Gout and Hyperuricemia: Treatment Update and Pharmacist’s Role
RELEASE DATE
March 5, 2020
EXPIRATION DATE
March 31, 2022
FACULTY
Cortney Mospan, PharmD, BCACP, BCGP
Assistant Professor of Pharmacy
Wingate University Levine College of Health Sciences
Wingate, North Carolina
FACULTY DISCLOSURE STATEMENTS
Dr. Mospan has no actual or potential conflict of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT
 Pharmacy
Pharmacy
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-20-026-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER:
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients' conditions and possible contraindications or dangers in use, review of any applicable manufacturer's product information, and comparison with recommendations of other authorities.
GOAL
To provide pharmacists with comprehensive knowledge about gout and hyperuricemia incidence, presentation, risk factors, and appropriate acute and chronic management strategies.
OBJECTIVES
After completing this activity, the participant should be able to:
- Review the pathophysiology and etiology of gout, as well as signs and symptoms of acute attacks.
- Discuss differences in recommendations from several organizations’ guidelines for the pharmacologic treatment of gout.
- Recognize appropriate treatment selections for acute gouty arthritis and chronic urate-lowering therapy.
- Identify strategies pharmacists can use to help improve patient adherence to gout treatment.
ABSTRACT: Gout is the most common form of inflammatory arthritis in the United States, and its prevalence has more than doubled over the past 20 years. Evidence suggests that elevated uric acid levels may contribute to the risk of cardiovascular disease, among many other comorbidities. The management of gout is suboptimal, with many patients not receiving urate-lowering therapy and not titrated to their target serum uric acid goal. Pharmacists can help enhance patient adherence and achievement of target uric acid goals, and they can play a key role in improving gout management and reducing associated cardiovascular risk.
Over the past 20 years, the incidence of gout has more than doubled, accompanied by an increase in comorbid conditions such as diabetes, hypertension, renal disease, dyslipidemia, and morbid obesity.1 The most recent (2015-2016) National Health and Nutrition Examination Survey (NHANES) estimated that 3.9% of U.S. adults (9.2 million people) have gout, making this condition the most common type of inflammatory arthritis in the country.2,3 Prevalence was stable between 2007 and 2016.
Men are more likely to have gout, with a prevalence of 5.2% versus 2.7% in women. The condition occurs less frequently in women because of the uricosuric properties of estrogen, but decreased estrogen production in postmenopausal women results in an increase in gout development in this population.4
Gout incidence increases with age, ranging from 0.7% in patients aged 20 to 39 years to 8.8% in patients aged 60 years and older, and peaks between the sixth and seventh decades of life. By age 75 years, 13.3% of men and 3.3% of women have been diagnosed with gout.5 Prevalence is considerably lower in Hispanic patients (2%) compared with patients of white, African American, or other ethnicity.2
Since the 1950s, gout has been recognized to be associated with cardiovascular (CV) disease, diabetes, hypertension, and kidney disease. Since then, numerous epidemiologic studies have identified a relationship between serum uric acid (SUA) levels and many CV conditions, including coronary artery disease, cerebrovascular disease, metabolic disease, preeclampsia, and vascular dementia.6 However, the clinical significance of this association has been debated based on the Framingham Heart Study’s assertion that uric acid is not a risk factor for CV disease.7 In a prospective cohort study spanning 1992 to 2017, Pérez Ruiz and colleagues found that the mortality rate was significantly higher in gout patients who failed to reach the recommended SUA goal (<6 mg/dL), even after adjustment for age, sex, CV risk factors, and previous CV events (hazard ratio 2.33; 95% CI, 1.60-3.41).8 In fact, with every 1-mg/dL increase in SUA, the relative risk of all-cause death increases by 39%.9
