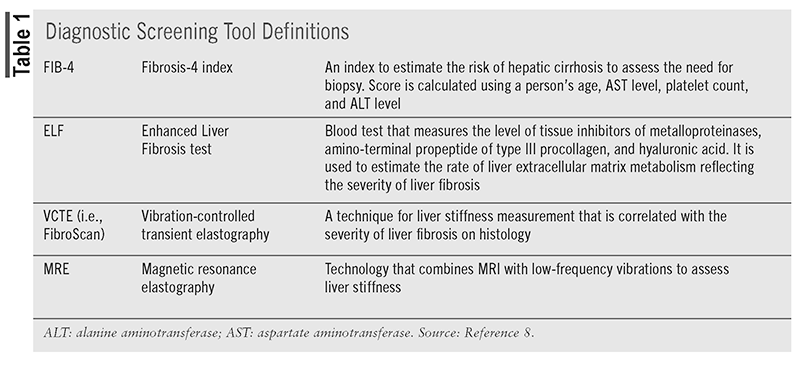
Screening patients with T2DM and suspected NAFLD-related advanced hepatic fibrosis using FIB-4 can be cost-effective and allows for the prediction of outcomes such as progression to cirrhosis or decompensation.3
Initial evaluation of patients with NAFLD should include a history, physical examination, laboratory studies, and ruling out other causes of hepatic steatosis or steatohepatitis. History should include weight, comorbidities, recent and current medications, family history of T2DM, NAFLD, or cirrhosis, screening for obstructive sleep apnea, and alcohol use. Laboratory testing should include hepatic panel, CBC with platelets, fasting glucose and hemoglobin A1C, fasting lipid panel, creatinine, urine microalbumin, and hepatitis C.3
Patients with a moderate (FIB-4 1.3-2.67) or high (FIB-4 >2.67) risk of advanced disease based on FIB-4 should undergo secondary risk assessment. Secondary assessments include vibration-controlled elastography (VCTE) or magnetic resonance elastography (MRE). Also, the Enhanced Liver Fibrosis (ELF) test is used as a secondary assessment. If secondary risk assessment is still consistent with an intermediate or high risk of fibrosis, patients should be referred to specialty care for further evaluation.3
Diagnosing, Grading, and Staging
Diagnosis of NAFLD and NASH typically requires imaging or biopsy to confirm fat deposition in the liver in a patient with little to no alcohol use and no other potential causes.3 Patients who have nonalcoholic fatty liver, the mildest type of NAFLD, have more than 5% of their hepatocytes exhibiting macrovesicular fat deposition but no cellular damage.3 The presence of inflammation and cellular damage (with or without fibrosis) defines NASH.3 Patients with cirrhosis, the most severe disease, exhibit bands of fibrotic scarring that can eventually develop into nodules.3
Other etiologies of hepatic steatosis also should be excluded: Wilson disease, celiac disease, alcohol use (typically defined as >21 standard drinks of alcohol per week in men and >14 drinks per week in women), hepatitis B or C infection, medications (amiodarone, tamoxifen, methotrexate, diltiazem, corticosteroids), hemochromatosis, or autoimmune hepatitis.7
The downfall of biopsy is its invasive nature and its associated morbidity and costs. Results of a biopsy can also have some interobserver variability, depending on the pathologist and how inflammation or cellular damage is interpreted.4
Imaging modalities such as ultrasound, computed tomography, FibroScan (vibration-controlled transient elastography), and controlled attenuation parameter can be used to diagnose steatosis and also to assess changes or progression of liver disease.1
Once a biopsy is obtained, it is reported as grades of hepatic steatosis, hepatocyte ballooning, and necroinflammatory activity. The NAFLD activity score (NAS) is a score reported from 0 to 8 points that is the sum of these components, as seen in (TABLE 2).9 Another histologic scoring system for NAFLD is the steatosis, activity, and fibrosis (SAF) score, which includes steatosis (S: 0 to 3 points, 0 points rules out NAFLD); activity (A [includes ballooning]: 0 to 2 points; lobular inflammation: 0 to 2 points); and fibrosis (F: 0 to 4). A score of at least 1 point for steatosis usually indicates NAFLD, but a diagnosis of NASH should not be made on NAS alone and should be based on patterns as well as individual lesions. Ballooning and lobular inflammation are mandatory to diagnosis NASH.9
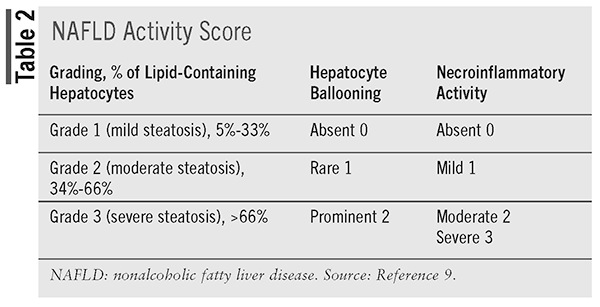
Liver fibrosis is staged on a fivepoint scale: no fibrosis (stage 0), pericellular fibrosis (stage 1), pericellular and portal fibrosis (stage 2), bridging fibrosis (stage 3), or cirrhosis (stage 4). Stages 3 and 4 are considered advanced fibrosis.9
TREATMENT
Once a diagnosis of NAFLD has been obtained, clinicians need to determine individualized treatment plans. Until recently, treatment of NAFLD and NASH has generally been limited to lifestyle and medications, mostly used off-label, to reduce weight or reduce steatosis. However, this year, the FDA approved another avenue for treatment, specifically for NASH.
