Exocrine Pancreatic Insufficiency
RELEASE DATE
December 1, 2019
EXPIRATION DATE
December 31, 2021
FACULTY
Danny H. Pham, PharmD Candidate 2021
Katie Ma, PharmD Candidate 2021
Analise Klassen, PharmD Candidate 2021
Laura Tsu, PharmD, BCPS, BCGP
Associate Professor of Pharmacy Practice
Chapman University School of Pharmacy
Irvine, California
FACULTY DISCLOSURE STATEMENTS
Mr. Pham, Mss. Ma and Klassen, and Dr. Tsu have no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT
Pharmacy
 Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-19-135-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER:
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients' conditions and possible contraindications or dangers in use, review of any applicable manufacturer's product information, and comparison with recommendations of other authorities.
GOAL
To provide participants with an overview of the pathophysiology and management of exocrine pancreatic insufficiency (EPI), with a focus on pancreatic enzyme replacement therapy (PERT).
OBJECTIVES
After completing this activity, the participant should be able to:
- Recall the common etiologies of EPI.
- Describe diagnostic methods used to diagnose EPI.
- Discuss nonpharmacologic treatment options for patients with EPI.
- Compare the different formulations of PERT.
ABSTRACT: Exocrine pancreatic insufficiency (EPI) is a condition characterized by inadequate production or delivery of pancreatic enzymes to the small intestine, which results in the insufficient breakdown and absorption of nutrients. Patients typically present with symptoms such as diarrhea, nausea, abdominal pain, and weight loss. The most common etiologies of EPI are cystic fibrosis and chronic pancreatitis. The Cystic Fibrosis Foundation and the Australasian Pancreatic Club have published guidelines on the management of EPI. Dietary restriction and smoking cessation are the most effective nonpharmacologic treatment methods for EPI. Pharmacologic treatment centers on pancreatic enzyme replacement therapy (PERT). PERT agents come in different formulations and concentrations of pancrelipase, which comprises the enzymes that normally digest fats for absorption in the body.
The pancreas is critical for the digestion, absorption, and metabolism of nutrients, and its exocrine functions are necessary for the breakdown of macronutrients. Exocrine pancreatic insufficiency (EPI) is a condition involving the inadequate production or delivery of pancreatic enzymes to the small intestine, leading to the insufficient breakdown and absorption of nutrients.
The most common causes of EPI are cystic fibrosis and chronic pancreatitis; celiac disease, Crohn's disease, and pancreatic cancer are less commonly involved.1 Patients with EPI typically present with gastrointestinal symptoms such as diarrhea, nausea, abdominal pain, and weight loss.2 Given that EPI has many different causes and is not typically recorded as a medical statistic, it is difficult to accurately determine its prevalence in the general patient population. No reliable data are currently available on EPI or its prevalence in terms of certain patient demographics, such as gender, age, and race.