Clinical inertia often occurs as a result of the provider’s lack of familiarity with treatment guidelines for gout and the belief that gout is a relatively benign disease without serious consequences like diabetes or hypertension. In a survey of 838 primary care providers, only about 50% indicated optimal treatment practices for acute gout management, and the rate was <20% for tophaceous gout and intercritical gout (gout in the presence of renal insufficiency).10 In a database study of 14,400 patients with gout, only 32% had uric acid monitoring data and just 29% had renal function testing data, demonstrating limited monitoring of necessary parameters for gout. Only 39% of patients had achieved an SUA goal of <6 mg/ dL, and 76% of patients treated for gout received a likely-suboptimal dosage of allopurinol <200 mg/dL, many of them lacking a dose adjustment for >1 year.11
Clinical Presentation
Acute gouty arthritis results from the precipitation of monosodium urate crystals in a joint space when they have exceeded their limit of solubility (SUA level of >6.8 mg/dL).3,12,13 Deposition of crystals in the joint triggers activation of the immune system, resulting in the release of neutrophils and several inflammatory cytokines, including interleukin-1 (IL-1) and may manifest in tophi.14 Tophi are visible nodules containing deposits of the monosodium urate crystals in a matrix of protein, mucopolysaccharides, and lipids in the joint space. The hallmark sign of gout is podagra (red, inflamed, swollen first metatarsophalangeal joint). Other commonly affected joints include larger lower-limb joints such as the knees, ankles, and midtarsal joints. Smaller joints (fingers) and more distal joints (wrists) may also be affected.15 In elderly patients, gout presentation may be atypical, with polyarticular joint presentation that primarily affects the upper limbs and a greater likelihood of tophi.16,17
Risk Factors for Gout
Comorbidities: Gout is commonly associated with metabolic syndrome. In patients with gout, the incidence of metabolic syndrome is 63%, compared with 25% in patients without gout. Interestingly, patients with diabetes have a 33% decreased risk of developing gout despite the presence of hyperglycemia and insulin resistance, which are components of metabolic syndrome. It is thought that hyperuricemia predisposition caused by hyperinsulinemia and insulin resistance is reversed by the uricosuric effects of glycosuria in diabetes. There is a dose-dependent relationship between increasing BMI and risk of incident gout. Hypertension is also an independent risk factor for incident gout.18
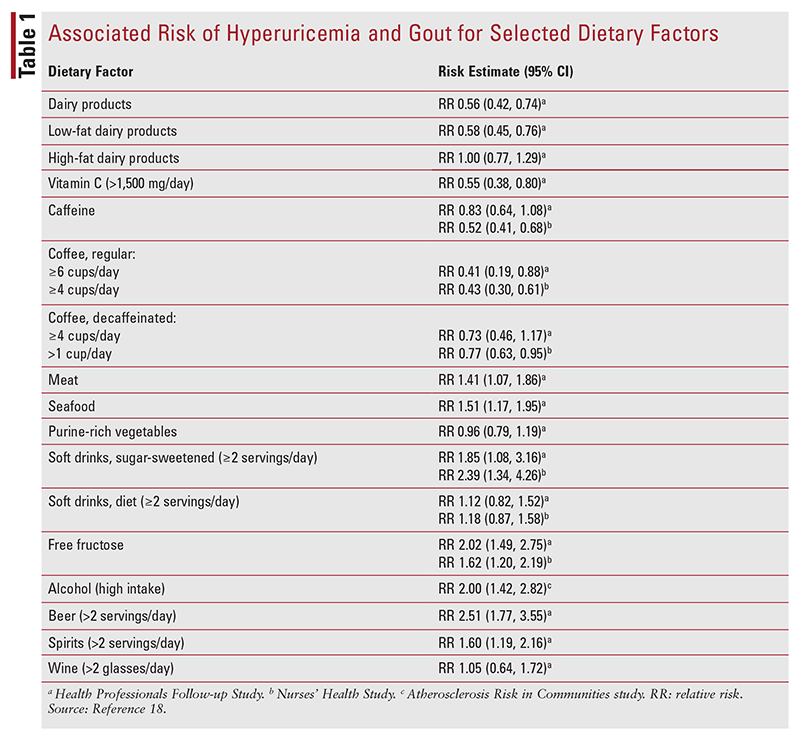
Diet and Lifestyle: The impact of diet on hyperuricemia and gout remains somewhat controversial. Foods high in purine content are associated with risk of hyperuricemia and gout, but the efficacy of low-purine diets in patients diagnosed with gout is less clear. In a study spanning 26 years and involving >44,000 adult male subjects without history of gout at baseline, Rai and colleagues evaluated the relationship between the Dietary Approaches to Stop Hypertension (DASH) and Western diets and risk of gout development.19 The DASH diet promotes a high intake of vegetables, nuts and legumes, fruits, low-fat dairy products, and whole grains and a low intake of sweetened beverages, red and processed meats, and sodium. The Western diet was defined as high intake of french fries, refined grains, red and processed meats, and sweets. A higher Western dietary pattern score was found to increase the risk of gout (relative risk [RR] 1.42; 95% CI, 1.16-1.74), whereas a higher DASH dietary pattern score conferred a lower risk of gout (RR 0.68; 95% CI, 0.57-0.80).19
Holland and McGill evaluated the influence of dietary education on SUA on patients receiving stable doses of urate-lowering therapy (ULT). After education at baseline and 3-month follow-up, there was no difference in SUA levels at 6 months between the treatment and control groups; however, the study involved only 30 patients.20
TABLE 1 provides RRs for various dietary factors that are associated with hyperuricemia and gout. Purine-rich foods theoretically increase the risk of gout by providing exogenous purines, which are broken down into uric acid.18 However, some purine-rich foods, such as asparagus, legumes, mushrooms, nuts, and oatmeal, appear not to increase the risk.21
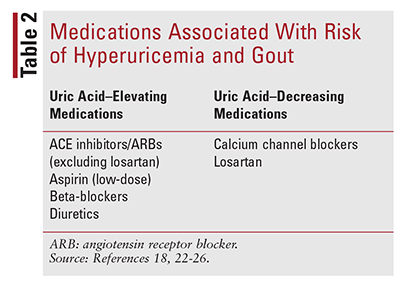
