Expanding Access to Naloxone: Role of the Pharmacist
 RELEASE DATE:
RELEASE DATE:
October 1, 2016
EXPIRATION DATE:
October 31, 2018
FACULTY:
Gerald Gianutsos, PhD, JD
Associate Professor of Pharmacology
University of Connecticut School of Pharmacy
Storrs, Connecticut
DISCLOSURE STATEMENTS:
Dr. Gianutsos has no actual or potential conflict of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT:
Pharmacy
 Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-16-089-H03-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE:
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@jobson.com
DISCLAIMER:
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients' conditions and possible contraindications or dangers in use, review of any applicable manufacturer's product information, and comparison with recommendations of other authorities.
GOAL:
To improve the pharmacist's understanding of the role of naloxone in preventing opiate overdose and the evolution of the regulations overseeing naloxone distribution.
OBJECTIVES:
After completing this activity, the participant should be able to:
- Describe the scope of the problem of opiate overdose.
- Explain the effect of naloxone as a rescue drug.
- Identify how federal and state laws regulate the distribution of naloxone.
- Discuss the expansion of permissible naloxone use and availability.
- Review the role of the pharmacist as a provider of naloxone.
- Recognize the pros and cons of increasing naloxone access.
ABSTRACT: The last few years have witnessed an explosive increase in deaths due to drug overdose, largely fueled by deaths from opiates, especially heroin and prescription pain relievers. A number of strategies have been proposed to reduce overdose by limiting the availability of the abused drugs through changes in prescribing practices and law enforcement efforts. However, another approach has been to increase the availability of the opiate antagonist naloxone, in order to prevent the potential lethality resulting from an overdose. Initially, the expansion had focused on emergency personnel and community organizations, but an increasing number of states have implemented programs promoting distribution of naloxone by pharmacists to addicts and third parties as well as Good Samaritan laws providing civil and criminal immunity for prescribing, dispensing, and use. It is important for pharmacists to understand the changes in state regulations concerning naloxone distribution in order to ensure safe usage.
Nearly half a million people in the United States have died from a drug overdose between 2000 and 2014, according to data from the CDC.1 The rate of drug overdose deaths increased by 137% during this period. Even more disturbing, more people died from drug overdoses in the U.S. in 2014 than during any previous year on record, and the number of overdose deaths in 2014 was almost one-and-a-half times higher than the number of deaths due to motor vehicle accidents.1 Fueling this alarming trend in overdose-related deaths are deaths due to opiates; the rate of opioid overdoses has tripled since 2000. In 2014, opioids were involved in 61% (totaling more than 28,000) of all drug overdose deaths.1
The CDC notes that there are two distinct but interrelated trends in the opioid overdose epidemic: a 15-year increase in overdose deaths involving prescription opioid pain relievers and a more recent surge in overdose deaths due to illicit opioids, driven largely by heroin.1 Heroin overdoses more than tripled in just 4 years.
Faced with this national epidemic, a number of strategies have been considered by regulators and other interested parties to reduce the supply of opiate products available to addicts or potential addicts. These strategies are listed in TABLE 1.2-4
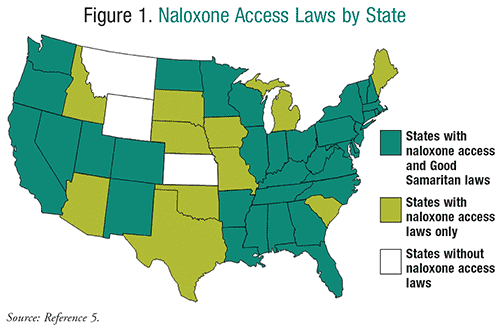
Another strategy, which is not aimed at reducing the supply of opiates but rather to reduce the risk of death following an opiate overdose, involves the expansion of access to the opiate antagonist naloxone. Beginning with New Mexico in 2001, 47 states plus the District of Columbia have enacted regulations that permit some form of expanded naloxone access as of June 2016 (the exceptions being Kansas, Montana, and Wyoming).5 This manuscript will review the problem of opiate overdose and the use of naloxone as a rescue drug for such overdose and will discuss the shifting regulatory landscape as it impacts pharmacists and other personnel.