Etiology
The etiology of EPI comprises a number of different conditions, both pancreatic and nonpancreatic.3 Pancreatic causes include chronic pancreatitis, cystic fibrosis, and obstruction of the pancreatic duct by a tumor or stricture. Nonpancreatic causes include gastrointestinal and pancreatic surgery, celiac or inflammatory bowel disease, Crohn's disease, autoimmune pancreatitis, inoperable pancreatic cancer, and diabetes.1 The most common causes of EPI are cystic fibrosis and chronic pancreatitis; approximately 85% of patients with cystic fibrosis have symptoms of EPI, and the condition is estimated to occur in 94% of patients with chronic pancreatitis.4,5
The occurrence of EPI in patients with untreated celiac disease ranges from 4% to 80%, but EPI has been reported to resolve after initiation of a glutenfree diet.1 Patients with inflammatory bowel disease have a 14% to 74% incidence of EPI, whereas 66% to 92% of patients with inoperable pancreatic cancer develop EPI. EPI symptoms also occur in type 1 and type 2 diabetes patients, ranging from 26% to 57% and 20% to 36%, respectively.1
Pathophysiology
Amylase, protease, and lipase are the main enzymes responsible for the breakdown of food into digestible components that the body can use.6 In EPI, there is an insufficient release of all three enzymes into the duodenum of the small intestine. The amount of enzymes released is inadequate to maintain normal digestive processes; therefore, the body is unable to digest and absorb the needed amounts of carbohydrates, fats, and proteins. Clinical symptoms do not present until lipase levels drop below 5% to 10% of normal postprandial levels, however.7 Pancreatic lipase is responsible for up to 90% of lipid breakdown in the body. Because of the significant role of pancreatic lipase, the maldigestion of fat is more significant in EPI compared with the digestion of carbohydrates or proteins.8
The progression of EPI differs from patient to patient, depending on the underlying etiology. Owing to the variety of causes of EPI, a wide array of clinical symptoms are associated with this condition. The most common symptoms of EPI are abdominal pain, flatulence, diarrhea, steatorrhea, and weight loss.2 Because of the malabsorption of fat, patients with EPI are unable to absorb fat-soluble vitamins such as A, D, E, and K. Untreated malabsorption puts patients at high risk for developing nutritional deficiencies, which can result in other health problems. Vitamin deficiencies and decreased levels of lipoproteins are associated with complications such as osteoporosis and bone fracture.9
Diagnosis
The symptoms of EPI are similar to those of other gastrointestinal diseases, making EPI difficult to diagnose. In clinical practice, the diagnosis of possible EPI begins with assessment by a provider, selfreported bowel movements, and presence of weight loss. However, reliance on patient-reported symptoms alone may lead to overdiagnosis or underdiagnosis. Numerous diagnostic tests are available, and their uses vary.
For initial evaluation, CT may be used to assess the pancreatic structure and determine gross structural changes.10 These results may not be specific enough, rendering it necessary to conduct further testing via MRI or endoscopic US.2 Sometimes, EPI may occur in patients with a normal pancreas without visible structural changes; in these cases, direct pancreatic function testing such as secretin-cholecystokinin stimulation and endoscopy may be used to achieve highly accurate results.10 However, these tests have significant drawbacks in terms of cost and invasiveness, which limit their clinical use.
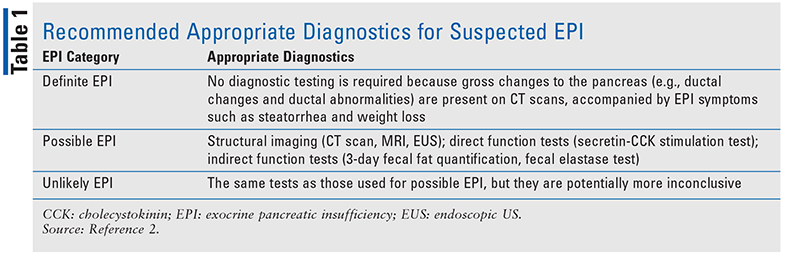
Indirect and noninvasive tests have grown more common in the past 20 years.11 Of the various procedures, fecal, breath, and blood tests are less expensive and easier to use. Fecal fat quantification is considered a gold standard for diagnosis of fat malabsorption.12 However, this test requires 3-day collection of the patient's feces while the patient adheres to a strict diet of less than 100 g of fat per day. Because this test is difficult for both the patient and laboratory personnel, it is rarely used in clinical settings. Fecal elastase is a pancreatic enzyme, and its concentration in the feces is measured via enzyme-linked immunosorbent assay. Fecal elastase tests provide higher sensitivity than direct-method tests. Fecal elastase has good sensitivity for moderate EPI (75%) and high sensitivity for severe EPI (95%).13 The main benefits of fecal elastase testing are that it is noninvasive, less time-consuming, and unaffected by pancreatic enzyme replacement therapy (PERT; discussed below).14 For a summary of how to determine whether a test is needed and which one should be used, see TABLE 1; the 2015 Australasian Guidelines for the Management of Pancreatic Exocrine Insufficiency (discussed below) divides suspected EPI into three categories and recommends appropriate diagnostics for each category.2