Medications: A list of medications associated with hyperuricemia and gout risk is given in TABLE 2. The risk of developing gout with diuretic use rises with both duration of therapy and increasing dose.22 Development of hyperuricemia with diuretic use typically occurs within 5 or 6 months of initiation of the medication.23 Other antihypertensives have been associated with hyperuricemia, though to a lesser extent.18,24,25 Antihypertensives that have uricosuric effects include losartan and calcium channel blockers. A risk of recurrent gout attacks exists for low-dose aspirin (100 mg or less) that is not maintained at higher doses (325 mg or greater).26
Age-Related Factors: Other factors that can increase the risk of gout in older adults come into play during middle age (age 45-64 years). These include increased adiposity, beer and protein intake, suboptimal kidney function, smoking, hypertension, and (as noted above) diuretic use.5
Diagnosis
Although pharmacists are not typically involved in the diagnosis of disease states, it is important to be familiar with the criteria for acute gouty arthritis should a patient present to the pharmacy or clinic with symptoms suggestive of it, in order to refer the patient for further evaluation. Gout is typically diagnosed based on clinical presentation. While aspiration of the joint fluid and identification of urate crystals (thin, needle-like appearance) are required for a confirmatory diagnosis, this is rarely done in practice. Clinical diagnosis is based on rapid development of monoarticular arthritis with associated swelling and redness.15 The American College of Rheumatology (ACR)–European League Against Rheumatism (EULAR) diagnostic criteria are available at https://goutclassificationcalculator.auckland.ac.nz.
Treatment Guidelines
Initiation of ULT: The various guidelines lack consensus regarding which patients qualify for ULT, with the American College of Physicians (ACP) guidelines being the least aggressive and the EULAR guidelines being the most aggressive. The ACP does not recommend ULT initiation after the first attack or in patients who have infrequent attacks.27 This is somewhat in alignment with the ACR guidelines, which recommend ULT in patients who have tophi, two or more flares per year, stage 2 or higher chronic kidney disease (CKD), and history of urolithiasis; these criteria reflect more frequent and severe disease.28,29 The EULAR guidelines differ in recommendin g consider ation of ULT initiation from the first attack, although a definitive diagnosis is required. This approach based on the growing impact of hyperuricemia on CV health.30
When ULT is indicated, recommendations of which agent to use vary significantly between guidelines. The ACP guidelines do not give a preference for ULT initiation.27 The ACR guidelines recommend xanthine oxidase inhibitors (XOI) as first-line ULT but gives equal consideration to both allopurinol and febuxostat.28,29 The EULAR guidelines, however, recommend allopurinol because of its affordability.30
Treat-to-TargetApproach: In the most recent NHANES data, only 33% of patients with gout were receiving ULT despite the ACR and EULAR guidelines’ recommendation to treat to a target SUA level of <6 mg/dL to prevent further gout exacerbations.2 Both the EULAR and ACR guidelines allow for a more aggressive target SUA level of <5 mg/dL in patients with more severe disease (e.g., tophi, frequent attacks) to promote further crystal dissolution and prevent acute attacks.28-30 In contrast, the ACP guidelines do not recommend a treat-to-target approach or ULT initiation owing to insufficient evidence that it improves outcomes or adherence. According to the ACP, there is a lack of clarity regarding whether the benefits outweigh the risks associated with medication escalation and repeated monitoring.27 However, the treat-totarget approach has been shown to reduce the risk of having two or more flares per year by 67% and the risk of tophi by 79%.31
Treatment Options
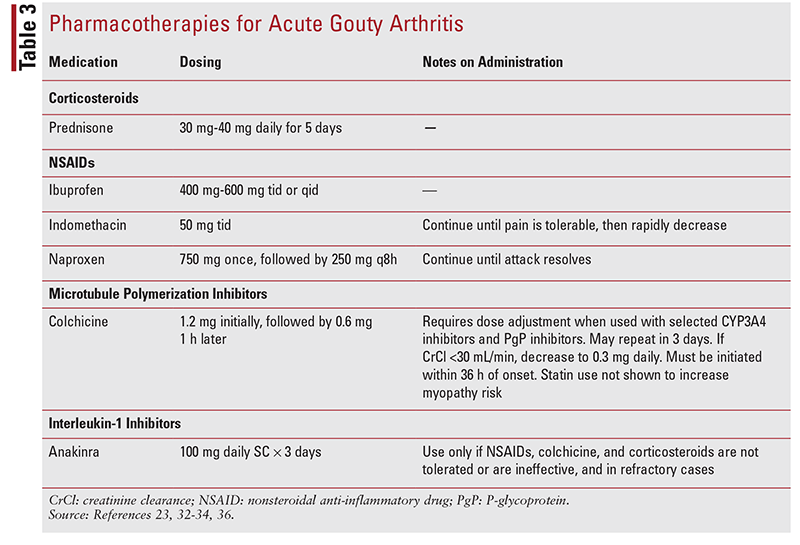
Treatment options for acute gouty arthritis include nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, colchicine (a microtubule polymerization inhibitor), and IL-1 inhibitors.20,28,32-34 Topical therapies have no role in acute gouty arthritis. While not exhaustive, TABLE 3 includes common therapies based on cost, convenience, and provider familiarity. Neither the ACR nor the EULAR guideline has a treatment preference for acute gouty arthritis; however, the ACP guidelines recommend corticosteroids as first-line treatment as long as there is no contraindication.27-30 This difference is not surprising given when each of these guidelines was published. The ACR guidelines were published in 2012, whereas the ACP guidelines were published in 2017. Updated ACR guidelines are currently under peer review.35
