Managing Modifiable Risk Factors in Chronic Coronary Disease
RELEASE DATE
February 1, 2024
EXPIRATION DATE
February 28, 2026
FACULTY
Meg Conger Ason, PharmD, BCACP, CPP
Clinical Pharmacist
Atrium Health
Huntersville, North Carolina
Jennifer LaPreze, PharmD, BCACP, CPP
Clinical Pharmacist
Atrium Health
Fort Mill, South Carolina
Caroline McDaniel, PharmD, CPP
Clinical Pharmacist
Atrium Health
Shelby, North Carolina
FACULTY DISCLOSURE STATEMENTS
Drs. Conger Ason, LaPreze, and McDaniel have no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT
 Pharmacy
Pharmacy
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-24-022-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients' conditions and possible contraindications or dangers in use, review of any applicable manufacturer's product information, and comparison with recommendations of other authorities
GOAL
To review the modifiable risk factors and treatment for chronic coronary disease, with a focus on dyslipidemia management.
OBJECTIVES
After completing this activity, the participant should be able to:
- Discuss the modifiable risk factors for chronic coronary disease.
- Explain how the management of comorbid disease states can lower the risk of cardiovascular disease.
- Recognize the importance of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors in improving cardiovascular risk.
- Identify therapies that target additional mechanisms in lowering LDL cholesterol and cardiovascular risk.
ABSTRACT: Chronic coronary disease (CCD) impacts >20 million people in the United States and contributes significantly to U.S. healthcare costs. Patients’ decisions about medications, lifestyle, and other modifiable risk factors can greatly affect their risk of cardiovascular (CV) events. Diet and exercise, as well as tight control of hyperlipidemia, hypertension, and diabetes, can reduce the incidence of myocardial infarction, cerebrovascular events, and other CV diseases. Statins (3-hydroxy-3-methylglutaryl coenzyme reductase inhibitors) are the foundation of therapy to improve CV risk by lowering cholesterol levels. Newer drug classes have emerged that show promise for lessening the CV disease burden. The pharmacist’s role in CCD management involves ascertaining that guideline therapies are used appropriately and educating patients about risk, lifestyle, and medication therapy management.
Coronary artery disease (CAD) is the most common type of heart disease in the United States.1 It is the leading cause of morbidity and mortality worldwide, with an estimated prevalence of >127 million people aged ≥20 years in 2020.2 The average direct and indirect costs associated with cardiovascular (CV) disease in the U.S. were estimated to be $407.3 billion in 2018 and 2019.2
PATHOPHYSIOLOGY
CAD is characterized by atherosclerotic plaque formation that causes narrowing and limiting of the blood flow within the epicardial arteries.3 Atherosclerosis is caused by endothelial dysfunction, inflammation, and oxidative-stress processes. Fatty streaks are formed by subendothelial deposition of foam cells. Foam cells are formed when the intimal layer breaks and monocytes move into the subendothelial space, becoming macrophages that take in oxidized LDL particles. Growth factors increase the number of foam cells, whereas T cells, when activated, release cytokines. This process results in plaque formation. If no insult occurs to the endothelium, a fibrous cap forms and becomes calcified, limiting blood flow and potentially causing anginal symptoms. If plaque rupture occurs, a thrombosis can form, causing partial or total occlusion of the impacted artery.4,5
CAD is classified as stable ischemic heart disease (SIHD) or acute coronary syndrome (ACS). SIHD is also referred to as chronic coronary disease (CCD) in the most recent guideline.6 In the U.S., 20.1 million individuals live with CCD and 11.1 million persons have chronic stable angina.6 Angina occurs is when myocardial oxygen demand exceeds the oxygen supply, resulting in chest discomfort.7 Stable angina is when chest discomfort is predictable, able to be replicated during certain testing, and relieved by rest or nitroglycerin.7 Because the focus of this article is modifiable risk factors in CCD, ACS management and angina treatment will not be discussed.
Primary goals in CCD treatment include improving quality of life, minimizing ischemic symptoms, and reducing the risk of CV events and death. To attain these goals, risk-factor modification and optimal medical therapy are needed.