The Problem
Increased deaths due to opioids were noted in virtually every drug category in 2014—heroin deaths increased by 26% while deaths due to synthetic opioid analgesics increased by 80%. An exception to this trend is deaths due to methadone, which remained relatively stable.6
The drug overdose deaths involve natural and semisynthetic opioids, especially the most commonly prescribed opioid analgesics, oxycodone and hydrocodone.6 Deaths from this class of opioids declined in 2012 compared with 2011, and held steady in 2013, suggesting a leveling off of the problem, but 2014 saw a 9% increase.1 In addition, there has been a sharp increase in deaths due to heroin overdose, coinciding with a general increase in heroin use nationwide.7 Evidence suggests that the strongest risk factor for heroin initiation and use is previous misuse of prescription opioids, especially among persons who report recent dependence or abuse.7
It will be no surprise to pharmacists that opioid prescribing has increased; 76 million opiate prescriptions were issued in 1991 and rose to 207 million in 2013.8 During this period, hydrocodone became the leading prescribed medication and oxycodone became the most retailed prescription opioid by weight.9 Oxycodone (OxyContin) was approved in 1995, and sales increased 69-fold from $44.8 million in 1996 to $3.1 billion by 2010.9 The increase in prescribing is attributed to a number of factors including an increased focus on inadequate pain management by physicians; new pain management standards; aggressive marketing practices by pharmaceutical companies; and an increasing emphasis in medicine on metrics of patient satisfaction,8 as well as the more familiar problems of diversion, theft, and improper prescribing.
It has been postulated that efforts to curb opioid prescribing, resulting in restricted prescription opioid access, have been a major factor in fueling heroin use and overdose. However, several lines of evidence dispute this. One recent analysis of drug overdose deaths in 28 states occurring between 2010 and 2012 found that increases in heroin death rates were not associated with reductions in prescription opiate death rates; instead, increases in heroin overdose death rates were associated with increases in prescription opioid overdose death rates.10 Another recent analysis suggests that the shift toward heroin use among some nonmedical users of prescription opioids was already occurring before the emphasis on curbing prescription opioid abuse by measures including the rescheduling of hydrocodone combination products and introduction of abuse-deterrent formulations.11 Consequently, it is likely that there are multiple and possibly interacting factors contributing to the changing patterns of heroin use and overdose deaths.10
Major drivers in the upward trend in heroin overdose include the increased availability of heroin, the high purity of street heroin, and its relatively low cost compared with diverted prescription opioids.10,12 The costs of heroin have dropped as the supply has increased; the amount of heroin seized at the border with Mexico quadrupled in 2013 compared with the early 2000s.13
In addition to an increased supply of heroin, the dramatic increase in deaths involving synthetic opioids coincided with law enforcement reports of increased availability of illicitly manufactured fentanyl. Fentanyl-like drugs are traditionally mixed into or sold as heroin, often to unsuspecting heroin users. The DEA reports that large volumes of counterfeit prescription drugs are making their way into the U.S. market, many of which contain lethal amounts of fentanyl and fentanyl-related compounds.6 Laboratory analysis of seized samples indicated that the tablets contained a variety of fentanyl doses; one sample contained between 0.6 and 6.9 mg of fentanyl per tablet (2 mg of fentanyl may be lethal for nontolerant opioid users).6 Laboratories in China mass produce fentanyl and market them to drug trafficking groups in Mexico, Canada, and the U.S. Nationwide, law enforcement officials reported an increase in fentanyl availability, seizures, and known overdose deaths higher than at any other time since the drug was first developed in 1959. Over 700 fentanyl-related deaths were reported in the U.S. between late 2013 and 2014; the CDC reported that deaths from all synthetic opioids increased 79% between 2013 and 2014 with a substantial portion of the increase related to illicit fentanyl (including the suspected cause of death of the singer Prince).6
Changing Demographics: Along with an increase in numbers, the demographics of heroin overdose have also been transformed.1,9,12 In the past decade, the gaps in heroin use rates between groups such as men and women, persons with low and higher incomes, and Medicaid and private insurance beneficiaries have narrowed. The largest increases in heroin use occurred in demographic groups that historically have had lower rates of heroin use, doubling among women and more than doubling among non-Hispanic whites, while declining among African Americans.12,13
Young adults have been affected more than other age groups. Between 2002 and 2013, the use increased 109% in the 18- to 25-year-old group compared with a 58% increase in those aged ≥26 years.12,13 The highest rates of heroin overdose deaths in the U.S. in 2000 occurred in black Americans aged 45 to 64; today the highest rates are in Caucasians aged 18 to 44.12,13 Similarly, large metropolitan areas have historically had the highest rates of drug overdoses, but today more deaths are occurring in rural and suburban areas,14 where access to emergency medical services (EMS) may be slower. In 2014, the five states with the highest rates of drug overdose deaths were West Virginia (35.5 deaths per 100,000), New Mexico (27.3), New Hampshire (26.2), Kentucky (24.7), and Ohio (24.6).1 States that have seen statistically significant increases in the rate of drug overdose deaths between 2013 and 2014 included Alabama, Georgia, Illinois, Indiana, Maine, Maryland, Massachusetts, Michigan, New Hampshire, New Mexico, North Dakota, Ohio, Pennsylvania, and Virginia.1,14 Most heroin users also abuse other drugs. Nine in 10 people who use heroin use it with at least one other drug, often with at many as three other drugs.13
Opioid Overdose
An overdose of an opiate can result in respiratory depression, limiting adequate oxygenation of blood. This reduces oxygen availability to the brain and heart, leading to unresponsiveness, anoxia, cyanosis, and possible death.8 The respiratory depression, which is reversible until death occurs, can take 1 to 3 hours and can be reversed with the pharmacologic antidote naloxone.8 Many factors can contribute to an increased risk of an overdose.3 Street drugs can vary in potency and purity; an increase in mortality is positively correlated with spikes and fluctuations in purity.3 Similarly, street drug users cannot reliably determine the identity of what they are consuming due to adulteration with other substances including toxic products (as with fentanyl).15 Time away from drugs, such as time spent in prison or rehabilitation, can also lower an individual’s tolerance and lead to the inadvertent use of an inappropriate dose.3
Naloxone
In light of the seriousness of the problem of opiate overdose, strategies in addition to reducing supply have been proposed. Among them is to make the opiate rescue drug, naloxone, more readily available.