Corticosteroids were previously believed to be less effective than NSAIDs for pain resolution, but recent evidence has shown their efficacy to be equal to that of NSAIDs.36 Prednisolone 35 mg/day has been found to provide pain relief for acute gouty arthritis equivalent to that of naproxen 500 mg twice daily.37 Alternative dosing of prednisone or prednisolone 0.5 mg/ kg/day may be used for a 10-day course of therapy. Tapering is not required, but high doses may be used for 2 to 5 days and then tapered over 7 to 10 days. Additionally, oral methylprednisolone dose packs may be considered.37,38
Colchicine has fallen out of favor because of cost increases, gastrointestinal (GI) side effects, and drug interactions. IL-1 inhibitors should be used only after all first-line treatment options have failed.28-30 NSAIDs are best used in patients aged <60 years without major CV, renal, and GI comorbidities. Any NSAID may be used, provided that a dosage sufficiently high for anti-inflammatory effects is given; however, for acute gout, celecoxib is not an appropriate selection because the daily dosage of 800 mg required for inflammatory benefit poses severe CV risk and exceeds the maximum daily dosage of 200 mg in the drug’s labeling.36,39 The ACR guidelines permit combination therapy of first-line treatment options (NSAID, corticosteroid, colchicine) if the response to monotherapy is inadequate; however, it is recommended that corticosteroids and NSAIDs not be used together based on concerns about synergistic GI toxicity.23
These treatment options are also recommended when ULT is initiated in order to prevent acute gouty arthritis exacerbations or recurrence. When antiinflammatory prophylaxis is initiated with ULT, NSAIDs, colchicine, and prednisone or prednisolone are appropriate treatment options, but low-dose colchicine and low-dose NSAIDs are first-line.23,28,29 Any NSAID may be used at the lowest effective dose. Colchicine 0.6 mg once or twice daily is appropriate. Daily corticosteroids are recommended only when colchicine and NSAIDs are ineffective, contraindicated, or not tolerated. Doses of 10 mg or less should be used. When colchicine is used for prophylaxis, it cannot be used during an acute attack to terminate the attack.36 The presence of tophi helps determine the extent of acute gouty arthritis prophylaxis. If tophi are present, prophylaxis should be continued for 6 months after the SUA goal is reached; if no tophi are present, prophylaxis should continue for 3 months after the SUA goal is reached.23,28,29
ULT Options
In November 2017, the FDA first alerted the public to preliminary safety results of a trial that identified an increased risk of heart-related death with febuxostat compared with allopurinol. This trial, Cardiovascular Safety of Febuxostat or Allopurinol in Patients with Gout and Cardiovascular Comorbidities (CARES), was required by the FDA when it approved febuxostat in 2009. Since that time febuxostat has carried a Warning and Precaution about CV events because the clinical trials that led to FDA approval found a higher rate of heart-related problems (heart attacks, stroke, heart-related death) in patients treated with febuxostat versus allopurinol.40 The CARES study found that febuxostat was noninferior to allopurinol for major adverse CV events (MACE; i.e., CV death, nonfatal myocardial infarction, nonfatal stroke, and urgent revascularization due to unstable angina)—that is, it was no worse than allopurinol for resultant MACE. However, death from any cause and CV death occurred more frequently in the febuxostat group.41 As a result, in February 2019, the FDA concluded that febuxostat confers an increased risk of death compared with allopurinol. Therefore, a boxed warning was added to the prescribing information for febuxostat. FDA guidance states that febuxostat use should be reserved for patients who have failed or who do not tolerate allopurinol. Febuxostat should not be initiated firstline as ULT in patients who have not previously tried allopurinol.42
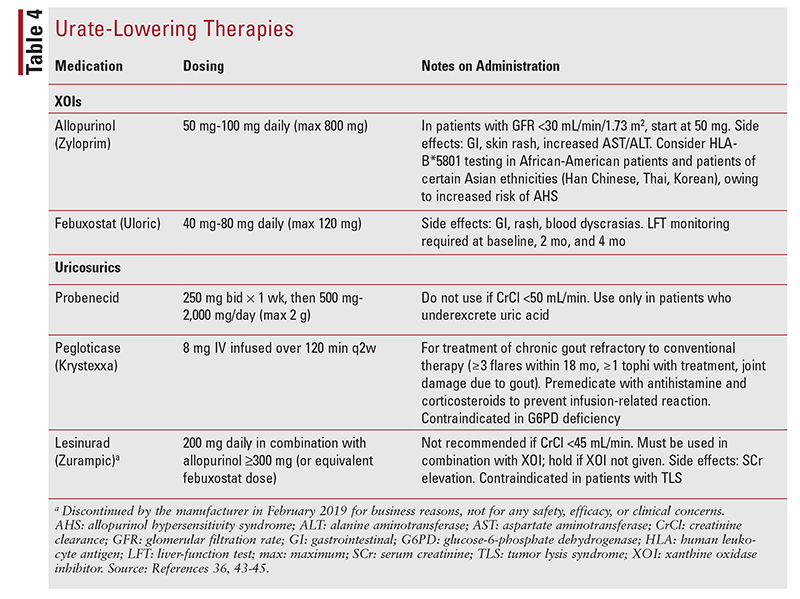