Guidelines
The most relevant and current guidelines addressing EPI are the Pancreatic Enzymes Clinical Care Guidelines, published by the Cystic Fibrosis Foundation, and the Australasian Guidelines for the Management of Pancreatic Exocrine Insufficiency, published by the Australasian Pancreatic Club.2,15 The latter guidelines were published in October 2015, and the Cystic Fibrosis Foundation guidelines were revised from the original 1995 version to include new information based on more recent literature on PERT.16,17 Both guidelines recommend PERT for treatment of EPI and make similar dosing recommendations. The consensus recommendation for dosing is that the PERT dose be based on either the patient's weight or the grams of fat ingested. Although patients may prefer the convenience of weight-based dosing, dosing based on ingested fat more effectively imitates the normal pancreatic response to meals. Both guidelines also recommend initiating the medication at a lower dose and titrating up as necessary. It is generally acknowledged that dosing above a certain range does not result in improved clinical condition and that patients who require doses in this range should be evaluated for lack of response to PERT.15 Although both guidelines agree on the basics of PERT, the Australasian guidelines are much more comprehensive in discussing the etiology, pathophysiology, and nonpharmacologic and pharmacologic treatments for EPI. The Australasian guidelines also expand on treatment recommendations for specific cases of EPI, such as acute pancreatitis, chronic pancreatitis, and the use of PERT after bowel restriction, gastric surgery, or pancreatectomy. Finally, the Australasian guidelines discuss the use of PERT in the presence of specific conditions, including irritable bowel syndrome, diabetes mellitus, and celiac disease. These guidelines also discuss the two pancreatic-enzyme agents available in Australia and New Zealand—Creon and Panzytrat—whereas the Cystic Fibrosis Foundation guidelines provide a more general discussion of pancreatic-enzyme agents, with no mention of specifics or differences in how each agent works. The Australasian guidelines are far more comprehensive overall and can supply healthcare providers with recommendations for patient-specific parameters and comorbidities.
Nonpharmacologic Treatment
Dietary restriction and smoking cessation should be implemented in all patients with EPI because these interventions have been shown to be the most effective nonpharmacologic methods for treating EPI symptoms. In the past, a low-fat diet (<20 g of fat daily) was recommended in order to prevent steatorrhea, a common symptom of EPI. However, the lowfat diet is no longer recommended because it often causes deficiencies of lipid-soluble vitamins that are already malabsorbed in EPI patients and is lower overall in total energy.12 Patients should be advised to follow a normal-fat diet, with careful attention to symptoms such as steatorrhea on a diet with more fat (>30% total energy from fat). Recent research has determined that patients do not require a low-fat diet while receiving adequate PERT; in fact, they should be encouraged to add high-fat snacks (e.g., nuts, cheese and crackers, cake, biscuits) and full-fat dairy products to their diet to increase energy intake. Adequate enzyme therapy, when given along with a high-fat diet, actually enables better absorption and digestion of fat compared with enzyme therapy given with a low-fat diet.2 It should be recommended that patients complaining of steatorrhea and other adverse effects consume small, frequent meals instead of large, high-calorie meals. Although meal size, meal frequency, and nutrient supplementation vary from patient to patient, consuming small, frequent meals throughout the day will result in a smaller chance of malabsorption and steatorrhea compared with consuming a few large, high-calorie meals. However, if a patient is already tolerating his or her meals well, it is not necessary to alter the meal frequency.2 Patients should be referred to a dietitian to manage nutrient intake because maintaining an appropriate diet is important for all EPI patients.