Pharmacology: Naloxone is an N-allyl derivative of oxymorphone and thebaine.16 It was first synthesized in 1960 and introduced into clinical practice in 1967 as a pure competitive opiate antagonist.17 Earlier drugs such as nalorphine and levallorphan were partial agonists which, unlike naloxone, produced some respiratory depression.16 Naloxone became the preferred drug for reversing opioid toxicity due to the lack of any agonist activity, resulting in minimal adverse effects when administered in the absence of an opioid. In non-opiate-dependent individuals, the adverse effects of naloxone include pain, flushing, and mild cardiovascular changes (e.g., hypotension, hypertension, tachycardia, irregular heartbeat).16
There are three families of receptors (mu, delta, and kappa) for endogenous, natural, and synthetic opiates, which bind with varying affinity to these receptors.18 Naloxone possesses high affinity for the mu opiate receptor.16 Opiate agonists, such as morphine and oxycodone, also bind with high affinity to the mu receptor, which is responsible not only for their analgesic effect, but also the abuse liability and the potentially lethal respiratory depression that is observed in overdose.19 Consequently, by competing with this binding site, naloxone can reverse the respiratory depression but can also precipitate a withdrawal response in a dependent individual.
Pharmacokinetics: Naloxone is well absorbed when given via parenteral routes, including IV and IM/SC injection or when nebulized.16 Once absorbed, naloxone readily crosses the blood-brain barrier because of its high lipophilicity and achieves a substantially higher brain-serum ratio than that of morphine.16 The onset of action of naloxone in adults is <2 minutes when administered via IV, and its apparent duration of action is on the order of 20 to 90 minutes; significantly, this is a much shorter duration than that of many opioid agonists.19
Naloxone has a very low oral bioavailability since it undergoes extensive first-pass hepatic metabolism; therefore, oral administration is not effective in reversing opioid intoxication.16 The primary hepatic metabolite is the inactive naloxone-3-glucuronide. Bioavailability of naloxone by the intranasal and sublingual routes is also relatively low.16
Dosing: The standard recommended dose of naloxone is 0.4 mg IV, which originated from the anesthesiology literature of the 1960s when excessive postoperative opioid anesthesia was reversed with naloxone in non-dependent surgical patients.16 Current recommendations for reversing opioid intoxication call for using an initial dose ranging from 0.04 up to 2 mg IV.16
Overall, dosing of naloxone in emergency situations is empirical since most of the critical variables that would determine the effective dose will be unknown to the rescuer.19 These include the amount of the opioid the patient has taken or received, the relative affinity of naloxone for the mu opioid receptor and the opioid that is to be displaced, the weight of the patient, and the degree of penetrance of the opioid analgesic into the central nervous system (CNS).19 Additionally, absorption of opiates may be erratic depending on the route of administration and the opiate-induced decrease in gastro-intestinal motility may prolong oral drug absorption.19
The general rule for adults is to use an initial naloxone dose of 0.4 mg (IV), and, if there is no response, the dose should be increased every 2 minutes to a maximum of 15 mg. If the respiratory depression does not respond to 15 mg, the assumption that the respiratory depression is due to an opiate overdose should be reconsidered.19 The effect of a single exposure to naloxone is usually transient due to its short duration of action compared to many opioid agonists.19 Naloxone-precipitated opiate withdrawal is usually brief and, while unpleasant, is not ordinarily life-threatening; low doses may restore breathing without provoking withdrawal.19,20
The majority of opioids have durations of action longer than that of naloxone, necessitating additional medical attention after respiration is restored, raising fears that, once resuscitated, patients will refuse further medical care and relapse into fatal respiratory complications. However, some reports have concluded that these fears may be unwarranted.20,21
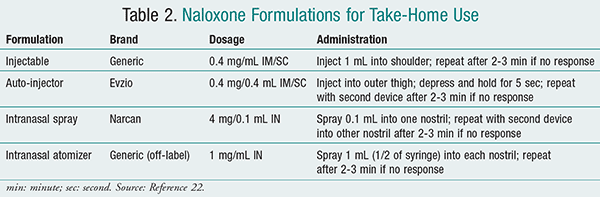
