Management of IBS With Concomitant Major Depressive Disorder
RELEASE DATE
December 1, 2020
EXPIRATION DATE
December 31, 2022
FACULTY
Austin De La Cruz, PharmD, BCPP
Clinical Assistant Professor
University of Houston College of Pharmacy
Houston, Texas
Carol Baby, PharmD
VA North Texas Health Care System
Dallas, Texas
FACULTY DISCLOSURE STATEMENTS
Drs. De La Cruz and Baby have no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT
 Pharmacy
Pharmacy
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-20-149-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER:
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients’ conditions and possible contraindications or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
GOAL
To educate pharmacists about the prevalence, pathophysiology, and treatment options available for irritable bowel syndrome (IBS), with a focus on individuals with a concomitant diagnosis of major depressive disorder.
OBJECTIVES
After completing this activity, the participant should be able to:
- Recognize the prevalence and pathophysiology of IBS.
- Describe the clinical presentation of IBS.
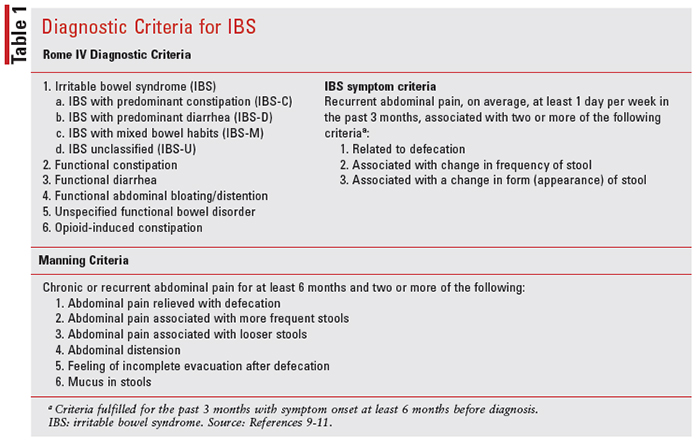
- Summarize the Rome IV and Manning diagnostic criteria related to IBS.
- Design appropriate pharmacologic treatment options for major depressive disorder based on a patient’s IBS clinical presentation and individual factors.
ABSTRACT: Irritable bowel syndrome (IBS) is a gastrointestinal disorder that is commonly associated with psychiatric disorders, such as major depressive disorder. When considering pharmacologic treatment options for the treatment of IBS, multiple factors should be considered, including the predominance of constipation or diarrhea, the presence of abdominal pain, and changes related to bowel function. Since various mental-health disorders have been associated with IBS, it is common practice for individuals to be placed on antidepressants in order to help manage these mental-health–related concerns. Unfortunately, there is a lack of clinical guidance on the recommendations for specific antidepressant treatment options that should be tailored to patients with IBS. There is, however, some data that suggest a more symptom-targeted therapeutic approach that may prove to be beneficial for both disorders.
Irritable bowel syndrome (IBS) is a common gastrointestinal (GI) disorder that results in chronic and irregular bowel movements. This disorder is typically characterized by recurrent complaints of lower abdominal pain, gas, and irregular defecation, which may include either constipation (IBS-C) or diarrhea (IBS-D). IBS is regarded as one of the most frequently diagnosed GI conditions. Studies show that 15% to 20% of Americans suffer from this disorder, and only about 25% of those affected seek medical care.1 When left untreated, IBS can have a substantial negative impact on an individual’s quality of life, which may result in missed workdays, decreased functioning and productivity, and an increased burden on the utilization of healthcare resources.
IBS has been traditionally characterized as a brain-gut disorder due to the strong association between IBS and an individual’s coexisting psychiatric diagnosis. Studies show that there is an increased prevalence of psychiatric disorders such as anxiety, depression, and personality disorders that commonly occur among adults with IBS.2 Often during the management of depression, GI-related complications are ignored or disregarded as typical medication-related side effects. GI-related concerns, however, should not be discounted in patients with a mental-health–related diagnosis or for those receiving psychiatric pharmacotherapy. It is important that a patient receive a full evaluation and diagnosis in order to tailor an appropriate treatment regimen that does not exacerbate any existing GIrelated symptoms. There are many treatment options available for major depressive disorder, including selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), atypical antidepressants, and tricyclic antidepressants (TCAs). At this time, however, there is little guidance regarding the use of these drugs to treat the symptoms of major depressive disorder in a patient with concomitant IBS.