RISK FACTORS
CAD is multifactorial, and contributing aspects can be categorized into modifiable and nonmodifiable factors. Modifiable risk factors include tobacco use, obesity, type 2 diabetes, hypertension, dyslipidemia, and chronic kidney disease. Nonmodifiable factors include age, sex, race, family history, and genetics.4,8 In both men and women, the incidence of CAD rises with age.
EVALUATION AND TESTING
To accurately assess risk factors and evaluate severity of disease, it is important for the clinician to obtain a thorough history, including family history, from the patient. Testing is another important piece of the puzzle. Some tests that may be used to evaluate or diagnose CAD include ECG, echocardiogram (ECHO), chest x-ray, stress testing, cardiac catheterization, and coronary artery calcium (CAC) score.4,5 The clinician will determine which tests are appropriate based on the patient’s presentation.
ECG, which measures the electrical activity of the heart, provides information regarding the heart’s rate, rhythm, and axis. An ECG can show ventricular hypertrophy, bundle branch blocks, and axis deviation in the chronic setting as well as T-wave and ST-segment changes during ACS.4 ECHO (ultrasound of the heart) shows the wall motion, valvular regurgitation and stenosis, and size of the heart’s chambers.4 ECHO may be used to assess response to therapy and can help diagnose certain pathologies, including pulmonary embolism.4 Stress testing can be exercise-based or pharmacologic; it can help diagnose CAD or be used for risk stratification. Cardiac catheterization is an invasive procedure that is considered the gold standard for evaluating ischemic CAD. The 2021 American Heart Association/American College of Cardiology (AHA/ACC) chest pain guidelines contain additional information regarding the role of invasive testing and revascularization.4,9
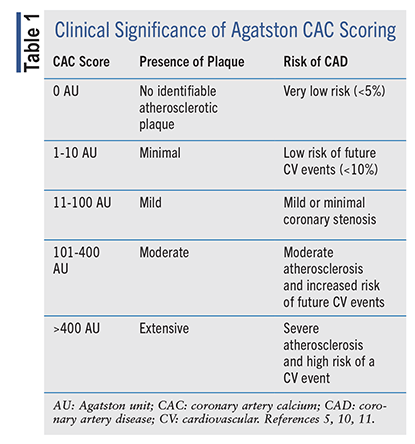
The CAC score is a noninvasive test that uses cardiac CT to assess the amount of calcium in the coronary arteries.5 CAC is related to coronary atherosclerosis, except in patients with renal insufficiency, who are more likely to develop medial calcification.5 The CAC score provides clinicians with additional information about a patient’s risk for obstructive CAD and future CV events, and it can help guide choices in therapy or additional testing.10,11 In patients without diabetes who have a 10-year atherosclerotic CV disease (ASCVD) risk of 7.5% to 20%, the ACC Solution Set Oversight Committee recommends evaluating a CAC score.12 CAC score is typically not used in patients aged <40 years, as they usually do not have calcified plaque.10,11 There are several methods for assessing CAC score. The Agatston method is commonly used, with the score being a product of the calcified atherosclerosis area and density weighting factor. The Agatston scoring system, including clinical significance, is presented in TABLE 1.5,10,11,13