In addition to dietary intervention, smoking cessation should be encouraged in EPI patients. Smoking is a risk factor for pancreatic cancer, acute pancreatitis, and chronic pancreatitis, which are already concerns for patients with EPI. Continued smoking also contributes to the progression of chronic pancreatitis through earlier development of calcifications in the pancreas. Finally, patients should be directed to abstain from alcohol, especially if they have alcohol-related EPI. Consumption of alcohol will cause faster deterioration of exocrine pancreatic function and should therefore be avoided.12
Pharmacologic Treatment
The main pharmacologic treatment for EPI is PERT. PERT is given in conjunction with food to improve fat absorption, and it can be used to manage symptoms of EPI. However, because PERT does not treat the underlying cause of the disease, it is intended to be a long-term therapy. With PERT, oral ingestion of the missing enzymes counters the decrease in production or delivery of pancreatic enzymes to the small intestine and helps digest ingested foods. Replacement pancreatic enzymes are extracted from porcine pancreas and are typically administered in enteric-coated or extended-release oral formulations.12 The pancreatic enzymes catalyze the breakdown of dietary fats, proteins, and sugars, mimicking the body's digestive enzymes in order to help prevent EPI symptoms such as malnutrition and weight loss.18
PERT is indicated in patients with symptoms of EPI, such as steatorrhea or weight loss, but it has also been shown to have benefits in patients who are asymptomatic.12 Patients who are allergic to pork products should be cautioned before starting PERT. PERT carries a risk of viral transmission because the enzymes are derived from pigs and could harbor viruses.18
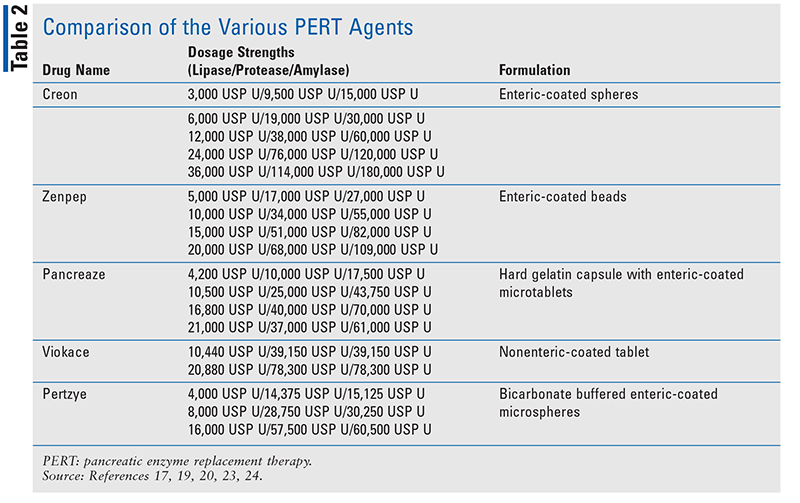
The term pancrelipase, which describes the group of enzymes in the formulation, consists of a mixture of amylases, lipases, and proteases. There are currently five commercially available oral formulations of pancrelipase (Creon, Zenpep, Pancreaze, Viokace, and Pertzye), which differ in terms of such properties as particle size, dosing range, and pharmacokinetics. Because of their different formulations and strengths, PERT agents are not interchangeable; the prescribed drug should be started at the lowest possible dose and titrated up as appropriate for the patient's individual efficacy and safety needs. The prescriber should wait a few days between dose adjustments to determine whether further titration is needed.