Pathophysiology
The exact pathophysiologic theory that results in the symptomatology of IBS is not fully understood at this time. Multiple mechanisms have been postulated, including dysfunctional GI motility, visceral hypersensitivity, intestinal inflammation, food sensitivity, psychosocial dysfunction, intestinal microbiota alterations, and neurohormonal dysregulation, to name a few. Recent studies hypothesize that the foundational pathophysiologic concept results from a disturbance in the brain-gut axis, which is the central line of communication that exists between the brain and the GI tract.3 This dysregulation may be the principal reason that 50% to 90% of individuals with IBS have a coexisting mental-health disorder, including panic disorder, generalized anxiety disorder, social phobia, posttraumatic stress disorder, or major depressive disorder.4
A healthy and fully functioning GI tract may be a pivotal factor in determining an individual’s psychiatric-related health, especially since 95% of the serotonin in our body is located in the gut.5 Serotonin (5-HT) plays a significant role in our enteric nervous system and is responsible for secretion, sensitization, and motility via serotonin type 3 (5-HT3) and serotonin type 4 (5-HT4) receptors.6 In about half of cases, IBS originates in the gut, not the brain, resulting in IBS symptoms starting first, followed by subsequent psychologic distress that may develop into a full diagnostic mental-health disorder.7
Screening and Diagnosis
IBS is a chronic and often lifelong disorder that requires proper diagnosis and care due to the high occurrence of symptom relapse. Patient-reported symptoms associated with IBS range from abdominal pain, bloating, changes in stool form, and consistency, to variable patterns of diarrhea and constipation. Additionally, lower abdominal pain is often reported, which can be extremely debilitating. IBS is often challenging to diagnose due to overlapping symptoms with other GI disorders, such as nonulcer dyspepsia and celiac disease.8 Because of the variabilities in symptomatology, two diagnostic criteria are often utilized to help diagnose IBS, including the Manning criteria and Rome IV criteria (see TABLE 1). Providers may further perform interventional tests, such as a sigmoidoscopy or colonoscopy, to rule out other GI disorders. Additional laboratory tests may also be ordered to monitor the stool for occult blood, ova, and parasites, complete blood count, erythrocyte sedimentation rate, and serum electrolytes.6
Management of IBS
A complete medication history should first be performed, and all current medications (prescriptions, OTC, complementary) should be assessed for the possibility of worsened constipation or diarrhea. Nonpharmacologic treatment options for the management of IBS should be considered first-line for mild episodes that may occur less frequently in order to avoid unnecessary medication-related side effects. Oftentimes, an individual may be able to identify certain food triggers that worsen IBS-related symptoms. Caffeine, alcohol, artificial sweeteners, and lactose are known to cause GI irritation in select individuals.6
A trial of trigger-food avoidance may be a reasonable first step, prior to pharmacologic interventions. A restricted diet low in fermentable oligo-, di-, and monosaccharides and polyols (FODMAPs) may be beneficial in reducing IBS-related hypersensitivity and pain according to several small studies.3 FODMAPs include foods such as wheat, beans, dairy, and high fructose corn syrup. Standard practice includes a short period of restricting FODMAPs from the diet, followed by an ordered reintroduction of FODMAPs to determine tolerability.12 With this technique, there is a risk of an inadequate diet in the short term that may extend to a long-term deficiency if the diet continues to be significantly restricted. Ultimately, appropriate nutrient intake is recommended in patients with IBS despite the restrictions in nutrient-rich foods.12 These recommendations, albeit helpful for a certain subgroup of patients, are not universal due to the prevalence of treatmentrelated failures, in addition to the variability of symptoms among different patients.