GUIDELINE-DIRECTED MANAGEMENT AND THERAPY
Primary goals in CCD treatment include improving quality of life, minimizing ischemic symptoms, and reducing the risk of CV events and death. To attain these goals, risk-factor modification and optimal medical therapy are needed to prevent disease progression (TABLE 2).6 It is important to seek patients’ engagement and agreement on modifications to optimize risk management. Healthcare professionals can provide information to help patients individualize their decisions, taking into account socioeconomic, cultural, and educational factors. Lifestyle and medication choices are individualized, and shared decision-making will help maintain patient motivation and follow-through.6,14
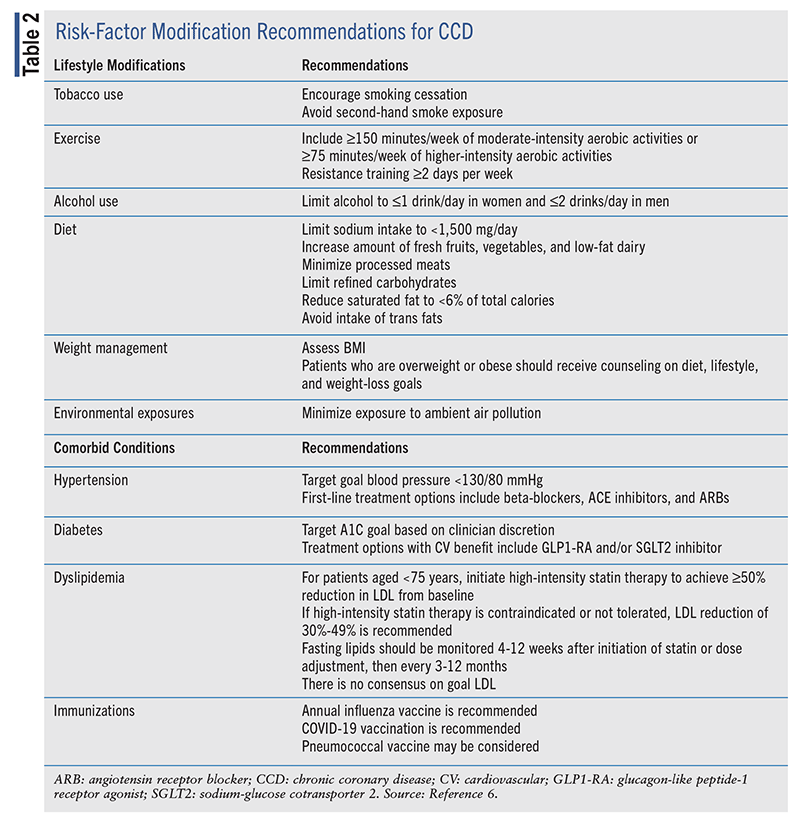
Lifestyle Management
Healthy lifestyle behaviors reduce the risk of CV events and mortality.6 A diet that is high in fiber and limits saturated fat, sodium, and refined carbohydrates provides significant health benefits.6 Increasing physical activity in patients without limitations also can help with risk reduction. The use of nonprescription medications and dietary supplements, including omega-3 fatty acids, calcium, and vitamins C, D, and E has not been shown to reduce the risk of acute CV events.6 Tobacco use should be assessed at each visit, with smoking-cessation therapies offered, including behavioral therapy. Although short-term e-cigarette use may be considered to assist with smoking cessation, the long-term safety is unknown.6
Hypertension Management
Hypertension affects >60% of patients with CCD.6 The risk of CV events doubles for every 20-mmHg increase in systolic blood pressure (BP) or 10-mmHg increase in diastolic BP.6,15 Every 10-mmHg reduction in systolic BP reduces CV risk by 17%.15,16 For patients with CCD, the goal BP is <130/80mmHg, and all-cause mortality is reduced by 27% in those who achieve it.6,17 Drug therapy for hypertension can be determined by other indications the individual patient may have.
Beta-blockers, ACE inhibitors, and angiotensin receptor blockers (ARBs) are considered first-line therapy for CCD. Beta-blockers are recommended in patients who have had a recent myocardial infarction (MI) or have ongoing angina. Beta-blockers for CCD include timolol, propranolol, bisoprolol, nadolol, metoprolol succinate, metoprolol tartrate, and carvedilol. Atenolol was noted to be inferior.6 In patients with CCD and left ventricular ejection fraction (LVEF) ≤40% with or without MI, beta-blockers are recommended to reduce the risk of future major adverse CV events (MACE), including nonfatal stroke, nonfatal MI, and CV death. Metoprolol succinate, carvedilol, or bisoprolol titrated to target doses is recommended in patients with CCD and LVEF <50%. Reassessment of long-term beta-blocker use may be appropriate if there is not a clinical indication for therapy, including the absence of left ventricular systolic dysfunction.6