TABLE 4 provides dosing for ULT options. Both allopurinol and febuxostat should be initiated at a low dosage and titrated every 2 to 5 weeks until the SUA level is <6 mg/dL.43,44 It has long been debated whether febuxostat is a more clinically effective XOI to prevent the formation of uric acid than allopurinol given that febuxostat inhibits both oxidized and reduced forms of xanthine oxidase whereas allopurinol inhibits only the reduced form.45 Clinical trials have found that more patients treated with febuxostat reach their SUA goal and achieve that goal significantly faster.46,47 A key consideration regarding these studies is that allopurinol often is not used at a dosage comparable to that for febuxostat; many trials use an allopurinol dosage of 100 mg to 300 mg/day, compared with 80 mg/day for febuxostat. The maximum dosage of allopurinol is 800 mg/day, and tophaceous gout often requires 400 mg to 600 mg to reach the SUA goal.36 Additionally, misguided renal dosing strategies for allopurinol are often followed. Allopurinol should be first-line treatment unless the patient has human leukocyte antigen-B*5801. Febuxostat should be reserved for patients with allopurinol hypersensitivity or intolerance.
Febuxostat was previously preferred for use in patients with renal disease as well. A long-held misconception is that the renal dose adjustment required for allopurinol addresses the increased levels of allopurinol’s active metabolite—oxypurinol—and perceived heightened risk of allopurinol hypersensitivity syndrome (AHS) with increasing oxypurinol levels. It is thought that AHS risk is most closely associated with higher starting doses of allopurinol (>100 mg).4,48 Emerging evidence suggests that allopurinol protects against, and is associated with a lower risk of, renal disease compared with febuxostat used for the treatment of ULT. Higher dosages of allopurinol and longer duration of therapy enhance the reduction in risk.49-51
Hyperuricemia is a risk factor for progression of kidney disease.51 A 30% improvement in estimated glomerular filtration rate (eGFR) is more likely in patients with stage 2 or 3 CKD, and achieving an SUA level of 6 mg/dL or lower provides a 37% risk reduction of disease worsening.51,52 Higher doses than those indicated for renal dosing modifications often are required to reach recommended uric acid targets, as <50% of patients with CKD achieve their SUA target with current renal dosing guidelines. Additionally, increasing the allopurinol dosage in CKD patients who tolerate a low initial dosage has never been proven to increase AHS risk.4,48,53
The most recent ULT is lesinurad (Zurampic), which has also been marketed as a combination product containing 200 mg lesinurad and 300 mg allopurinol (Duzallo). In February 2019, the manufacturer discontinued both products for business reasons; this decision was not due to any efficacy, safety, or clinical concerns with lesinurad.36 The prescribing information states that lesinurad should be used only in patients who cannot achieve their SUA goal with an XOI alone.54 Lesinurad must be given with allopurinol 300 mg daily or febuxostat 80 mg daily. If the XOI is not given, lesinurad should be held as well. This is due to the boxed warning for acute kidney injury, which is more likely to occur when lesinurad is given alone.54
Another medication in this class, verinurad, is currently in development, and similar renal dosing is expected.55
Additional medications with urate-lowering effects may be added in patients with comorbidities. According to ACR guidelines, patients with refractory gout or gout that is not at goal with maximum allopurinol dosage may receive fenofibrate, which can lower uric acid levels as much as 20% to 30%, for additional urate-lowering benefit.28,29,36 When fenofibrate was used in combination with XOI therapy, SUA levels showed a significant reduction, with an average decrease of 2.40 mg/dL versus 1.81 mg/dL for XOI monotherapy.56 This treatment is best for gout patients who have triglyceridemia that warrants pharmacotherapy. Losartan is another medication that can reduce uric acid levels. This is not a class effect, and a review of eight studies found that only losartan consistently lowered SUA (an average reduction of 0.8-1 mg/dL could be expected); however, the clinical significance, if any, has not been determined.57 In patients with concomitant diabetes and/or hypertension who qualify for ACE inhibitor therapy or angiotensin receptor blocker therapy, losartan would be the optimal selection.