For constipation-predominant IBS, patients might consider dietary fiber as the first nonpharmacologic approach. Both soluble and insoluble fibers have been studied for their effect on IBS.13 Soluble fibers include oats, barley, flaxseeds, oranges, carrots, and beans, and insoluble fibers include wheat bran, whole grains, broccoli, cabbage, and grapes. Soluble fibers in particular appear to play a positive role in improving symptoms of IBS. It is reasonable to advise patients to include small amounts of soluble fiber into their diet over the course of a few weeks. If symptoms improve, this can be continued.13
Insoluble fibers do not appear to show benefits and therefore should not be recommended for the management of IBS since they may lead to worsening abdominal pain and bloating.7,13 Total fiber intake of 20 to 30 grams per day is recommended, although dose-dependent bloating, distention, and flatulence can affect overall tolerability and compliance. Not all patients will benefit from fiber supplementation, such as those with severely delayed colon transit or obstructed defecation.10 The desired outcome should be bulkier stools that are more easily passed.6
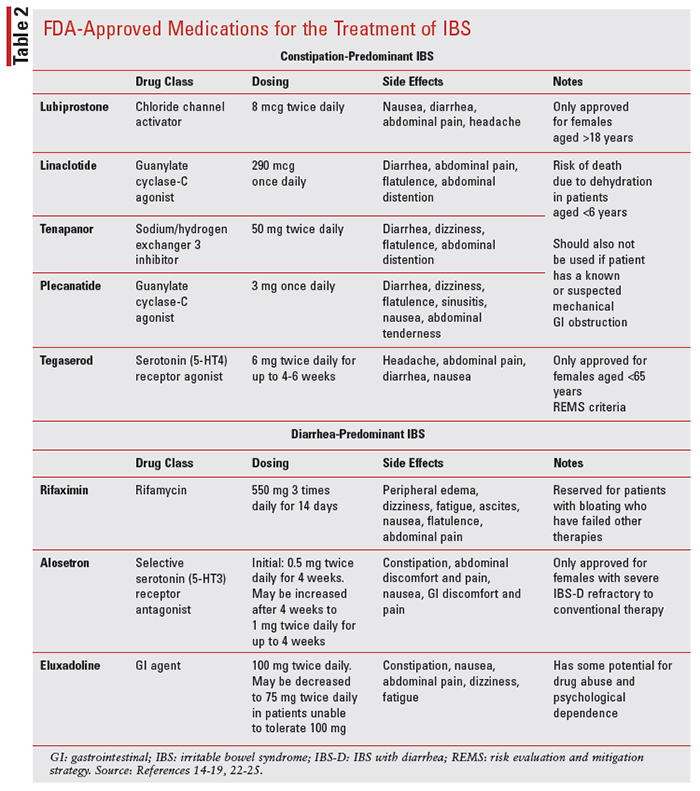
Pharmacologic treatment options for IBS depend on the predominance of constipation or diarrhea as well as the level of severity of those symptoms (see TABLE 2). There are not enough data to make a definitive decision as a second-line treatment option after fiber failure for constipation-predominant individuals; however, polyethylene glycol (PEG) is an osmotic agent that has shown superiority to both placebo and lactulose when used in adults and children.6,10 Side effects of PEG include nausea, vomiting, flatulence, and abdominal cramping.6 Caution should be taken with saline laxatives (e.g., magnesium citrate, magnesium sulfate, and sodium phosphate), which have limited data to show safety and efficacy. For symptom relief, stimulant laxatives (e.g., bisacodyl) have demonstrated clinical benefits for stool frequency and other related symptoms. Side effects may include severe abdominal cramping, nausea, vomiting, and electrolyte disturbances. Long-term laxative use is not encouraged for severe constipation and should be used at the lowest required dose.6
Lubriprostone, linaclotide, and plecanatide are three prosecretory agents that have demonstrated efficacy over placebo by improving constipationrelated symptoms of IBS-C in adults. Lubriprostone is FDA approved for chronic idiopathic constipation, opioid-induced constipation, and IBS-C in women aged 18 years and older. The most common adverse effects include nausea, diarrhea, and abdominal pain.14 Linaclotide is FDA approved for chronic idiopathic constipation and IBS-C. Linaclotide is contraindicated in patients younger than age 6 years due to dehydration-related deaths and in patients with known or suspected mechanical GI obstruction. Additionally, safety and efficacy have not been established in patients younger than age 18 years and, therefore, linaclotide is not recommended in this population. Common adverse effects of linaclotide include diarrhea, abdominal pain, flatulence, and abdominal distention.15
Plecanatide is currently FDA approved for chronic idiopathic constipation and IBS-C. It shares a similar mechanism with linaclotide by increasing the amount of fluid in the large intestines, which results in increased number of bowel movements and decreased abdominal pain through guanylate cyclase-C agonism. The most common adverse effect is diarrhea, which occurred in 5% of patients who were studied.16 The FDA recently approved tenapanor in 2019 for the treatment of IBS-C. Tenapanor, a novel treatment, is a minimally absorbed small molecule that acts locally by inhibiting the sodium hydrogen exchanger 3 in the gut. This drug is also used in patients with chronic kidney disease on dialysis to reduce serum phosphorus. The most common adverse effect reported was diarrhea. Like linaclotide and plecanatide, tenapanor is also contraindicated in patients younger than age 6 years. Additionally, safety and efficacy have not been established in patients younger than age 18 years and, therefore, is not recommended in this age group.17