ACE inhibitors and ARBs are recommended in patients with CCD who have LVEF ≤40% or conditions such as diabetes, chronic kidney disease, or hypertension, if not contraindicated. If additional BP-lowering therapies are needed, calcium channel blockers, mineralocorticoid receptor antagonists, or thiazide diuretics may be considered.6
Diabetes Management
Patients with diabetes have double the risk of developing CAD compared with those who do not have diabetes.15 The ideal A1C to reduce macrovascular complications in patients with diabetes has not been demonstrated. An A1C of ≤7% is reasonable for most patients and has a proven reduction in microvascular complications.15,18 However, an A1C of ≤6.5% may be suitable for patients with a longer life expectancy, and an A1C of ≤8% may be appropriate for patients with multiple comorbid conditions.19
Historically, metformin has been the initial drug of choice for diabetes management. Per 2023 American Diabetes Association recommendations, diabetes medications with proven reduction in CV events should be initiated in patients with CAD. These medications, which include glucagon-like peptide-1 receptor agonists (GLP1-RAs) and sodium-glucose cotransporter 2 (SGLT2) inhibitors, are summarized in TABLE 3.18,19
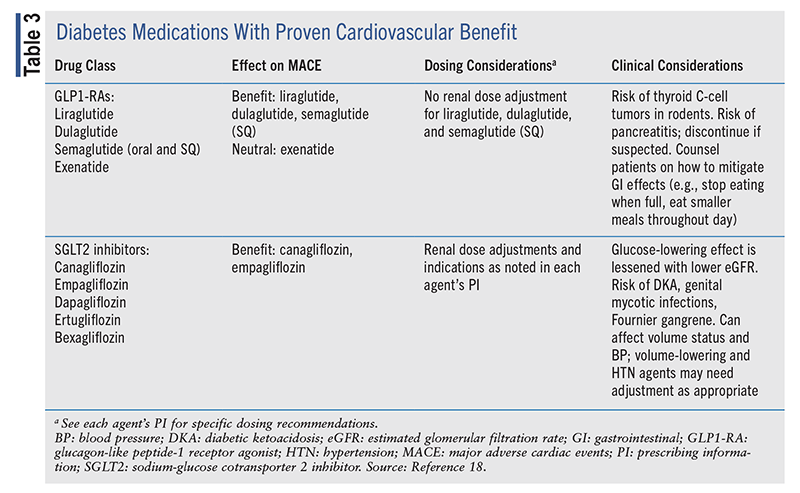
The injectable GLP1-RAs liraglutide, semaglutide, and dulaglutide have proven reductions in CV events in patients with established ASCVD. In the trials LEADER, SUSTAIN-6, and REWIND, which studied liraglutide, injectable semaglutide, and dulaglutide, respectively, researchers noted a significant decrease in the primary composite of CV events.20,21 Semaglutide and dulaglutide also demonstrated a reduction in stroke, whereas liraglutide reduced all-cause mortality and CV death.20 The SGLT2 inhibitors canagliflozin and empagliflozin reduced the primary CV composite in the CANVAS and EMPA-REG trials, respectively, whereas dapagliflozin did not. 22,23 Dapagliflozin, however, demonstarted a decrease in hospitalization for heart failure.24
Dyslipidemia Management
In the pharmacotherapy assessment, it is important to educate patients about risk-reduction cholesterol targets and medication options so that they can participate in decisions about strategies and which agents to use. In addition to lifestyle changes, combination drug therapy is becoming increasingly necessary as evidence emerges about the ideal degree of LDL-lowering for ASCVD risk reduction. It is agreed that lower LDL targets lead to a lower risk of ASCVD risk without safety concerns about intensifying treatment; however, there is no consensus on optimal LDL targets. LDL goals of <100 mg/dL have given way to recommendations for levels <70 mg/dL, <55 mg/dL, and even lower.25 The AHA/ACC Joint Committee recommends lowering the LDL level by as much as 50% to reduce ASCVD risk, but it has not established a target threshold.6