Dietary Supplements: Patients often seek natural therapies for rheumatologic disorders. Patients frequently report using cherry extract; however, there is inadequate evidence of its safety and effectiveness, and cherry extract should not be recommended for use. Vitamin C, celery seed, and turmeric have been found to be safe, but only vitamin C has data supporting its effectiveness as a treatment option. Dosages of 500 mg/day or more are effective for preventing acute flares. Celery seed has been used for acute pain treatment during a flare, but there is no recommended dosage, and no data suggest that it is an effective treatment strategy. Turmeric also has no efficacy data, but a dosage of 1,000 mg/day has been found to be effective for other arthritic disorders.58-60
The Pharmacist’s Role
Gout is commonly thought of as an insignificant disease, but it costs the healthcare system approximately $27.4 million each year, ranging from $3,000 to $13,514 per patient.61,62 Patient adherence to gout medications has been found to be consistently poor. Among seven chronic conditions, adherence was lowest for gout medications, with an estimated 63% of cases remaining uncontrolled and 19% untreated.61-63 Lack of disease control is predictive of an increase in gout-associated costs and healthcare-resource utilization and results in an estimated increase in absenteeism by five workdays annually.61,62
The pharmacist can play a key role in gout treatment. Many patient misperceptions exist concerning gout, and it is critical for pharmacists to educate patients about medication benefits, side effects, risks of elevated uric acid, and the importance of adherence to follow-up laboratory testing. Eight percent of patients who discontinued ULT did so based on the misperception that their ULT caused acute gout attacks and resulted in worsening of their gout. Also, significant gaps in therapy have been identified, many of them in the first year of therapy. Nearly 70% of patients have a significant interruption (>60 days) during their first year of therapy.61 As a key member of the healthcare team, the pharmacist can notify providers when patients do not stay adherent or refill gout medications, should monitor for side effects, and can help prescribers avoid clinical inertia (discussed below) and continue to titrate to a target SUA level of <6 mg/dL. Hispanic patients, who have the lowest incidence of gout, have the greatest prevalence of ULT use (57.7%), compared with 26.5% of African American patients and 35% of white patients.2 It is important for pharmacists to ensure that all patient populations are being assessed for ULT appropriateness.
Pharmacists can improve patient adherence to ULT by being aware of factors that are associated with success in adherence and helping patients incorporate them into daily life. Common success factors include assimilation of ULT into the daily routine, moderate intake of enjoyable purine-containing foods, and association of ULT with flare avoidance. Barriers to adherence that pharmacists should work with patients to create solutions for include medication cost and side effects, the misperception that medication is needed only for acute attacks, and forgetfulness.64,65
Clinical inertia is prevalent in gout. In a database study of more than 14,000 patients with a diagnostic code for gout, just 39% had an SUA level noted in their chart and only 29% had any renal-function testing. A little more than one-third of patients (39%) had an SUA level in line with ACR guidelines (<6 mg/ dL), and those who were treated were often on a stable allopurinol dose of just 200 mg/dL for more than 1 year.11 Pharmacist-managed gout programs have shown that pharmacist management of ULT titration has a high success rate for getting patients to their SUA goal. In a pilot study, 82% of patients achieved an SUA level of <6 mg/dL.66
Conclusion
Gout is emerging as a chronic condition that has unrecognized potential for CV risk elevation and is often suboptimally treated. Over the past 20 years, the incidence of gout has more than doubled, accompanied by an increase in comorbid conditions such as diabetes, hypertension, renal disease, and dyslipidemia. The pharmacist can assist prescribers in optimizing medication selection, performing diseasestate monitoring, and titrating patients to their SUA goals.
REFERENCES
- Elfishawi MM, Zleik N, Kvrgic Z, et al. The rising incidence of gout and the increasing burden of comorbidities: a population-based study over 20 years. J Rheumatol. 2018;45:574-579.
- Chen-Xu M, Yokose C, Rai SK, et Contemporary prevalence of gout and hyperuricemia in the United States and decadal trends: the National Health and Nutrition Examination Survey, 2007-2016. Arthritis Rheumatol. 2019;71:991-999.
- Hainer BL, Matheson E, Wilkes Diagnosis, treatment, and prevention of gout. Am Fam Physician. 2014;90:831-836.
- Vargas-Santos AB, Neogi Management of gout and hyperuricemia in CKD. Am J Kidney Dis. 2017;70:422-439.
- Burke BT, Köttgen A, Law A, et al. Gout in older adults: the Atherosclerosis Risk in Communities study. J Gerontol A Biol Sci Med Sci. 2016;71:536-542.
- Feig DI, Kang DH, Johnson Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811-1821.
- Culleton BF, Larson MG, Kannel WB, Levy D. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med. 1999;131:7-13.