Tegaserod is a 5-HT4 partial agonist that was originally FDA approved for the treatment of IBS-C in women. Tegaserod was later removed from the market in 2008 due to the risks of severe adverse effects, including the increased risk of heart attack or stroke. On April 1, 2019, the company received approval for tegaserod reintroduction into the market for IBS-C in women younger than age 65 years.18 Tegaserod is only available through a strict prescribing program in which providers are to contact the FDA Division of Drug Information to receive the medication directly from the manufacturer. Common adverse effects include abdominal pain, diarrhea, headache, and nausea.19
Diarrhea-predominant individuals may consider OTC loperamide, which is indicated for the control and symptomatic relief of acute and nonspecific diarrhea in patients aged 2 years or older with chronic diarrhea associated with inflammatory bowel disease. Even though loperamide does not have an FDA-approved indication for IBS, it is recommended by the American Gastroenterological Association over no drug treatment since it reduces stool frequency and improves stool consistency. It is important to note that loperamide does not reduce the global symptoms of IBS.20 Loperamide is a muopioid agonist that binds opiate receptors in the gut and thereby slows intestinal motility by affecting water and electrolyte movement through the bowel. Loperamide has black box warnings of torsades de pointes and sudden death if higher than recommended doses are used. High doses should be avoided due to the risk of serious cardiac events. Loperamide is contraindicated in pediatric patients younger than age 2 years. Common adverse effects include dry mouth, flatulence, and abdominal cramps.21
Rifaximin is an FDA-approved medication for the treatment of IBS-D in adults. It is a short-term treatment that is taken three times a day for 2 weeks. Rifaximin has a unique mechanism of action and is classified as a semisynthetic derivative of the antibiotic rifampin and acts by binding to the betasubunit of bacterial DNA-dependent RNA polymerase, blocking one of the steps in transcription. This mechanism results in the inhibition of bacterial protein synthesis and, consequently, inhibits the growth of bacteria. The efficacy of rifaximin was established in three randomized, multicenter, double-blind, placebo-controlled trials. The first two trials were identical, and both trials demonstrated statistically significant improvement of rifaximin versus placebo (41% vs. 31%, P = .0125; 41% vs. 32%, P = .0263) for adequate relief of IBS symptoms during the month following 2 weeks of treatment. The third trial also showed improvement in abdominal pain responders, resulting in a 30% or greater reduction in abdominal pain in 51% of rifaximin patients versus 42% of placebo patients (95% CI: 9% [1.6-17]). Rifaximin treatment can be repeated up to two times if symptoms return. Common adverse effects of rifaximin include nausea, fatigue, peripheral edema, flatulence, abdominal pain, and ascites.22