Per 2022 ACC recommendations, the CAC score can be a useful tool in assessing a patient’s risk and need for therapy. If the CAC score is 0 AU, statin use may be held, with the patient reassessed in 5 to 10 years if no other high-risk conditions develop. If the score is between 1 and 99 and is below the 75th percentile for age, sex, and race, a statin is recommended for patients aged ≥55 years. If the CAC is ≥100 AU or the result is above the 75th percentile for age, sex, and race, a statin is recommended.12
Statins
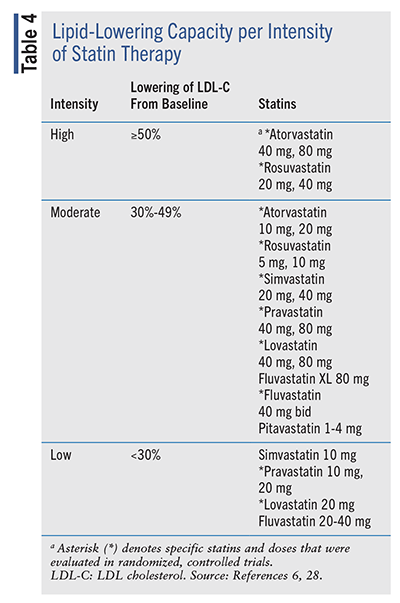
Three-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, or statins, are the foundation of therapy to lower cholesterol levels in primary and secondary prevention of CCD.6 Statins work by inhibiting hepatic HMG-CoA reductase, which is the rate-limiting step in cholesterol biosynthesis.26 Once the statin binds to the reductase enzyme, activity is decreased, thus reducing the intracellular synthesis of cholesterol.26 Statins decrease total cholesterol, LDL cholesterol (LDL-C), and triglycerides and increase HDL cholesterol. Hyperlipidemia treatment recommendations include use of a high-intensity statin to attain the LDL-C reduction of ≥50%.6
Clinical trials have determined the effect of statin therapy on MACE.6 In 2010, the Cholesterol Treatment Trialists’ Collaboration performed a meta-analysis of 26 randomized, controlled trials (RCTs) to evaluate the efficacy and safety of more intensive lowering of LDL-C. Five of the RCTs studied more intensive versus less intensive statin regimens, and the other 21 RCTs compared statin versus control groups.27 In the RCTs comparing statin regimens, the more intensive regimen led to a significant reduction in MACE (rate ratio 0.85; 95% CI, 0.82-0.89).27 In these studies, high-intensity statin therapy was shown to reduce MACE 15% more than moderate-intensity statin therapy.6 In all 26 trials, a significant reduction in major vascular events was found per 1-mmol/L reduction in LDL-C.27 Reduction in MACE occurred across all populations irrespective of age, and findings demonstrated that the greater the LDL-C reduction, the greater reduction in MACE.27
Lipid-Lowering Capacity: The lipid-lowering capacity per intensity of statin therapy is quantified in TABLE 4.28 As mentioned earlier, the goal in patients with CAD is to lower LDL-C by ≥50%. Although this reduction is expected, response varies among patient populations.6
Tolerability Issues: Although statin therapy is well tolerated overall, in clinical practice intolerance is not uncommon.29 Statin intolerance is defined as having one or more adverse effects from statin therapy that subside upon dose reduction or discontinuation of the statin.29 To confirm that a patient has statin intolerance, the patient must have tried two statins, one of them at the lowest approved daily dose.29 Statin intolerance can lead to suboptimal lipid-lowering and negative clinical effects, increasing the patient’s risk for adverse CV outcomes.