Creon: Creon (AbbVie Inc.) is a delayed-release capsule that was FDA approved in 2009. It is indicated to treat EPI due to cystic fibrosis or other conditions.18 Creon capsules contain enteric-coated spheres of the medication, and the capsule is designed to release the enzymes at a pH of 5.5 or greater. Patients who are unable to swallow capsules may open them and mix the contents into a soft food, such as applesauce. An open-label clinical trial that investigated the efficacy of Creon found a mean body-weight increase of 2.7 ± 3.4 kg (P <.0001) and saw improvements in abdominal pain, flatulence, stool frequency, and consistency.19
Zenpep: Zenpep (Allergan USA, Inc.), a delayed-release capsule, was approved in 2009 for the treatment of EPI.20 The capsules are filled with enteric-coated beads containing the enzymes and should be taken orally without being broken or chewed.21 If a patient cannot swallow the capsules, the contents may be mixed with a soft food, such as applesauce. In a randomized, double-blind, controlled, crossover trial, Zenpep was shown to have a higher coefficient of fat absorption compared with placebo (88.3% vs. 62.8%, respectively; P <.001) and coefficient of nitrogen absorption (87.2% vs. 65.7%, respectively; P <.001).21
Pancreaze: Pancreaze (McNeil Pediatrics) is a delayed-release capsule that was FDA approved in 2010.22 It is indicated to treat EPI due to cystic fibrosis or other conditions. Pancreaze is a hard gelatin capsule that contains enteric-coated microtablets; the capsules should be taken whole, without crushing or chewing.22 If a patient cannot swallow the capsules, the microtablets may be mixed into a slightly acidic soft food, such as applesauce. A randomized, placebo-controlled trial examining the efficacy of Pancreaze showed better fat absorption measured by the mean and standard deviation change of the coefficient of fat absorption between Pancreaze (–1.5 ± 5.88%; P <.001) and placebo (–34.1 ± 23.03%).23 Protein absorption was also shown to improve in patients taking Pancreaze versus placebo.23
Viokace: Viokace (Aptalis Pharma US, Inc.), a tablet approved in 2012, is the only drug that is indicated for EPI in combination with a proton pump inhibitor (PPI), such as omeprazole.24 Unlike other PERT agents, Viokace is indicated only for the treatment of EPI due to chronic pancreatitis or pancreatectomy. Because the drug is not enteric-coated, it must be administered in combination with a PPI to prevent degradation in the stomach. The medication should be taken whole, with food. In a randomized, double-blind, placebo-controlled, parallel-group study comparing Viokace with placebo, Viokace was shown to increase fat and nitrogen absorption significantly more than placebo.24
Pertzye: Pertzye (Digestive Care, Inc.) is a delayed-release capsule that was FDA approved in 2012 for the treatment of EPI.25 The capsules contain bicarbonate-buffered, enteric-coated microspheres of various sizes. Pertzye may be taken whole or the contents may be mixed with a soft food; it also may be administered via the feeding port of a gastrostomy tube by combining the contents with a soft food, such as applesauce.25 In a randomized, doubleblind, placebo-controlled, crossover trial comparing Pertzye with placebo, the mean coefficient of fat absorption with Pertzye was 82.5% versus 46.3% for placebo (P <.001).26
Relizorb: Relizorb (Alcresta Inc.), which is not a PERT drug, is also indicated for the treatment of EPI. It is not an oral therapy but rather an immobilized lipase cartridge that is used to hydrolyze fats in enteral formula.27 Relizorb connects to the patient's existing enteral feeding tube and digests fats ex vivo, allowing the delivery of absorbable fatty acids and glycerides. Relizorb is used for enteral feeding only; it should not be connected to any IV or parenteral lines, and medications should not be administered through the cartridge. It is designed for one-time use and therefore should be replaced after it is used. In a clinical study that examined efficacy of fat absorption with Relizorb, plasma levels of the omega-3 fatty acids docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) significantly increased for Relizorb versus placebo. In the Absorption and Safety with Sustained Use of Relizorb Evaluation study, which prospectively investigated patients receiving PERT who had low levels of DHA and EPA (indicating malnutrition), Relizorb was shown to improve DHA and EPA levels compared with PERT given alone.28
Dosing and Monitoring Parameters: Dosing recommendations for all PERT agents follow the Cystic Fibrosis Foundation guidelines, which suggest that therapy be initiated at the lowest possible dose and increased to achieve optimal results.15 The main active ingredient in PERT that is measured when determining a patient's dosage is lipase, which is dosed either by grams of fat ingested or by weight.15 Dosing by grams of fat ingested is more accurate and more closely mimics the normal physiologic process of a healthy individual's digestive enzymes, but dosing by weight is simpler and is used more often in children and older adults. Adults typically require 500 to 4,000 lipase units per gram of fat ingested, or 500 to 2,000 lipase units per weight in kg per meal, and 250 to 1,250 lipase units per weight in kg per snack.15 Given the need to dose by either weight or food ingestion, the choice of PERT agent is typically determined by lipase counts of the lowest dose available for a particular brand, with uptitration afterward to prevent malnutrition. Doses exceeding 6,000 lipase units per kg per meal have been associated with fibrosing colonopathy; therefore, doses should be planned to be less than 2,500 lipase units per kg per meal.18 There have been no reported cases of overdose, but, regardless, prescribers should still adhere to the guidelines in order to prevent adverse effects associated with high doses of PERT, such as fibrosing colonopathy. Fibrosing colonopathy can lead to abdominal pain, distention, vomiting, and constipation.29 No dose adjustment is necessary for hepatic or renal impairment, but patients should be monitored closely for signs of toxicity and side effects.