Alosetron is a highly selective 5-HT3 antagonist indicated for women with severe IBS-D who have not responded adequately to conventional therapy. Alosetron showed improvement in abdominal pain and IBS-related global symptoms. Due to the risk of severe adverse reactions such as ischemic colitis and serious complications of constipation, alosetron is only to be used by those physicians enrolled in the Prometheus Prescribing Program. The Risk Evaluation and Mitigation Strategy program restrictions are primarily due to the risks of hospitalizations, blood transfusions, and death that may occur with alosetron therapy. The most common adverse reactions in clinical studies were constipation, nausea, abdominal discomfort, and pain.23
Eluxadoline is a novel mixed mu- and kappareceptor agonist and delta-receptor antagonist. It is indicated for the treatment of IBS-D in adults. Given its opioid-receptor activity, eluxadoline is currently listed as a schedule IV controlled substance, and patients should be closely monitored for the potential of drug abuse and dependence. In clinical trials, eluxadoline improved abdominal stool consistency, frequency, and quality-of-life metrics. Eluxadoline is contraindicated in patients without a gallbladder and in those with known or suspected structural disease of the pancreas, alcoholism, severe hepatic impairment, or a history of severe constipation. The most common adverse effects include constipation, nausea, and abdominal pain.24
Antispasmodics are used to treat abdominal pain and spasms in all IBS subtypes and may be helpful in IBS-D.10 Dicyclomine is currently FDA approved for the treatment of IBS. Dicyclomine relieves smoothmuscle spasms of the GI tract through anticholinergic effects as well as through a direct effect upon smooth muscle. Adverse effects include dry mouth, dizziness, blurred vision, and nausea.25 Hyoscyamine is often used in clinical practice despite the lack of evidence supporting its efficacy. OTC peppermint oil has also been found to be generally effective and well tolerated in patients with IBS. Common adverse effects include heartburn, nausea, and vomiting.26
Probiotics such as bifidobacterium infantis have also shown improvements in abdominal pain, bloating, and bowel movement difficulty compared with placebo. Disodium cromoglycate, fecal microbiota transplantation, herbal therapies, and complementary therapies have all been postulated as well; however, none of these treatment modalities have been extensively studied for this indication.10
Antidepressant Use in IBS
Proper pharmacologic treatment that considers the patient’s mental-health disorder and IBS-related complications is essential. As previously mentioned, patients with IBS are more likely to have depression, anxiety, and somatization disorders compared with the general population, so the chance that a healthcare provider will interact directly with a patient with concomitant diagnoses is unquestionable.3 Since antidepressants may have GI-related effects that can either improve or worsen the patient’s already existing IBSrelated symptoms, it becomes imperative to select an appropriate, well-tolerated agent from the start.
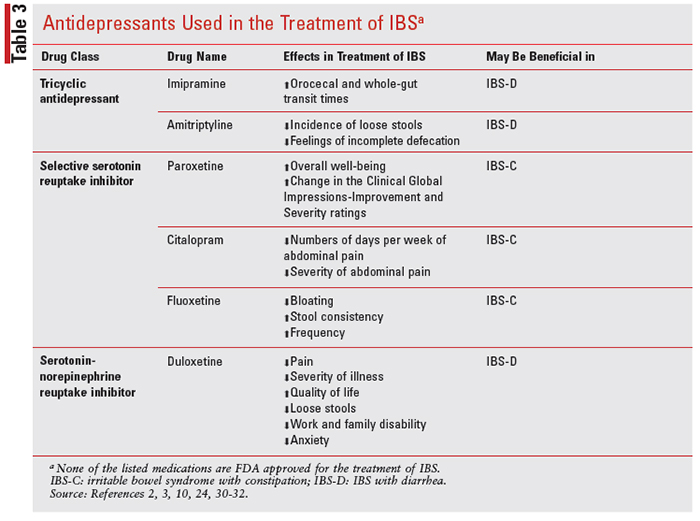
Most major depressive disorder treatment guidelines recommend SSRIs, SNRIs, bupropion, or mirtazapine as first-line treatment options for patients with mild-to-moderate depression, in addition to psychotherapy as adjunctive treatment. TCAs tend to be reserved as a third-line treatment option due to the increased risk of neurologic-, anticholinergic-, and cardiovascular-related adverse effects. Although antidepressants are not considered first-line treatment options for IBS, there are many trials that have looked at their efficacy in the management of IBS symptoms given their interaction with the serotonin neurotransmitters in the digestive system (see TABLE 3).