Dosage Forms: Traditional naloxone hydrochloride is available as a solution for IV injection containing 0.4 mg/mL, but alternative dosage forms have become available (TABLE 2).22 One is an auto-injector (Evzio) for IM or SC use; the prefilled single-dose injector contains 0.4 mg/0.4 mL naloxone hydrochloride.23 Once activated, the auto-injector automatically inserts the needle IM or SC and delivers the 0.4 mg naloxone hydrochloride dose. The needle then retracts fully into its housing, the black base locks in place, and a red indicator appears in the viewing window. Electronic visual and audible instructions signal that the device has delivered the intended dose of naloxone and instructs the user to seek emergency medical attention.23
The FDA recently approved Narcan (naloxone hydrochloride) Nasal Spray following a fast-track designation and priority review.24 One spray delivers a dose of 4 mg of naloxone into one nostril. The nasal spray provides advantages over IV administration, including ease of use (little training is required and there is no searching for a vein or other injection site); eliminates the risk of an accidental needlestick; and prevents the reuse of a potentially contaminated needle. In clinical trials conducted to support the approval of the naloxone-containing nasal spray, the intranasal delivery of the drug achieved approximately the same or higher levels of naloxone as a single dose of a naloxone IM injection, and achieved these levels in approximately the same time frame.24 However, there are reports suggesting that in the experience of emergency medical technicians, the mean time between naloxone administration and a clinical response was longer for intranasal administration, that its effectiveness may be lower, and that nasal administration required multiple administrations compared with injections.21
Naloxone Programs
Since naloxone effectively reverses an opiate overdose, there have been a number of calls for making naloxone more readily available. Experts have proposed expanding access to naloxone since at least the early 1990s, and programs distributing naloxone to heroin users have operated in Germany, Italy, and the United Kingdom for the past two decades.25,26 In the U.S., it is believed that the first actual provision of take-home naloxone occurred in Chicago in 1996.26 Following the death of one of its founding members in May 1996, the Chicago Recovery Alliance began providing training and dispensing naloxone kits as early as autumn 1996. Because of the demand arising from a fourfold increase in drug-induced deaths reported by the Medical Examiner’s Office from 1996 to 2000, naloxone distribution was converted into a formal program with a standardized training curriculum in 2001.26 The first state program was enacted that same year in New Mexico (discussed later).
Today, it is common for states to provide naloxone to first responders, in particular EMS. However, EMS providers follow protocols for medical care and administration of medications that may be regionally specific.21 The primary symptoms that prompt naloxone admin-istration by EMS providers are breathing difficulties and lack of responsiveness.21
As previously mentioned, 47 states now authorize expanded access to naloxone (FIGURE 1).5 Early expansion beyond paramedical per- sonnel to include other first responders (police and firefighters) has become more commonplace.16,21 Further expansion to high-risk sites (e.g., community-based programs, drug treatment facilities, prisons, syringe exchanges, Veterans Administration [VA] healthcare systems, primary care clinics, and schools) has also occurred.16,25,27 Most of these laws were enacted within the past 5 years and, in addition to promoting the use of naloxone, include training and education on recognizing and preventing overdoses.28 The number of laypersons receiving naloxone kits from organizations increased by 187% between 2010 and 2014, and the number of reported reversals increased by 160% over that period; some 30 states have at least one organization providing naloxone.27 More recently, naloxone has been provided directly to addicts and family members through pharmacies. In some states, pharmacists are permitted to dispense naloxone directly to laypeople without a prescription.25
Providing a prescription drug to a third-party individual (i.e., someone other than the patient/addict) is generally prohibited by laws that require a prescription to be issued only if a physician-patient relationship has been established.28 Moreover, there is a concern by healthcare providers over the risk of criminal, civil, or professional liability. States have responded to these concerns by the implementation of programs designed to promote the use of naloxone as a rescue drug.