Pharmacologic Therapy
Resmetirom: On March 14, 2024, the FDA approved resmetirom under the accelerated approval pathway for treatment for adults with NASH with moderate-to-advanced liver fibrosis (fibrosis stages F2 to F3) in conjunction with diet and exercise.10,11 This is the first medication to be FDA approved for this indication. Resmetirom is a liver-directed, thyroid hormone receptor beta (THR-B)–selective agonist.11
There is an association between the development of NAFLD and thyroid dysregulation. T3 and T4 levels have been associated with an increased occurrence of advanced liver fibrosis by biopsy in euthyroid patients. Thyroid-stimulating hormone also displays a positive linear relationship with the risk of NAFLD.12,13
The two main thyroid receptors include THR-A and THR-B. THR-A is expressed mainly in myocardial atria, adipose tissue, and the brain, while THR-B is mainly in the liver and heart ventricles. THR-B plays a role in lowering cholesterol and triglyceride levels, increasing bile acid synthesis, and fat oxidation.14 Because resmetirom is a THR-B selective agonist, it can help decrease fat and triglyceride content in the liver.
Currently, there are three ongoing phase III randomized, controlled trials investigating the safety and efficacy of resmetirom in NAFLD, NASH and fibrosis, and well-compensated NASH cirrhosis: MAESTRO-NAFLD-OLE, MAESTRO-NASH, and MAESTRO-NASH-Outcomes. MAESTRO-NASH recently published findings.15,16
In MAESTRO-NASH, 966 adults with biopsyconfirmed NASH and liver fibrosis (stage FIB, F2, or F3) were randomly assigned to receive 80 mg resmetirom, 100 mg resmetriom, or placebo once daily for 52 weeks. The two primary endpoints were NASH resolution with no worsening of fibrosis and improvement in fibrosis by at least one stage with no worsening of the NAFLD activity score.16
NASH resolution was defined as an achievement of hepatocellular ballooning score of 0, a lobular inflammation score of 0 or 1, and a reduction in NAFLD activity score by 2 or more points. A secondary endpoint was the percent change from baseline at Week 24 in low-density lipoprotein (LDL) cholesterol level.16
Resolution of NASH with no worsening of fibrosis occurred in 25.9% of the 80-mg group and 29.9% of the 100-mg group compared with 9.7% in the placebo group (P <.001 for both comparisons with placebo).16
Improvement in fibrosis by at least one stage with no worsening of disease activity scores occurred in 24.2% of the 80-mg group and 25.9% of those in the 100-mg group versus 14.2% in the placebo group (P <.001). LDL cholesterol levels were reduced by resmetirom from baseline to Week 24: 13.6% in the 80-mg group; −16.3% in the 100-mg group; and 0.1% in the placebo group (P <.001).16
The safety of long-term use of resmetirom has not been assessed. The MAESTRO-NASH trial is planned to continue to 54 months to evaluate liverrelated outcomes.16
Dosing and Administration: Resmetirom is available as 60-mg, 80-mg, and 100-mg tablets. The dosing of oral resmetirom is based on actual body weight. The approved dosing for individuals weighing less than 100 kg is 80 mg once daily; for patients who weight 100 kg or more, the dosing is 100 mg once daily regardless of administration of food. There is no dose adjustment for patients with mild or moderate renal impairment, but it has not been studied in individuals with severe renal impairment. Dosing is impacted by certain medications and is discussed in the Drug Interaction subsection of the prescribing information.11
There are no specific contraindications; however, resmetirom should be avoided in individuals with decompensated cirrhosis and with moderateto- severe hepatic impairment (Child-Pugh Class B or C). Hepatic impairment can increase plasma concentration and increase the risk of adverse effects. Resmetirom safety and efficacy have not been established in pediatric patients or with use in pregnancy and lactation.11
Adverse Effects and Warnings: The most common adverse effects were diarrhea, nausea, vomiting, constipation, abdominal pain, dizziness, and pruritus.