Current guidelines do not recommend dose adjustments or indicate when patients should follow up with prescribers, but in general patients should follow up shortly after initiating PERT or changing the dosage. Prescribers should wait a couple of days after altering the dosage to monitor the efficacy of the current regimen. Weight is an important monitoring parameter because increased fat and nutrient absorption can cause the patient to gain weight. Patients should also be monitored for abdominal symptoms to minimize adverse events. In children receiving PERT, growth should be monitored in order to determine the drug's efficacy.30
Adverse Effects: Because all of the PERT agents have the same active ingredient—i.e., pancrelipase—their adverse effects are the same. Common adverse effects are headache, neck pain, nasal congestion, and abdominal pain.30 Nausea, blurred vision, constipation, and urticaria are rare side effects. More severe adverse effects include fibrosing colonopathy, irritation in the mouth, hyperuricemia, and allergic reactions.18 Starting with a lower dose of enzymes could help prevent fibrosing colonopathy; ensuring that no drug remains in the mouth could help prevent oral irritation; and exercising caution when using PERT in patients with gout or renal impairment could help prevent hyperuricemia.18 Pancrelipase enzymes have no contraindications and do not have any drug-drug interactions. The drug formulations generally have food interactions because they are delayed-release, so the drug should not be administered with alkaline foods such as avocado, onions, and tofu, which would decrease efficacy.
Special Populations: Infants with EPI typically require lower doses of enzymes; the Cystic Fibrosis Foundation guidelines recommend 450 to 900 lipase units per gram of fat, or 2,000 to 4,000 lipase units per 120 mL of formula or when breastfeeding.15 This is because infants generally ingest more fat per kg of body weight than adults do.
PERT agents have not been included in animalreproduction studies and are Pregnancy Category C. It is not known whether pancrelipase can cause fetal harm when administered to a pregnant woman.18 The risk versus benefit to the individual patient should be considered before a PERT is prescribed to a pregnant woman with EPI. Given that the oral absorption of pancrelipase is low, it should generally be safe for pregnant women.
It is not known whether pancrelipase is secreted in breast milk; therefore, as with prescribing in pregnancy, the patient's individual risks and benefits of starting the drug should be considered before it is prescribed.18 Considering that the oral absorption of pancrelipase is low, it should generally be safe for breastfeeding women.