State Regulations: The laws vary widely among the different states and have been categorized into three domains25: 1) laws intended to increase naloxone prescribing and distribution; 2) laws intended to increase pharmacy naloxone access; and 3) laws intended to encourage overdose witnesses to summon emergency responders or administer naloxone. Some of the variables include the type of individual who can be issued a prescription for naloxone, whether laypeople can administer the medication, and the nature of immunity provided to individuals who prescribe, dispense, or administer naloxone or who report an overdose emergency.
Of the 47 programs, 39 states provide immunity to prescribers, 41 remove criminal liability for possession of naloxone, 42 shield a lay administrator from civil liability, 37 authorize naloxone prescriptions to third parties, and 33 authorize standing orders for naloxone prescriptions.29 A few also permit prescribing by pharmacists. The laws vary, but most contain several key components.25
The most widespread changes have expanded the scope of prescribing to permit healthcare providers who are authorized to prescribe naloxone to their own patients to also prescribe naloxone to an addict’s family members, caregivers, and other individuals who might be in a position to assist in the event of an overdose. Many states also permit naloxone prescribing via a standing order; at least 12 states implemented a standing order protocol for naloxone as of 2014, under a collaborative practice arrangement or similar structure.5
In general, in the case of the standing order, a prescription for naloxone is issued by a prescriber to be provided to any person who meets certain specified criteria, instead of the usual case of writing a prescription for a specific individual, and generally a pharmacist is permitted to dispense naloxone to any person authorized to receive it via the standing order.25 In at least four states (Connecticut, Idaho, North Dakota, and New Mexico), there are laws permitting some (those with specialized training) or all pharmacists to prescribe naloxone on their own authority, which does not require the delegation of prescriptive authority by another medical professional before dispensing can occur. Pharmacists practicing in some federal agencies, such as the Indian Health Service and the VA, have been granted prescribing authority.24 In jurisdictions where the pharmacist is the prescriber, he or she is generally bound by the same requirements that apply to physicians and other prescribers. There are six additional states (California, Illinois, Nevada, Ohio, Oregon, and Vermont) that permit pharmacists to dispense naloxone under a statewide protocol issued by one or more professional boards.25
At least seven states require pharmacists to receive training or education before dispensing naloxone to a patient who does not have a patient-specific prescription.25 The typical training is a 2-hour continuing education (CE) course. While benefitting patient safety, the training requirement also restricts the number of sites where naloxone could be available. At least 15 states also require a patient who does not have a personal prescription for naloxone to receive training or education before the medication can be dispensed. It has been noted that the training requirement may act as a barrier to providing naloxone access to third parties, depending on the nature and length of the training.25
In addition to permitting third parties, including lay people, to receive and administer the drug, many of the new state laws provide some form of limited immunity against criminal and civil liability, and nearly all states grant a limited immunity to healthcare professionals who prescribe naloxone.5,25 Immunity in many states has been expanded to others who may observe or be present during an overdose situation and to administer naloxone in the case of an emergency. These laws, usually referred to as Good Samaritan laws, are intended to encourage bystanders who witness a patient who has overdosed to administer the opiate antidote and/or call for medical assistance without fear of being arrested for drug-related crimes.29 Originally designed to encourage bystanders to contact 911, these laws have expanded to carrying and administering naloxone.
As in the case of other naloxone-related laws, these vary among the states; some provide broad immunity protection while others consider seeking medical assistance for a person experiencing an overdose as an affirmative defense, or as a mitigating factor during sentencing.29 For example, the Massachusetts statute states that “a person acting in good faith may receive and possess a naloxone prescription, and may administer naloxone to an individual appearing to experience an opiate-related overdose.” It also states that “a person who, in good faith, seeks medical assistance for someone experiencing a drug-related overdose shall not be charged or prosecuted for possession of a controlled substance…if the evidence for the charge of possession of a controlled substance was gained as a result of the seeking of medical assistance.”30 In most states the Good Samaritan statutes will not protect someone who is distributing or selling drugs.