Warnings include hepatotoxicity and gallbladderrelated adverse reactions. Individuals receiving resmetirom should have their liver enzymes monitored and be assessed for signs of hepatotoxicity. If hepatotoxicity is suspected, resmetirom should be discontinued and the patient monitored. If laboratory results return to baseline, clinical decision-making skills will need to be used to decide if therapy should be resumed.
Gallbladder-related adverse events, including cholelithiasis, acute cholecystitis, and obstructive pancreatitis, occurred more in the resmetiromtreated patient group compared with placebo in clinical studies. If cholelithiasis is suspected, additional testing and follow-up is needed, and resmetirom should be discontinued until the cholelithiasis has resolved.11
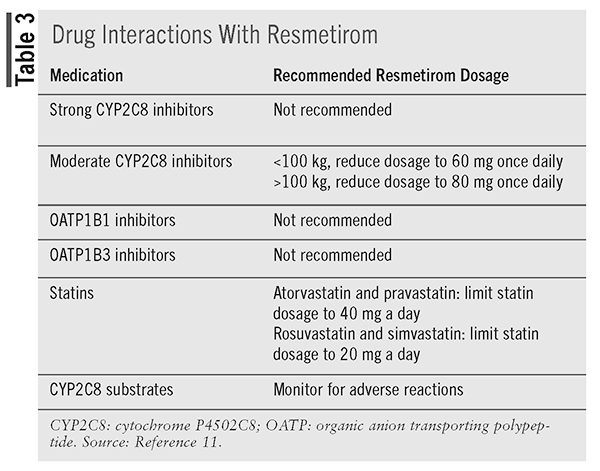
Drug Interactions: Resmetirom is a cytochrome P4502C8 (CYP2C8) substrate and is not recommended with the use of strong CYP2C8 inhibitors, such as gemfibrozil. Recommended dose adjustment is based on an individual’s weight if using a moderate CYP2C8 inhibitor, such as clopidogrel.11
Resmetirom is a weak CYP2C8 inhibitor, and when coadministered with CYP2C8 substrates, patients should be monitored for related adverse reactions.11
Resmetirom also is an organic anion transporting polypeptide (OATP)1B1 and OATP1B3 substrate. Resmetirom has an impact on other medications, including statins, and can increase the plasma concentrations of atorvastatin, pravastatin, rosuvastatin, and simvastatin.11
Drug interactions and recommendations are noted in (TABLE 3).11
Additional Agents
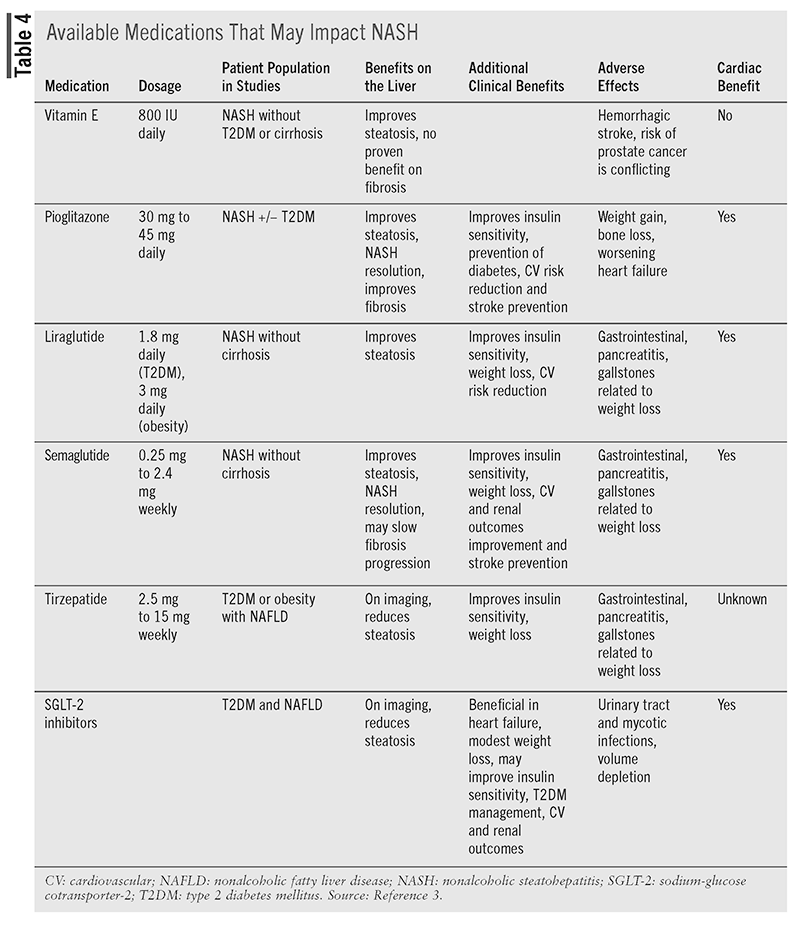
Even though they have not been approved by the FDA to treat NAFLD, medications that improve obesity, diabetes, and other comorbid conditions have been included in guidelines as potential treatment options for selected patients, as shown in (TABLE 4).3 Glucagon-like peptide-1 (GLP-1) receptor agonists such as semaglutide, liraglutide, and tirzepatide and the thiazolidinedione pioglitazone can be used in selected patients to help with NASH. Vitamin E has been shown to improve NASH in patients without diabetes. Sodium-glucose cotransporter-2 inhibitors such as empagliflozin and dapagliflozin have limited data on improvement in patients with NASH, but they are considered potential options. None of these agents have shown benefit in fibrosis, and they have not been studied in cirrhosis.3

Nonpharmacologic Treatment
Lifestyle: Patients also can improve liver disease, both NAFLD and NASH, with treatment and strict adherence to lifestyle changes. Because of the chronic nature of the disease, without treatment patients can eventually develop complications. Those can include liver failure or hepatocellular carcinoma, kidney disease, increased risk of diabetes, or cardiovascular disease.4