Muscle-related adverse effects, including myalgia, myopathy, myositis, and rhabdomyolysis, are the most common side effects noted by patients experiencing statin intolerance. The most-reported complaint with statin therapy is myalgia.29 It is now reasonably accepted to try alternate-day dosing of statins, especially atorvastatin or rosuvastatin, because these regimens are equally efficacious for lowering LDL-C.30
Statins may be categorized as either hydrophilic or lipophilic.31 The hydrophilic statins include pravastatin and rosuvastatin, and the lipophilic statins include simvastatin, fluvastatin, lovastatin, pitavastatin, and atorvastatin. Lipophilic statins are associated with more intolerances because they enter the cells by passive diffusion and can become widely distributed to different tissues.31 The solubility of the statin can also impact metabolism; hydrophilic statins are easier for the liver to excrete, thus making them more tolerable.31
Additional Therapies
As research on LDL thresholds for minimizing ASCVD risks has continued to evolve, additional therapies (TABLE 5) have become important for helping patients, particularly those with homozygous and heterozygous hyperlipidemias, reach those thresholds. In 2022, the ACC Solution Set Oversight Committee released a report on the utility of newer nonstatin therapies for lowering LDL and ASCVD risk, including guidance on their use. For most patients who require additional LDL-lowering, firstline add-on agents include ezetimibe and the proprotein convertase subtilisin/kexin type 9 (PCSK9) monoclonal antibody inhibitors.12
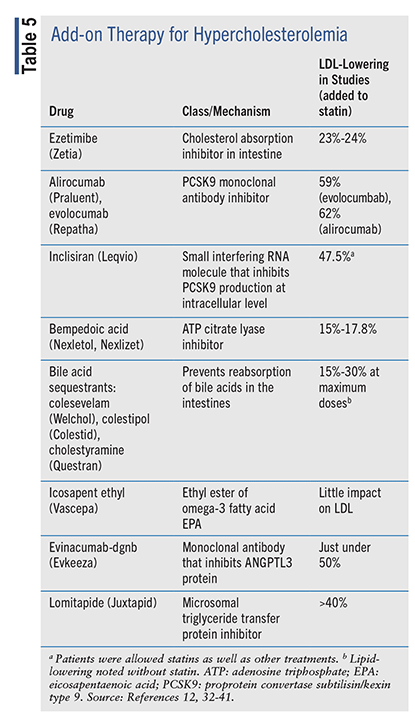
Ezetimibe: This effective oral drug, which was approved in 2002 and is available in generic form, inhibits the absorption of cholesterol in the small intestine, leading to reduced total cholesterol, LDL, and apolipoprotein B levels.12 In 2015, the IMPROVE-IT study showed that the addition of ezetimibe to a statin in patients with recent ACS resulted in modestly lower LDL levels and lower rates of the composite outcome: CV death, nonfatal MI, unstable angina requiring rehospitalization, coronary revascularization (≥30 days after randomization), or nonfatal stroke.32 The ACC Solution Set Oversight Committee report recommends ezetimibe in patients who need <25% additional lowering beyond statins and in those who cannot administer, afford, or tolerate PCSK9 drugs.12 Potential side effects of ezetimibe include persistent elevations in liver enzymes, especially when given in combination with statins. Cases of myopathy and rhabdomyolysis have been reported with ezetimibe with or without concomitant statins.12
PCSK9 Monoclonal Antibody Inhibitors: This drug class includes alirocumab and evolucumab. The PCSK9 protein marks the LDL receptor for degradation, and the blockade of PCSK9 keeps the receptor functioning; that, in turn, allows more LDL particles to be removed from circulation and can dramatically lower the LDL level.12 These drugs have been shown to reduce LDL by as much as 58% (alirocumab) and 64% (evolocumab) when added to maximally tolerated statins.12 The ODYSSEY Outcomes trial demonstrated that alirocumab also decreases the incidence of coronary heart disease death, MI, ischemic stroke, or hospitalization for unstable angina in patients with existing ASCVD.33 The FOURIER trial found that evolocumab lessened the risk of CVD death, MI, stroke, revascularization, or hospitalization for unstable angina in similar patients.34 Both trials have contributed to the theory that lower LDL levels than recommended previously can reduce CV events with little risk. PCSK9 monoclonal antibody inhibitors are self-administered SQ with an autoinjector every 2 to 4 weeks, and the at-home administration adds to ease of use. Injection-site reactions are among the potential side effects. As evidenced by the ODYSSEY Outcomes and FOURIER trials, these agents do not have any negative effects on cognition.12
Inclisiran: Inclisiran is a long-acting small interfering RNA molecule that inhibits PCSK9 production at an intracellular level.12 This agent was FDA approved in 2021 as an adjunct to diet and other therapies in patients with heterozygous familial hypercholesterolemia or elevated ASCVD risk who need additional LDL-lowering.12 Inclisiran is a 284-mg SQ injection that is administered in a healthcare professional’s office at day 1, 3 months, 6 months, and every 6 months thereafter.35 The most common side effects are infection-site reactions, joint pain, and respiratory infection.35 CV morbidity and mortality data for inclisiran are unknown, but studies are ongoing.12 As of yet, there have been no head-to-head studies between inclisiran and the monoclonal antibody inhibitors.12 It is not recommended for inclisiran and PCSK9 monoclonal antibody inhibitors to be used together.