As a class, antidepressants were studied in a metaanalysis that included 18 randomized, controlled trials involving 1,127 patients. In this meta-analysis, both the TCAs and SSRIs showed total improvement compared with placebo and appeared to be effective treatments for IBS. The number needed to treat for TCAs and SSRIs was 4.5 and 5, respectively. Although this may not be clinically relevant, it indicates that there may be a slight benefit for TCAs compared with SSRIs. (The number needed to treat is the number of patients required to treat to prevent one additional bad outcome.) While observing the eight trials in the meta-analysis that reported overall adverse events, the incidence of adverse events was significantly higher in the antidepressant groups, especially the TCA group, compared with placebo, with both drowsiness and dry mouth being the most common complaints in the TCA group. In total, 36.4% of patients assigned to antidepressants experienced adverse events, compared with 21.1% of those taking placebo.27
In patients with IBS-D, TCAs at low doses have shown benefits by controlling the perception of visceral pain and altering GI transit time.1 Additionally, some patients who are taking TCAs commonly experience anticholinergic effects, such as constipation, dry mouth, blurred vision, and urinary retention. For a patient with IBS-D, this side effect of constipation may be beneficial. In one trial, IBS-D patients were given imipramine for 4 days, which resulted in prolonged orocecal transit time and whole-gut transit times, both of which are used to measure gut motility.2 In a separate study, amitriptyline was compared with placebo and was shown to be significantly more effective in decreasing the incidence of loose stools and the feeling of incomplete defecation.3 Additionally, a 2-month study that included 50 IBS-D patients found that 10 mg of amitriptyline was effective in reducing the incidence of loose stool and feeling of incomplete defecation. Patients also showed a greater complete response, which included loss of all symptoms compared with placebo.28 Not only is amitriptyline effective in adults with IBS, but it has also improved the quality of life in adolescents with IBS when taken for 6 to 13 weeks.6
As mentioned previously, TCAs have anticholinergic properties that may slow the GI tract, leading to benefits with chronic diarrhea in addition to modulating the perception of visceral pain.3,6 Because of TCAs’ significant anticholinergic properties, they should not be used in patients with both pain and constipation (IBS-C).
Compared with TCAs, there is less evidence to support the use of SSRIs or SNRIs with individuals who have IBS-D with recurring abdominal pain. One study evaluating the use of the SSRI, paroxetine, showed a significant improvement in overall wellbeing with paroxetine but no significant change in abdominal pain, bloating, or social functioning.24 A separate study observed the use of paroxetine controlled-release and also showed no significant difference in composite pain scores. The paroxetine study, however, showed a significant change in the Clinical Global Impressions-Improvement and Severity ratings.29 A third SSRI study showed that citalopram significantly reduced the numbers of days per week and severity of abdominal pain. Significant improvements in urgency and straining were seen at week 6.30,31 In contrast, a meta-analysis that observed the efficacy of citalopram showed conflicting data. However, in this same meta-analysis, fluoxetine showed improvement of IBS-C symptoms such as bloating, stool consistency, and frequency.30 There are a lack of data available for the use of SNRIs in IBS, but one study showed that duloxetine significantly improved pain, severity of illness, quality of life, loose stools, work and family disability, and anxiety, independent of its antidepressant effects in patients with IBS.10,32
TCAs and SSRIs appear to have some data in providing relief in IBS, especially if IBS symptoms are pain related, where TCAs appear to be more effective. Adverse events can potentially limit both classes as appropriate pharmacologic agents. More studies that focus on SSRIs and SNRIs in patients with IBS are needed to determine the clinical use of these classes. Pharmacologic treatment agents should be selected based on the patient’s presentation, predominance of constipation or diarrhea, and individual symptomatology in order to personalize therapy. An appropriate antidepressant would treat the patient’s depression while providing relief of IBS-related symptoms and not exacerbate the patient’s overall GI symptoms.