Because NAFLD is strongly linked with obesity, poor dietary choices, and decreased physical activity, lifestyle changes have long been the backbone of NAFLD treatment. An expert panel of hepatologists commissioned by the American Gastroenterological Association Institute of Clinical Practice Updates Committee and the American Gastroenterological Association Governing Board has compiled a peer-reviewed list of best practice lifestyle recommendations to help treat the disease. The recommendations for treating NAFLD focus on weight loss, reduction of calorie intake, elimination or reduction of alcohol consumption, and exercise (SIDEBAR 1).4,17
Trials providing evidence for the benefits of lifestyle modification on patients with NAFLD are small and typically short. In general, research on dietary changes suggest that higher-protein diets, weight loss, and diets that lower hepatic triglyceride content may be beneficial for patients with NAFLD.18 For example, one study compared levels of steatosis in patients on a low-fat diet versus a Mediterranean diet over 12 weeks. Both groups of patients saw improvements in steatosis but experienced variations in other markers, including lipid levels, weight, and other factors. Studies such as this one have led to a recommendation for patients with NAFLD to follow the Mediterranean diet.19
Research has also focused on exercise’s impact on patients with liver disease. Sedentary behavior is linked with the development of T2DM, obesity, and NAFLD. Activity, both moderate-intensity aerobic exercise and resistance or strength training, has been shown to improve steatosis, independent of weight loss.18 High-intensity aerobic exercise does not confer any additional benefit over moderate-intensity aerobic exercise.18
In addition to helping treat liver disease and weight reduction, lifestyle changes may help lower cardiovascular risks and treat or avoid development of other metabolic disorders, including diabetes, hyperlipidemia, hypertension, and obesity.17
CONCLUSION
NAFLD and NASH are complicated diseases with complex contributing factors and etiologies. Liver experts have moved this year to increase awareness, improve research, and remove stigmatizing terminology by creating new nomenclature that stresses the disease’s metabolic links. They hope that this will strengthen future treatments and allow for patients’ access to appropriate diagnosis and treatment. An additional tool to use in treating severe forms of the disease, resmetirom, has recently been approved for use in patients with NASH. Researchers hope that increased awareness and new treatment options will help to drive improvements in patient health and lower medical burdens.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
- Cotter TG, Rinella M. Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology. 2020;158(7):1851-1864.