Bempedoic Acid: This agent was FDA approved in 2020 as an adjunct to maximally tolerated statin therapy in adults with ASCVD or heterozygous familial hypercholesterolemia.12 Bempedoic acid inhibits adenosine triphosphate citrate lyase, resulting in upregulation of LDL receptors and improved clearance of LDL-C.12 This prodrug is activated by very-long-chain acyl CoA synthetase-1, which is an enzyme found in the liver but not in skeletal muscle cells. This is thought to make bempedoic acid a potentially effective option for patients with sensitivity to statins.12
In the CLEAR Outcomes study, bempedoic acid demonstrated LDL-lowering of approximately 21 percentage points and a statistically significant reduction in composite primary endpoint events: death from CV causes, nonfatal MI, nonfatal stroke, or coronary revascularization.36 It had no significant effects on individual outcomes for fatal or nonfatal stroke, death from CV causes, and death from any cause.36 Additionally, bempedoic acid was studied as an add-on to ezetimibe in the CLEAR Tranquility study and as monotherapy in the CLEAR Serenity study. When bempedoic acid was combined with a statin, an additional 15% to 17.8% in LDL-lowering occurred. Adding ezetimibe and bempedoic acid to a statin resulted in an additional 38% LDL-lowering beyond the statin alone.12 Clinically significant adverse effects include increased risk of hyperuricemia, tendon rupture, atrial fibrillation, and benign prostatic hyperplasia.36 Bempedoic acid should be avoided with concomitant simvastatin >20 mg daily or pravastatin 40 mg daily.12
Bile Acid Sequestrants: Bile acid sequestrants, such as colesevelam, colestipol, and cholestyramine, are nonabsorbed polymers that bind bile acids and prevent their reabsorption in the intestine.37 They have been approved for use in adults with hyperlipidemia and in boys and postmenarchal girls aged 10 to 17 years with heterozygous familial hyperlipidemia.37 Bile acid sequestrants can provide a small amount of LDL-lowering when added to statins. However, tolerability, the risks of pancreatitis and gastrointestinal complications, and the heavy pill burden make these drugs a lower-priority choice for lipid-lowering.12
Icosapent Ethyl: Icosapent ethyl, a highly purified eicosapentaenoic acid ethyl ester, is an add-on agent that is recommended to help reduce the risk of MACE in patients with elevated triglycerides despite maximal use of statins.6 The REDUCE-IT trial demonstrated a significant decrease in the composite endpoint CV events in patients taking icosapent ethyl 2 g twice daily.38 Icosapent ethyl typically does not impact LDL.
Familial Hypercholesterolemias
Adults and children with homozygous familial hypercholesterolemia have notably higher LDL levels and risk of CCD and CV events. New medications are emerging to treat these rare conditions, including evinacumab and lomitapide. These medications also offer LDL-lowering in the 40% to 50% range.12,39
THE PHARMACIST’S ROLE
The role of the pharmacist in CCD management lies in ascertaining that guideline therapies are used appropriately and in educating patients about managing risks, lifestyle, and medication therapy. With many clinicians prescribing multiple types of CCD therapies, pharmacists are centrally positioned to make sure that their patients are prescribed the most appropriate treatment. Given their regular accessibility to patients, pharmacists have a key opportunity to help patients navigate tolerability and adherence issues or cost and accessibility concerns. As therapy options expand, the pharmacist’s evolving role is to foster optimal treatment in their patients with CCD.
CONCLUSION
CCD is a complex disease state with a significant cost burden for patients if risk factors are not managed and treatment is not optimized. Patients can improve outcome and quality of life by implementing medications as well as lifestyle changes including exercise, weight loss, smoking cessation, and other interventions. Pharmacists are integral in guiding patients through this shared decision-making process.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
- CDC. Coronary artery disease. July 19, 2021. www.cdc.gov/heartdisease/coronary_ad.htm. Accessed November 30, 2023.
- Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics—2023 update: a report from the American Heart Association. Circulation. 2023;147(8):e93-e621.
- Herrington W, Lacey B, Sherliker P, et al. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res. 2016;118(4):535-546.