- Pérez Ruiz FP, Richette P, Stack AG, et Failure to reach uric acid target of <0.36 mmol/L in hyperuricaemia of gout is associated with elevated total and cardiovascular mortality. RMD Open. 2019;5:e001015.
- Ioachimescu AG, Brennan DM, Hoar BM, et Serum uric acid is an independent predictor of all-cause mortality in patients at high risk of cardiovascular disease: a preventive cardiology information system (PreCIS) database cohort study. Arthritis Rheum. 2008;58:623-630.
- Harrold LR, Mazor KM, Negron A, et al. Primary care providers’ knowledge, beliefs and treatment practices for gout: results of a physician questionnaire. Rheumatology (Oxford). 2013;52:16231629.
- Maravic M, Hincapie N, Pilet S, et Persistent clinical inertia in gout in 2014: an observational French longitudinal patient database study. Joint Bone Spine. 2018;85:311-315.
- Neogi Clinical practice. Gout. N Engl J Med. 2011;364:443452.
- Terkeltaub R. Update on gout: new therapeutic strategies and options. Nat Rev Rheumatol. 2010;6:30-38.
- Gonzalez EB. An update on the pathology and clinical management of gouty arthritis. Clin Rheumatol. 2012;31:13-21.
- Haines A, Bolt J, Dumont Z, Semchuk Pharmacists’ assessment and management of acute and chronic gout. Can Pharm J (Ott). 2018;151:107-113.
- Camerota A, Mastroiacovo D, Bocale R, Desideri G. Therapeutic challenges in the management of chronic hyperuricemia and gout in the elderly. Ann Gerontol Geriatric Res. 2014;1:1012.
- Chowalloor PV, Keen HI, Inderjeeth Gout in the elderly. OA Elderly Med. 2013;1:2.
- Roddy E, Choi HK. Epidemiology of gout. Rheum Dis Clin North Am. 2014;40:155-175.
- Rai SK, Fung TT, Lu N, et al. The Dietary Approaches to Stop Hypertension (DASH) diet, Western diet, and risk of gout in men: prospective cohort study. 2017;357:j1794.
- Holland R, McGill Comprehensive dietary education in treated gout patients does not further improve serum urate. Intern Med J. 2015;45:189-194.
- Choi HK. Diet, alcohol, and gout: how do we advise patients given recent developments? Curr Rheumatol Rep. 2005;7:220-226.
- Hunter DJ, York M, Chaisson CE, et al. Recent diuretic use and the risk of recurrent gout attacks: the online case-crossover gout study. J Rheumatol. 2006;33:1341-1345.
- Wilson L, Saseen JJ. Gouty arthritis: a review of acute management and prevention. 2016;36:906-922.
- Juraschek SP, Appel LJ, Miller ER III. Metoprolol increases uric acid and risk of gout in African Americans with chronic kidney disease attributed to hypertension. Am J Hypertens. 2017;30:871-875.
- Vezmar Kovacevic S, Simisic M, Stojkov Rudinski S, et al. Potentially inappropriate prescribing in older primary care patients. PLoS One. 2014;9:e95536.
- Zhang Y, Neogi T, Chen C, et al. Low-dose aspirin use and recurrent gout Ann Rheum Dis. 2014;73:385-390.
- Qaseem A, Harris RP, Forciea MA. Management of acute and recurrent gout: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:58-68
- Khanna D, Fitzgerald JD, Khanna PP, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012;64(10):1431-1446.
- Khanna D, Khanna PP, Fitzgerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and anti-inflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64:1447-1461.
- Richette P, Doherty M, Pascual E, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis. 2017;76:29-42.
- Doherty M, Jenkins W, Richardson H, et al. Efficacy and cost-effectiveness of nurse-led care involving education and engagement of patients and a treat-to-target urate-lowering strategy versus usual care for gout: a randomised controlled trial. 2018;392:1403-1412.
- Prasad S, Ewigman B. Acute gout: oral steroids work as well as NSAIDs. J Fam Pract. 2008;57:655-657.
- Wilbur K, Makowsky M. Colchicine myotoxicity: case reports and literature review. Pharmacotherapy. 2004;24:1784-1792.
- Kwon OC, Hong S, Ghang B, et al. Risk of colchicine-associated myopathy in gout: influence of concomitant use of statin. Am J Med. 2017;130:583-587.
- American College of Rheumatology. Gout. rheumatology.org/Practice-Quality/Clinical-Support/Clinical-Practice-Guidelines/Gout. Accessed January 7, 2020.
- Duzallo and Zurampic. duzallohcp.com. Accessed February 14, 2020.
- Janssens HJ, Janssen M, van de Lisdonk EH, et al. Use of oral prednisolone or naproxen for the treatment of gout arthritis: a double-blind, randomised equivalence trial. 2008;371:1854-1860.
- Abhishek A, Valdes AM, Jenkins W, et al. Triggers of acute attacks of gout, does age of onset matter? A primary care based cross-sectional PLoS One. 2017;12:e0186096.