Model Programs: The first state to amend its laws to make it easier for medical professionals to prescribe and dispense naloxone was New Mexico in 2001.31 The state also made it easier for lay people to administer naloxone without fear of legal repercussions. New Mexico recognized the growing problem of opiate overdose when prescription opiate drug–related deaths overtook deaths from heroin in the state, and it became the first state to authorize pharmacists to prescribe naloxone in 2014. One rationale for granting prescriptive authority to pharmacists is the rural character of the state, where the ubiquitous presence of community pharmacies would increase access to potentially lifesaving measures.31
With regard to New Mexico as a model for naloxone distribution by pharmacists, several components should be noted.31 First, pharmacists need to complete an approved training program consisting of 2 hours of live CE every 2 years. The patient is screened and evaluated by the pharmacist for the risk of overdose, and the patient must complete and sign a consent form before the naloxone can be dispensed. The regulations list 12 criteria for overdose risk, including being prescribed long-acting opioids; opiate use for more than 30 days; opioids prescribed to elderly (>65 years) or teenaged patients; use of opioids in patients with concurrent diseases such as renal dysfunction, liver disease, respiratory infection, sleep apnea, chronic obstructive pulmonary disease (COPD), and emphysema or other respiratory/ airway disease that can lead to potential airway obstruction; patients who may have difficulty accessing EMS (e.g., due to distance, remoteness, lack of transportation, homelessness, lack of phone services); or “patients as determined by the Pharmacist using their professional judgment.” In addition, with consent, the pharmacist is to notify the patient’s primary care provider within 15 days of the original prescription.31
Finally, the pharmacist is expected to provide educational material and training to the patient, including face-to-face education on the proper use of the naloxone rescue kit and a plan for overdose prevention and adverse effects. The patient must identify a designated rescue person, who is also encouraged to attend the training. If the kit is used, and the patient requests a new prescription, the pharmacist is to conduct a thorough evaluation regarding the events leading to the use and to determine whether appropriate medical follow-up was completed, as required.31
In Rhode Island, the program began as a pilot collaborative practice agreement initially involving a physician, a clinical pharmacist, and Walgreens, such that a pharmacist with appropriate advanced training could provide naloxone to anyone who requested the drug.32 The pilot program has expanded, and a regulation issued in 2014 states: “Naloxone (Narcan) may lawfully be prescribed and dispensed to an individual at risk of experiencing an opiate-related overdose or a family member, friend or other person in a position to assist a person at risk of experiencing an opiate-related overdose. Any such prescription shall be regarded as being issued for a legitimate medical purpose in the usual course of professional practice.”32 The last stipulation is a typical requirement for prescribing a drug.
The rationale for these kinds of third-party prescriptions is that people who use drugs or their family and friends are more likely to be present during the drug use or to discover the individual who is in distress and therefore be able to administer the naloxone in a more timely manner and increase the likelihood of a rescue.25 In fact, there are CDC data suggesting that at least in certain communities, 82.8% of the reported reversals were done by people who use drugs and 9.6% by family and friends of a user, while emergency service accounted for only 0.2% of the reversals.27 It is not yet clear if this is the common experience. However, many studies do support the observation that bystanders can have an impact on overdose mortality; such studies indicate a reversal rate of 75% to 100%.33
Impediments
The naloxone programs are not without their critics. As mentioned earlier, there are some safety concerns with using naloxone as a rescue drug, including precipitated opiate withdrawal, a recurrence of respiratory depression when the short-acting antagonist wears off, and the possible inability of a layperson to deal with these issues if they arise.
As noted earlier, traditionally a prescription is only offered when a practitioner-patient relationship has been established. Ordinarily, this would entail “sufficient contact with the patient to allow an informed professional evaluation of the patient’s conditions and needs… [and] would normally involve examining of the patient as necessary to determine that the medication is indicated and safe for the patient, taking a history on matters relevant to the care, discussing the treatment plan and its alternatives with the patient, and taking steps to ensure adequate follow-up care.”3 Under the conditions of third-party recipients, the prescribing practitioner may not know the intended recipient and, in the case of an emergency, the individual administering the drug may not know the patient.