- Shahjehan RD, Bhutta BS. Coronary artery disease. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan–.
- Shreya D, Zamora DI, Patel GS, et al. Coronary artery calcium score—a reliable indicator of coronary artery disease? Cureus. 2021;13(12):e20149.
- Virani SS, Newby LK, Arnold SV, et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA guideline for the management of patients with chronic coronary disease: a report of the American Heart Association/ American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2023;148(9):e9-e119.
- Kannam JP, Aroesty JM, Gersh BJ. Chronic coronary syndrome: overview of care. UpToDate. www.uptodate.com/contents/chronic-coronary-syndrome-overview-of-care. Accessed November 30, 2023.
- Regmi M, Siccardi MA. Coronary artery disease prevention. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan–.
- Gulati M, Levy PD, Mukherjee D, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;144(22):e368-e454.
- Pedersen ER, Hovland S, Karaji I, et al. Coronary calcium score in the initial evaluation of suspected coronary artery disease. Heart. 2023;109(9):695-701.
- Kramer CM, Villines TC. Coronary artery calcium scoring (CAC): overview and clinical utilization. UpToDate.www.uptodate.com/contents/coronary-artery-calcium-scoring-cac-overview-and-clinical-utilization. Accessed January 16, 2024.
- Lloyd-Jones DM, Morris PB, Ballantyne CM, et al. 2022 ACC expert consensus decision pathway on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2022;80(14):1366-1418.
- Cherukuri L, Birudaraju D, Budoff MJ. Coronary artery calcium score: pivotal role as a predictor for detecting coronary artery disease in symptomatic patients. Coron Artery Dis. 2021;32(6):578-585.
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation.2019;140(11):e596-e646.
- Knuuti J, Wijns W, Saraste A, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes: the task force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J. 2020;41(3):407-477.
- Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol.2012;60(24):e44-e164.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127-e248.
- American Diabetes Association. Standards of Care in Diabetes—2023 abridged for primary care providers. Clin Diabetes. 2022;41(1):4-31.
- ElSayed NA, Aleppo G, Aroda VR, et al; American Diabetes Association. Cardiovascular disease and risk management. Diabetes Care. 2023;46(Suppl 1):s158-s190.
- Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121-130.
- Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375(4):311-322.
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644-657.
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128.
- Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347-357.
- Handelsman Y, Jellinger PS, Guerin CK, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the management of dyslipidemia and prevention of cardiovascular disease algorithm—2020 executive summary. Endocr Pract. 2020;26(10):1196-1224.
- Bansal AB, Cassagnol M. HMG-CoA reductase inhibitors. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan–.
- Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170 000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;139(25):e1082-e1143.
- Cheeley MK, Saseen JJ, Agarwala A, et al. NLA scientific statement on statin intolerance: a new definition and key considerations for ASCVD risk reduction in the statin intolerant patient. J Clin Lipidol. 2022;16(4):361-375.
- Awad K, Mikhailidis DP, Toth PP, et al. Efficacy and safety of alternate-day versus daily dosing of statins: a systematic review and meta-analysis. Cardiovasc Drugs Ther. 2017;31(4):419-431.
- Climent E, Benaiges D, Pedro-Botet J. Hydrophilic or lipophilic statins? Front Cardiovasc Med. 2021;8:687585.
- Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387-2397.
- Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097-2107.
- Sabatine MS, Giugliano RB, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713-1722.
- Leqvio (inclisiran). Novartis Pharmaceuticals Corp. www.leqvio.com. Accessed November 11, 2023.
- Nissen SE, Lincoff AM, Brennan D, et al. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N Engl J Med. 2023;388(15):1353-1364.
- Lent-Schochet D, Jialal I. Antilipemic agent bile acid sequestrants. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan–.
- Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380(11):11-22.
- Cuchel M, Bruckert E, Ginsberg HN, et al. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J. 2014;35(32):2146-2157.
- Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1489-1499.
- Ray KK, Troquay RPT, Visseren FLJ, et al. Long-term efficacy and safety of inclisiran in patients with high cardiovascular risk and elevated LDL cholesterol (ORION-3): results from the 4-year open-label extension of the ORION-1 trial. Lancet Diabetes Endocrinol. 2023;(11)2:109-119.