- Celebrex (celecoxib) package insert. New York, NY: Pfizer Inc; May 2019.
- FDA adds Boxed Warning for increased risk of death with gout medicine Uloric (febuxostat). www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-increased-risk-death-gout-medicine-uloricfebuxostat. Accessed January 15, 2020.
- White WB, Saag KG, Becker MA, et al. Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med. 2018;378:1200-1210.
- FDA to evaluate increased risk of heart-related death and death from all causes with the gout medication febuxostat (Uloric). www.fda. gov/media/108760/download. Accessed January 15, 2020.
- Gillen M, Valdez S, Zhou D, et al. Effects of renal function on pharmacokinetics and pharmacodynamics of lesinurad in adult volunteers. Drug Des Devel Ther. 2016;10:3555-3562.
- Gupta A, Sharma PK, Misra AK, Singh S. Lesinurad: a significant advancement or just another addition to existing therapies of gout? J Pharmacol Pharmacother. 2016;7:155-158.
- Koide H, Hira D, Tsujimoto M, et al. Previous dosage of allopurinol is a strong determinant of febuxostat efficacy. Biol Pharm Bull. 2017;40:681686.
- Cutolo M, Cimmino MA, Perez-Ruiz Potency on lowering serum uric acid in gout patients: a pooled analysis of registrative studies comparing febuxostat vs. allopurinol. Eur Rev Med Pharmacol Sci. 2017;21:41864195.
- Becker MA, Schumacher HR, Espinoza LR, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther. 2010;12:R63.
- Stamp LK, Chapman Urate-lowering therapy: current options and future prospects for elderly patients with gout. Drugs Aging. 2014;31:777786.
- Singh JA, Cleveland JD. Gout and the risk of age-related macular degeneration in the PLoS One. 2018;13:e0199562.
- Singh JA, Yu Are allopurinol dose and duration of use nephroprotective in the elderly? A Medicare claims study of allopurinol use and incident renal failure. Ann Rheum Dis. 2017;76:133-139.
- Levy G, Shi JM, Cheetham TC, Rashid N. Urate-lowering therapy in moderate to severe chronic kidney disease. Perm J. 2018;22:17-142.
- Sircar D, Chatterjee S, Waikhom R, et al. Efficacy of febuxostat for slowing the GFR decline in patients with CKD and asymptomatic hyperuricemia: a 6-month, double-blind, randomized, placebo-controlled trial. Am J Kidney Dis. 2015;66:945-950.
- Graham GG, Kannangara DR, Stocker SL, et al. Understanding the dose-response relationship of allopurinol: predicting the optimal Br J Clin Pharmacol. 2013;76:932-938.
- Zurampic (lesinurad) package insert. Cambridge, MA: Ironwood Pharmaceuticals, Inc; January
- Smith WB, Hall J, Berg JK, et al. Effect of renal impairment on the pharmacokinetics and pharmacodynamics of verinurad, a selective uric acid reabsorption inhibitor. Clin Drug Investig. 2018;38:703-713.
- Jung JY, Choi Y, Suh CH, et al. Effect of fenofibrate on uric acid level in patients with Sci Rep. 2018;8:16767.
- Wolff ML, Cruz JL, Vanderman AJ, Brown JN. The effect of angiotensin II receptor blockers on hyperuricemia. Ther Adv Chronic Dis. 2015;6:339-346.
- Singh JA, Shah N, Edwards NL. A cross-sectional internet-based patient survey of the management strategies for gout. BMC Complement Altern Med. 2016;16:90.
- Natural Medicines (comprehensive database). https://naturalmedicines. therapeuticresearch.com. Accessed February 13,
- Daily JW, Yang M, Park S. Efficacy of turmeric extracts and curcumin for alleviating the symptoms of joint arthritis: a systematic review and meta-analysis of randomized clinical J Med Food. 2016;19:717-729.
- Aung T, Myung G, FitzGerald JD. Treatment approaches and adherence to urate-lowering therapy for patients with gout. Patient Prefer Adherence. 2017;11:795-800.
- Morlock R, Chevalier P, Horne L, et Disease control, health resource use, healthcare costs, and predictors in gout patients in the United States, the United Kingdom, Germany, and France: a retrospective analysis. Rheumatol Ther. 2016;3:53-75.
- Briesacher BA, Andrade SE, Fouayzi H, Chan KA. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy. 2008;28:437-443.
- Nassar-Ghodsi N, Harrold LR. Overcoming adherence issues and other barriers to optimal care in gout. Curr Opin Rheumatol. 2015;27:134-138.
- Singh JA. Challenges faced by patients in gout treatment: a qualitative study. J Clin Rheumatol. 2014;20:172-174.
- Goldfien RD, Ng MS, Yip G, et al. Effectiveness of a pharmacist-based gout care management programme in a large integrated health plan: results from a pilot study. BMJ Open. 2014;4:e003627.