Note, for example, the Florida statute, which states that “such patient or caregiver is authorized to store and possess approved emergency opioid antagonists and, in an emergency situation when a physician is not immediately available, administer the emergency opioid antagonist to a person believed in good faith to be experiencing an opioid overdose, regardless of whether that person has a prescription for an emergency opioid antagonist.”34 One commentator has noted that “providing naloxone under those terms would amount to deputizing the lay person as a medical practitioner, which contravenes the basic idea of licensure and criminal laws that prohibit the unlicensed practice of medicine.”3
There are also concerns that naloxone distribution could potentially exacerbate opiate abuse and its consequences by instilling a false sense of security in drug addicts who may feel that a rescue is available in the event of an overdose and take more risks. In fact, Maine Governor Paul LePage recently vetoed a bill that would have allowed pharmacists in the state to dispense naloxone without a prescription, stating “Naloxone does not truly save lives; it merely extends them until the next overdose. Creating a situation where an addict has a heroin needle in one hand and a shot of naloxone in the other produces a sense of normalcy and security around heroin use that serves only to perpetuate the cycle of addiction.” It should be noted that drug overdose deaths in Maine increased by 31% in 2015.35 In addition, cost has also become an issue, with recent soaring prices and poor, if any, insurance coverage for naloxone rescue kits gaining the attention of Congress and others.36
Summary and Conclusions
Drug overdose deaths, especially those due to opiates such as prescription analgesics and heroin, continue to rise at an alarming rate. Most efforts to reduce the overdoses have focused on reducing the supply of opiates, either by modifying prescribing practices, increasing law enforcement efforts, or introducing abuse-deterrent formulations. Another strategy has been to increase accessibility to the opiate antagonist naloxone, as a means of reversing the potentially fatal respiratory depression produced by opiates, especially as safer and easier to use dosage forms become available. One means of increasing access has been to amend state laws to involve the pharmacist either through collaborative practice arrangements or by granting prescriptive authority, so that the pharmacist can dispense naloxone to persons at risk of overdose or to their family or friends. This approach directly involves the pharmacist as a factor in reducing drug overdose deaths and enhances the opportunities for pharmacists to use their professional judgment and to provide care and education to their community. Pharmacists also need to stay abreast of initiatives at the federal level to increase naloxone access as part of a multifaceted effort to treat the growing problem of addiction.
REFERENCES
- Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(50):1378-1382. www.cdc.gov/mmwr/preview/mmwrhtml/mm6450a3.htm. Accessed August 1, 2016.
- National Governors Association. Six strategies for reducing prescription drug abuse. September 13, 2012. www.nga.org/cms/home/nga-center-for-best-practices/center-publications/page-hsps-publications/six-strategies-for-reducing-pres.html. Accessed August 1, 2016.
- Burris S, Beletsky L, Castagna C, et al. Stopping an invisible epidemic: legal issues in the provision of naloxone to prevent opioid overdose. Drexel Law Rev. 2009;1(2):273-339. http://prescribetoprevent.org/wp-content/uploads/2012/11/burris_stoppinganinvisibleepidemic.pdf. Accessed August 1, 2016.
- Gianutsos G. Update on the prescription drug abuse epidemic for pharmacists and pharmacy technicians. Power-Pak. April 29, 2016. www.powerpak.com/course/preamble/112976. Accessed August 1, 2016.
- Network for Public Health Law. Legal interventions to reduce overdose mortality: naloxone access and overdose Good Samaritan laws. June 2016. www.networkforphl.org/_asset/qz5pvn/network-naloxone-10-4.pdf. Accessed August 1, 2016.
- DEA intelligence brief. Counterfeit prescription pills containing fentanyls: a global threat. July 2016. https://content.govdelivery.com/attachments/USDOJDEA/2016/07/22/file_attachments/590360/fentanyl%2Bpills%2B report.pdf. Accessed August 1, 2016.
- Jones CM, Logan J, Gladden RM, Bohm MK. Vital signs: demographic and substance use trends among heroin users—United States, 2002-2013. MMWR Morb Mortal Wkly Rep. 2015;64(26):719-725. www.cdc.gov/mmwr/preview/mmwrhtml/mm6426a3.htm. Accessed August 1, 2016.
- Hawk KF, Vaca FE, D’Onofrio G. Reducing fatal opioid overdose: prevention, treatment and harm reduction strategies. Yale J Biol Med. 2015;88(3):235-245.
- Unick GJ, Rosenblum D, Mars S, Ciccarone D. Intertwined epidemics: national demographic trends in hospitalizations for heroin- and opioid-related overdoses, 1993-2009. PLoS One. 2013;8:e54496. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0054496. Accessed August 1, 2016.
- Rudd RA, Paulozzi LJ, Bauer MJ, et al. Increases in heroin overdose deaths—28 states, 2010 to 2012. MMWR Morb Mortal Wkly Rep. 2014;63(39):849-854. www.cdc.gov/mmwr/preview/mmwrhtml/mm6339a1. htm. Accessed August 1, 2016.
- Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374:154-163.
- Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821-826.
- Cook L. The heroin epidemic in 9 graphs. U.S. News & World Report. August 19, 2015. www.usnews.com/news/blogs/data-mine/2015/08/19/the-heroin-epidemic-in-9-graphs. Accessed August 1, 2016.
- CDC. Drug overdose deaths hit record numbers in 2014. www.cdc.gov/media/releases/2015/p1218-drug-overdose.html. Accessed August 1, 2016.
- Cole C, Jones L, McVeigh J, et al. Cut: A Guide to Adulterants, Bulking Agents and Other Contaminants Found in Illicit Drugs. Liverpool, England: Liverpool John Moores University; April 2010. www.cph.org.uk/wp-content/uploads/2012/08/cut-a-guide-to-the-adulterants-bulking-agents-and-other-contaminants-found-in-illicit-drugs.pdf. Accessed August 1, 2016.