The Pharmacist's Role
Pharmacists can play a major role in the management of IBS by identifying common symptoms associated with IBS and counseling patients on the many treatment options available, which may include antidepressants. Given the high percentage of adults with IBS and concomitant mental illness, it would be advantageous for pharmacists to educate patients on the potential benefits and risks of using antidepressants for IBS. Additionally, since there are well-known psychiatric-related concerns that may develop with an IBS-related diagnosis, pharmacists should instruct their patients to seek out mental-health treatment if they are not being managed by a mental-health provider. Cognitive behavioral therapy and behavioral techniques aimed at modifying the patient’s avoidance behaviors may serve as proper adjunctive therapy to their existing antidepressant regimen. Even though there is limited literature surrounding the use of antidepressants in IBS, pharmacists must keep up to date with new literature in order to educate both patients and providers about the safest and most effective treatment options available.
Conclusion
There are a few treatment options that can be considered in a patient with IBS who has a concomitant major depressive disorder. One of the biggest challenges is choosing the right agent that will not exacerbate IBS symptoms. In patients with treatmentresistant IBS, antidepressant therapy may be beneficial, and treatment should be determined based on the patient’s diagnosed IBS type. There are some data that show the benefits of TCAs, such as imipramine and amitriptyline, for patients experiencing treatment-resistant IBS-D. TCAs have also been shown to prevent loose stools and improve the patient’s overall quality of life. It is important to note, however, that TCAs are commonly used as third-line treatment options for major depressive disorder due to the risks of adverse effects, so close monitoring should be performed if this class of medications is used for IBS. SSRIs and SNRIs have been studied in smaller trials, but more large-scale, high-quality studies are needed in order to observe the effects of these medications in the management of IBS with concomitant major depressive disorder.
Diarrhea-predominant individuals may consider OTC loperamide, which is indicated for the control and symptomatic relief of acute and nonspecific diarrhea in patients aged 2 years and older with chronic diarrhea associated with inflammatory bowel disease. High doses should be avoided due to the risk of cardiac events.
REFERENCES
1. Chang JY, Talley Nj. An Update on irritable bowel syndrome: from diagnosis to emerging therapies. Curr Opin Gastroenterol. 2011;27:72-78.
2. Cremonini F, Talley NJ. Irritable bowel syndrome: epidemiology, natural history, health care seeking, and emerging risk factors. Gastroenterol Clinc North Am. 2005;34:189-204.
3. Cashman MD, Martin DK, Dhillon S, et al. Irritable bowel syndrome: a clinical review. Curr Rheum Rev. 2016;12:13-26.
4. Lydiard RB. Irritable bowel syndrome, anxiety, and depression: what are the links? J Clin Psychiatry. 2001; 62(suppl 8):38-45.
5. Camilleri M. Serotonin in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes. 2009;16(1):53-59.
6. Fabel PH, Shealy KM. Diarrhea, constipation, and irritable bowel syndrome. In: DiPiro JT, Talbert RL, Yee GC, Patzke GR, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 10th ed. New York, NY: McGraw-Hill. http://accesspharmacy.mhmedical.com/content.asp x?bookid=1861§ionid=146059459. Accessed August 11, 2020.