- Soto A, Spongberg C, Martinino A, Giovinazzo F. Exploring the multifaceted landscape of MASLD: a comprehensive synthesis of recent studies, from pathophysiology to organoids and beyond. Biomedicines. 2024;12(2):397.
- Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, et al. AASLD practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77(5):1797-1835.
- Francque SM, Marchesini G, Kautz A, et al. Non-alcoholic fatty liver disease: a patient guideline. JHEP Reports. 2021;3(5):100322.
- Parola M, Pinzani M. Liver fibrosis in: NAFLD/NASH: from pathophysiology towards diagnostic and therapeutic strategies. Mol Aspects Med. 2024;95:101231.
- Rakel D. Nonalcoholic steatohepatitis/fatty liver. In: Integrative Medicine. 5th ed. Elsevier; 2023:427-431.
- Boschuetz N, Phan H, Said A. Nonalcoholic fatty liver disease (NAFLD). In: Conn’s Current Therapy. Elsevier; 2024:279-286.
- Cusi K, Isaacs S, Barb D, et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings. Endocr Pract. 2022;28(5):528- 562.
- Paternostro R, Trauner M. Current treatment of non-alcoholic fatty liver disease. J Intern Med. 2022;292(2):190-204.
- Commissioner of the FDA Approves First Treatment for Patients with Liver Scarring Due to Fatty Liver Disease. FDA. March 15, 2024. www.fda.gov/news-events/press-announcements/fda-approvesfirst- treatment-patients-liver-scarring-due-fatty-liver-disease.
- Highlights of Prescribing Information. www.madrigalpharma. com/wp-content/uploads/2024/06/NDA-217785_REZDIFFRAPI_ 14Mar2024_final-revised-clean-SPLPPI.pdf.
- Bano A, Chaker L, Plompen EPC, et al. Thyroid function and the risk of nonalcoholic fatty liver disease: the Rotterdam Study. J Clin Endocrinol Metab. 2016;101:3204-3211.
- Li R, Zhou L, Chen C, et al. Sensitivity to thyroid hormones is associated with advanced fibrosis in euthyroid patients with nonalcoholic fatty liver disease: a cross-sectional study. Dig Liver Dis. 2023;55(2):254-261.
- Sinha RA, Bruinstroop E, Singh BK, Yen PM. Nonalcoholic fatty liver disease and hypercholesterolemia: roles of thyroid hormones, metabolites, and agonists. Thyroid. 2019;29:1173-1191.
- Kokkorakis M, Boutari C, Hill M, et al. Resmetirom, the first approved drug for the management of metabolic dysfunction-associated steatohepatitis: trials, opportunities, and challenges. Metabolism. 2024;154:155835.
- Harrison SA, Bedossa P, Guy CD, et al. A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis. N Engl J Med. 2024;390:497-509.
- Younossi ZM, Corey KE, Lim JK. AGA Clinical Practice Update on Lifestyle Modification Using Diet and Exercise to Achieve Weight Loss in the Management of Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology. 2021;160(3):912-918.
- Hallsworth K, Adams LA. Lifestyle modification in NAFLD/NASH: facts and figures. JHEP Reports. 2019;1(6):468-479.
- Properzi C, O’Sullivan TA, Sherriff JL, et al. Ad libitum Mediterranean and low-fat diets both significantly reduce hepatic steatosis: a randomized controlled trial. Hepatology. 2018;68(5):1741-1754.