- Kim HK, Nelson LS. Reducing the harm of opioid overdose with the safe use of naloxone: a pharmacologic review. Expert Opin Drug Saf. 2015;14(7):1137-1146.
- Jasinski DR, Martin WR, Haertzen CA. The human pharmacology and abuse potential of N-allylnoroxymorphone (naloxone). J Pharmacol Exp Ther. 1967;157:420-426.
- Simon EJ. Opiate receptors in the central nervous system. Curr Dev Psychopharmacol. 1977;4:33-69.
- Boyer EW. Management of opioid analgesic overdose. N Engl J Med. 2012;367:146-155.
- Wermerling DP. Review of naloxone safety for opioid overdose: practical considerations for new technology and expanded public access. Ther Adv Drug Saf. 2015;6:20-31.
- Faul M, Dailey MW, Sugerman DE, et al. Disparity in naloxone administration by emergency medical service providers and the burden of drug overdose in US rural communities. Am J Public Health. 2015;105 (suppl 3):26-32.
- Prescribe to Prevent. Naloxone formulations. January 21, 2016. http://prescribetoprevent.org/wp2015/wp-content/uploads/Naloxone-product-chart.16_01_21.pdf. Accessed September 15, 2016.
- Evzio (naloxone hydrochloride injection) auto-injector prescribing information. Richmond VA: Kaleo, Inc; April 2014. http://evzio.com/pdfs/Evzio%20PI.PDF. Accessed August 1, 2016.
- FDA moves quickly to approve easy-to-use nasal spray to treat opioid overdose. FDA news release. November 18, 2015. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm473505.htm. Accessed August 1, 2016.
- Davis CS, Carr D. Legal changes to increase access to naloxone for opioid overdose reversal in the United States. Drug Alc Depen. 2015;157:112-120.
- Strang J, McDonald R, eds. Preventing opioid overdose deaths with take-home naloxone. European Monitoring Centre for Drugs and Drug Addiction; 2016. www.emcdda.europa.eu/system/files/publications/2089/TDXD15020ENN.pdf. Accessed August 1, 2016.
- Wheeler E, Jones TS, Gilbert MK, Davidson PJ. Opioid overdose prevention programs providing naloxone to laypersons—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(23):631-635. www.cdc.gov/mmwr/preview/mmwrhtml/mm6423a2.htm. Accessed August 1, 2016.
- National Conference of State Legislatures. Drug overdose immunity and Good Samaritan laws. www.ncsl.org/research/civil-and-criminal-justice/drug-overdose-immunity-good-samaritan-laws.aspx. Accessed August 1, 2016.
- The Policy Surveillance Program. Naloxone overdose prevention laws map. LawAtlas. http://lawatlas.org/query?dataset=laws-regulating-administration-of-naloxone#.U3YljijzDEU. Accessed August 1, 2016.
- Immunity from prosecution under Secs 34 or 35 for persons seeking medical assistance for self or other experiencing drug-related overdose. Mass Gen Laws. Ch 94C, §34A.
- New Mexico Board of Pharmacy Regulations. Pharmacist prescriptive authority of naloxone rescue kit protocol. www.rld.state.nm.us/uploads/filelinks/e3740e56e0fe428e991dca5bd25a7519/nrk_protocol_bop_dale_ tinker.pdf. Accessed August 1, 2016.
- State of Rhode Island Department of Health. Rules and regulations pertaining to opioid overdose prevention. March 2014. http://sos.ri.gov/documents/archives/regdocs/released/pdf/DOH/7687.pdf. Accessed August 1, 2016.
- Doyon S, Aks SE, Schaeffer S. Expanding access to naloxone in the United States. J Med Toxicol. 2014;10(4):431-434.
- The 2016 Florida Statutes. Emergency Treatment for Suspected Opiate Overdose. Title XXiX, Ch. 381 §381.887(3). www.leg.state.fl.us/statutes/index.cfm?App_mode=Display_Statute&URL=0300-0399/0381/0381.html. Accessed August 1, 2016.
- Miller K. LePage vetoes bill aimed at increasing access to overdose antidote. Portland Press Herald. April 20, 2016. www.pressherald.com/2016/04/20/lepage-vetoes-bill-aimed-at-increasing-access-to-heroin-anti-overdose-drug/. Accessed August 1, 2016.
- Karlin-Smith S. Price spikes for life-saving drug. Politico. May 16, 2016. www.politico.com/story/2016/05/drug-prices-addiction-223192. Accessed August 1, 2016.