Compliance and Complications of Inadequate Treatment: It is essential for pharmacists to provide EPI patients with information about PERT and the importance of adherence and correct dosing. PERT works optimally when administered with the foods that need to be digested, and many variables, such as food-intake quantity, fat percentage, and digestive factors, make it critical to determine the best dosage for the individual patient. Not only is the correct dosage necessary, but the patient must understand when to take the medication and the importance of taking it to remedy his or her EPI symptoms. If a patient is not receiving an adequate dosage of pancrelipase or is not adherent to the medication regimen, complications or worsening of the disease state could ensue. This may be seen subjectively via a patient's complaints of fatigue, abdominal pain, foul-smelling stools, or diarrhea, but it may also be seen objectively via weight measurements and laboratory testing to determine whether malnutrition has developed. The biggest concern of inadequate replacement of pancreatic enzymes is that the patient will not receive the necessary nutrients and vitamins from food intake, leading to weight loss and malnutrition.16
Comparisons: Because all PERT agents contain the same active ingredients (lipase, protease, and amylase), differences between them in adverse effects and efficacy are minimal. In one crossover trial that investigated Zenpep and Creon, both drugs had similar efficacy and safety.31 The biggest difference between the various drugs is the dosage of the enzymes, and this typically determines which PERT agent a patient is prescribed. TABLE 2 compares the different PERT drugs' formulations and dosage strengths.
Conclusion
EPI occurs most commonly in patients with chronic pancreatitis and cystic fibrosis, and it can lead to severe problems, such as malnutrition and abdominal pain, if left untreated. There is currently no cure for EPI, but diet supplementation in addition to PERT may be used to manage this condition and prevent it from being symptomatic. All of the various pancrelipase agents aim to replace the patient's missing digestive enzymes, and taking the medication helps the patient digest foods and absorb essential nutrients and vitamins. Pharmacists play a key role in providing patients with information about PERT and the importance of adherence so that they understand why they are taking the medication, how it works, and how to correctly dose and take the medication with meals. Because most patients will need PERT on a lifelong basis, their understanding of the prescribed drug can lead to better compliance and improved outcomes.
REFERENCES
- Singh VK, Haupt ME, Geller DE, et al. Less common etiologies of exocrine pancreatic insufficiency. World J Gastroenterol. 2017;23(39):7059-7076.
- Australasian Pancreatic Club. Australasian Guidelines for the Management of Pancreatic Exocrine Insufficiency. October 2015.
- Domínguez-Muñoz J. Pancreatic enzyme therapy for pancreatic exocrine insufficiency. Curr Gastroenterol Rep. 2007;9(2):116-122.
- Ahmed N, Corey M, Forstner G, et al. Molecular consequences of cystic fibrosis transmembrane regulator (CFTR) gene mutations in the exocrine pancreas. Gut. 2003;52(8):1159-1164.
- Lévy P, Domínguez-Muñoz E, Imrie C, et al. Epidemiology of chronic pancreatitis: burden of the disease and consequences. United European Gastroenterol J. 2014;2(5):345-354.
- Keller J, Layer P. Human pancreatic exocrine response to nutrients in health and disease. Gut. 2005;54(suppl 6):vi1-vi28.
- DiMagno EP, Go VL, Summerskill WH. Relations between pancreatic enzyme outputs and malabsorption in severe pancreatic insufficiency. N Engl J Med. 1973;288(16):813-815.
- Ferrone M, Raimondo M, Scolapio JS. Pancreatic enzyme pharmacotherapy. Pharmacotherapy. 2007;27(6):910-920.
- Sikkens EC, Cahen DL, Koch AD, et al. The prevalence of fat-soluble vitamin deficiencies and a decreased bone mass in patients with chronic pancreatitis. Pancreatology. 2013;13(3):238-242.
- Nikfarjam M, Wilson JS, Smith RC. Diagnosis and management of pancreatic exocrine insufficiency. Med J Aust. 2017;207(4):161165.
- Keller J, Layer P. Diagnosis of pancreatic exocrine insufficiency in chronic pancreatitis. Pancreapedia. https://pancreapedia.org/sites/ default/files/DOI%20V3.%20Formatted%20Keller%20and%20 Layer%209-04-15.pdf. Accessed November 8, 2019.
- Lindkvist B. Diagnosis and treatment of pancreatic exocrine insufficiency. World J Gastroenterol. 2013;19(42):7258-7266.