7. Ford AC, Lacy BE, Talley NJ. Irritable bowel syndrome. N Engl J Med. 2017;376:2566-2578.
8. National Collaborating Centre for Nursing and Supportive Care (UK). Irritable Bowel Syndrome in Adults: Diagnosis and Management of Irritable Bowel Syndrome in Primary Care [Internet]. London: Royal College of Nursing (UK); 2008 Feb. (NICE Clinical Guidelines, No. 61.) 6, Diagnosis. www.ncbi.nlm.nih.gov/books/ NBK51944/
9. Drossman DA, Hasler WL. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology. 2016;150:12571261.
10. Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology. 2016;150:1393-1407.
11. Manning AP, Thompson WG, Heaton KW, et al. Towards positive diagnosis of the irritable bowel. British Medical Journal. 1978;2:653654.
12. Staudacher HM. Nutritional, microbiological and psychosocial implications of the low FODMAP diet. J Gastroenterology and Hepatology. 2017;32:16-19.
13. Nagarajan N, Morden A, Bischof D, et al. The role of fiber supplementation in the treatment of irritable bowel syndrome. European Journal of Gastroenterology & Hepatology. 2015;27(9):1002-1010.
14. Sucampo Pharmaceuticals Inc. (2012). Lubiprostone: Package Insert. Bethesda, MD.
15. Forest Pharmaceuticals Inc. (2012). Linaclotide: Package Insert. St. Louis, MO.
16. Salix Pharmaceuticals Inc. (2020). Plecanatide: Package Insert. Bridgewater, NJ.
17. Ardelyx Inc. (2019). Tenapanor: Package Insert. Fremont, CA.
18. Druken T. FDA approves the reintroduction of Zelnorm (tegaserod) for irritable bowel syndrome with constipation (IBS-C) in women under 65. Finanzen.ch. 2019.
19. Novartis Pharmaceutical Coproration. (2002). Tegasorod: Package Insert. East Hanover, NJ.
20. Weinberg D, Smalley W, Heidelbaugh J, Sultan S. American Gastroenterological Association Institute Guideline on the Pharmacological Management of Irritable Bowel Syndrome. American Gastroenterological Association. 2014;1146-1148.
21. Johnson & Johnson Consumer Inc. (2016). Loperamide: Package Insert. USA: Food and Drug Administration
22. Salix Pharmaceuticals Inc. (2010). Rifaximin: Package Insert. Morrisville, NC, USA: Food and Drug Administration
23. GlaxoSmithKline. (2002). Alosetron: Package Insert. Research Triangle Park, NC.
24. Barshop K, Staller K. Eluxadoline in irritable bowel syndrome with diarrhea: rationale, evidence, and place in therapy. Ther Adv Chronic Dis. 2017;8(11):153-160.
25. Axcan Scandipharm Inc. (2006). Dicyclomine: Package Insert. Birmingham, AL.
26. Trinkley KE, Nahata MC. Medication management of irritable bowel syndrome. Digestion. 2014;89:253-267.
27. Ford AC, Lacy BE, Harris LA, et al. Effect of antidepressants and psychological therapies in irritable bowel syndrome: an updated systematic review and meta-analysis. Am Journal of Gastroenterology. 2019;114(1):21-39.
28. Vahedi H, Merat S, Momtahen S, et al. Clinical trial: the effect of amitriptyline in patients with diarrhea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2008;27:678-684.
29. Masand PS, Pae C, Krulewicz S, et al. A double-blind, randomized, placebo-controlled trial of paroxetine controlled-release in irritable bowel syndrome. Psychosomatics. 2009;50(1):78-86.
30. Trinkley K, Nahata M. Treatment of irritable bowel syndrome. Journal of Clinical Pharmacy and Therapeutics. 2010;(36):275-282.
31. Tack J, Broekaert D, Fischler B, et al. A controlled crossover study of the selective serotonin reuptake inhibitor citalopram in irritable bowel syndrome. Gut. 2006;55(8):1095-1103.
32. Brennan B, Fogarty K, Roberts J, et al. Duloxetine in the treatment of irritable bowel syndrome: an open label pilot study. Human Psychopharmacology: Clinical and Experimental. 2009;14(5):423-428.