- Chronic Pancreatitis German Society of Digestive and Metabolic Diseases (DGVS), Hoffmeister A, Mayerle J, et al. S3 consensus guidelines on definition, etiology, diagnosis and medical, endoscopic and surgical management of chronic pancreatitis German Society of Digestive and Metabolic Diseases (DGVS) [translated from German]. Z Gastroenterol. 2012;50(11):1176-1224.
- Domínguez-Muñoz JE, Hardt PD, Lerch MM, Löhr MJ. Potential for screening for pancreatic exocrine insufficiency using the fecal elastase-1 test. Dig Dis Sci. 2017;62(5):1119-1130.
- Schwarzenberg SJ, Dorsey J. Pancreatic enzymes clinical care guidelines. Cystic Fibrosis Foundation. www.cff.org/Care/ClinicalCare-Guidelines/Nutrition-and-GI-Clinical-Care-Guidelines/PancreaticEnzymes-Clinical-Care-Guidelines. Accessed September 17, 2019.
- Borowitz DS, Grand RJ, Durie PR. Use of pancreatic enzyme supplements for patients with cystic fibrosis in the context of fibrosing colonopathy. Consensus Committee. J Pediatr. 1995;127(5):681-684.
- Lahiri T, Hempstead SE, Brady C, et al. Clinical practice guidelines from the Cystic Fibrosis Foundation for preschoolers with cystic fibrosis. Pediatrics. 2016;137(4):e20151784.
- Creon (pancrelipase) package insert. North Chicago, IL: AbbVie Inc; March 2015.
- Gubergrits N, Malecka-Panas E, Lehman GA, et al. A 6-month, open-label clinical trial of pancrelipase delayed-release capsules (Creon) in patients with exocrine pancreatic insufficiency due to chronic pancreatitis or pancreatic surgery. Aliment Pharmacol Ther. 2011;33:1152-1161.
- Zenpep (pancrelipase) package insert. Irvine, CA: Allergan USA, Inc; March 2017.
- Wooldridge JL, Heubi JE, Amaro-Galvez R, et al. EUR-1008 pancreatic enzyme replacement is safe and effective in patients with cystic fibrosis and pancreatic insufficiency. J Cyst Fibros. 2009;8(6): 405-417.
- Pancreaze (pancrelipase) package insert. Titusville, NJ: McNeil Pediatrics; April 2010.
- Trapnell BC, Strausbaugh SD, Woo MS, et al. Efficacy and safety of PANCREAZE® for treatment of exocrine pancreatic insufficiency due to cystic fibrosis. J Cyst Fibros. 2011;10(5):350-356.
- Viokace (pancrelipase) package insert. Birmingham, AL: Aptalis Pharma US, Inc; March 2012.
- Pertzye (pancrelipase) package insert. Bethlehem, PA: Digestive Care, Inc; July 2017.
- Konstan MW, Accurso FJ, Nasr SZ, et al. Efficacy and safety of a unique enteric-coated bicarbonate-buffered pancreatic enzyme replacement therapy in children and adults with cystic fibrosis. Clin Investig (Lond). 2013;3(8):723-729.
- Relizorb (immobilized lipase) package insert. Newton, MA: Alcresta Inc; January 2018.
- Stevens J, Wyatt C, Brown P, et al. Absorption and Safety with Sustained Use of RELiZORB Evaluation (ASSURE) study in patients with cystic fibrosis receiving enteral feeding. J Pediatr Gastroenterol Nutr. 2018;67(4):527-532.
- Smyth RL. Fibrosing colonopathy in cystic fibrosis. Arch Dis Child. 1996;74(5):464-468.
- Pancrelipase: drug information. UpToDate. www.uptodate.com. Accessed August 28, 2019.
- Taylor CJ, Thieroff-Ekerdt R, Shiff S, et al. Comparison of two pancreatic enzyme products for exocrine insufficiency in patients with cystic fibrosis. J Cyst Fibros. 2016;15(5):675-680.