Managing Overactive Bladder Syndrome
RELEASE DATE
September 1, 2024
EXPIRATION DATE
September 30, 2026
FACULTY
Charles R. Breese, PhD
Professor of Pharmaceutical Sciences
Department of Pharmaceutical Sciences
William Carey University School of Pharmacy
Biloxi, Mississippi
Donna M. Breese, PharmD
Professor of Pharmacy Practice
Department of Pharmacy Practice
William Carey University School of Pharmacy
Biloxi, Mississippi
DISCLOSURE STATEMENTS
Faculty Disclosures: Dr. D. Breese and Dr. C. Breese have no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT
 Pharmacy
Pharmacy
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-24-092-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients’ conditions and possible contraindications or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
GOAL
To educate pharmacists about the latest American Urological Association/Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction (AUA/SUFU) guidelines on the pharmacologic and nonpharmacologic management of overactive bladder (OAB).
EDUATIONAL OBJECTIVES
After completing this activity, the participant should be able to:
- Identify the symptoms of OAB.
- Discuss the AUA/SUFU guideline recommendations on the treatment of OAB.
- Define the place in therapy for nonpharmacologic treatments for OAB.
- List the pharmacologic treatments for OAB and describe the unique issues associated with treating OAB.
ABSTRACT: Overactive bladder (OAB) syndrome is characterized by the symptoms of urgency (with or without urge incontinence), frequency, and nocturia. Patients experience the constant urge to urinate, even when the bladder is not full. Frequent trips to the bathroom and worrying about involuntary urination can affect a patient’s quality of life. Treatments include behavioral therapies (e.g., modified fluid intake, pelvic floor exercises), medications (e.g., antimuscarinic medications, β-3 adrenergic agonists), and surgery. Pharmacists can optimize the pharmacologic treatment of OAB by doing a thorough medication review, eliminating medications that may be contributing to the patient’s symptoms, and encouraging adherence.
Overactive bladder (OAB) is a clinical syndrome defined by the International Continence Society as urinary urgency (the sudden and intense urge to pass urine), usually with frequency (the need to urinate multiple times a day), and nocturia (the need to wake up frequently during the night to urinate) in the absence of infection or other obvious causes. Patients with OAB may also experience the sudden, intense urge to urinate, even when the bladder is not full. Urge urinary incontinence (UUI), the involuntary loss of urine with a feeling of urgency, may or may not accompany the symptom of urinary urgency in patients with OAB.1
OAB prevalence rates range from 7% to 27% in men and 9% to 43% in women.3 The prevalence and severity of OAB symptoms increase with age, with women being more likely than men to experience UUI with OAB; however, OAB affects men and women of all ages and is not simply a result of gender or aging. Despite having symptoms, many patients with OAB do not seek treatment for UUI or wait until their symptoms are particularly bothersome before seeking treatment.2,3
PATHOPHYSIOLOGY/PATHOGENESIS
Detrusor muscle contraction is essential for normal micturition; however, abnormalities associated with detrusor muscle hyperactivity can produce many of the symptoms observed in patients with OAB. The cholinergic (parasympathetic) system is involved with detrusor muscle contraction and bladder emptying, and the adrenergic (sympathetic) system is involved with relaxation of the bladder muscle and contraction of the sphincters that assist in bladder filling and continence.4 While both M2 and M3 receptors are found in the detrusor muscle of the bladder, the M3 muscarinic receptor subtype is thought to be the most important in regulating detrusor contraction and voiding.5 The detrusor muscle also has β-3 receptors that are involved with detrusor muscle relaxation and increased filling capacity of the bladder.6 Patients with hyperactivity of the detrusor muscle may have neurogenic and/or myogenic origins, but to date the cause of detrusor muscle hyperactivity is largely unknown.4
DIAGNOSIS
The American Urological Association/Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction (AUA/SUFU) guidelines recommend that the initial evaluation of OAB symptoms include a thorough medical history (including questions about urinary symptoms and review of medications), a physical examination (including anatomical factors and mobility impairment), and a urinalysis to rule out urinary tract infection and microhematuria.3 A validated lower urinary tract symptom questionnaire may be used to assess how bothersome symptoms are at baseline and for monitoring purposes during treatment. The Lower Urinary Tract Research Network Symptoms Index long version (LURN SI-29) or short version (LURN SI-10) and the Bristol Female Lower Urinary Tract Symptoms questionnaire are all validated for use in women. LURN SI-29 and LURN SI-10 were developed as patient-reported outcome measures to assess urinary symptoms in adult men and women. The LURN SI-29 and LURN SI-10 assess urgency, incontinence, voiding difficulty, nocturia, pain, frequency, and postmicturition symptoms over the past 7 days. The LURN SI-29 includes 27 questions for both men and women, and two questions that are gender-specific (one for women; one for men). It takes approximately 5 minutes to complete. The LURN SI-10, developed from the SI-29, includes 10 items used for an index total score and a summary item on the extent of bother. It is intended for use in clinical practice and takes approximately 2 minutes to complete. A voiding diary may also be helpful in assessing how bothersome symptoms are and how disruptive they may be to everyday life. A postvoid residual may be used in patients with OAB symptoms to help rule out incomplete emptying or urinary retention when voiding or emptying symptoms are present.3
To help guide the overall treatment plan, the patient should be assessed for comorbid conditions that may contribute to urinary frequency, urgency, or incontinence. Examples of these comorbid conditions may include constipation, menopause, sleep apnea, anxiety, depression, tobacco use, hypertension, obesity, glycosuria, diabetes, or pelvic organ prolapse.3
MINIMALLY INVASIVE TREATMENT APPROACHES
Traditionally, treatment of OAB has focused on a stepwise approach starting with noninvasive therapies as first line, pharmacologic therapies as second line, and minimally invasive therapies as third line when less invasive therapies are inadequate to manage symptoms or the patient is unwilling or unable to use a specific therapy. Guidelines suggest utilizing a shared decision-making process, and the clinician and the patient may choose to use noninvasive therapies alone or in combination with other noninvasive therapies, medications, and/or minimally invasive therapies.3
For most patients, treatment of OAB should begin with the same conservative, noninvasive therapies used for other types of urinary incontinence in women (see TABLE 1). These therapies usually have low risk of harm and few, if any, adverse effects. Incontinence management strategies (e.g., liners, pads, diapers, barrier creams, external catheters, vaginal inserts, and absorbent washable underwear) should be discussed with all patients with OAB and complaints of UUI. Behavioral therapies, including lifestyle modifications, bladder training, and pelvic floor muscle training (PFMT), should be considered for all patients with OAB who are willing and capable of utilizing them. Behavioral therapies alone may be sufficient for symptom management in some patients. For behavioral therapies to be successful, however, clinicians must provide adequate explanation and training and the patient must consistently utilize the therapy. Management of constipation, avoidance of excessive fluid intake, and reduced consumption of caffeine, carbonated beverages, diet beverages, and alcohol are important modifications for all patients with OAB. Patients who are overweight should be counseled on dietary modifications and exercise to encourage weight loss. Those who use tobacco products should be counseled on methods and aids for complete tobacco cessation. Bladder training, setting a voiding schedule (timed voiding), and using techniques such as relaxation, distraction, or pelvic floor muscle contractions may help suppress sensations of urgency that occur before the next scheduled void, which may reduce UUI episodes. Pelvic floor muscle exercises (Kegel exercises) help improve strength and control of the pelvic floor muscles to help prevent urine leakage. To benefit from the exercises, women must be taught to do them properly and must do them consistently.3,7
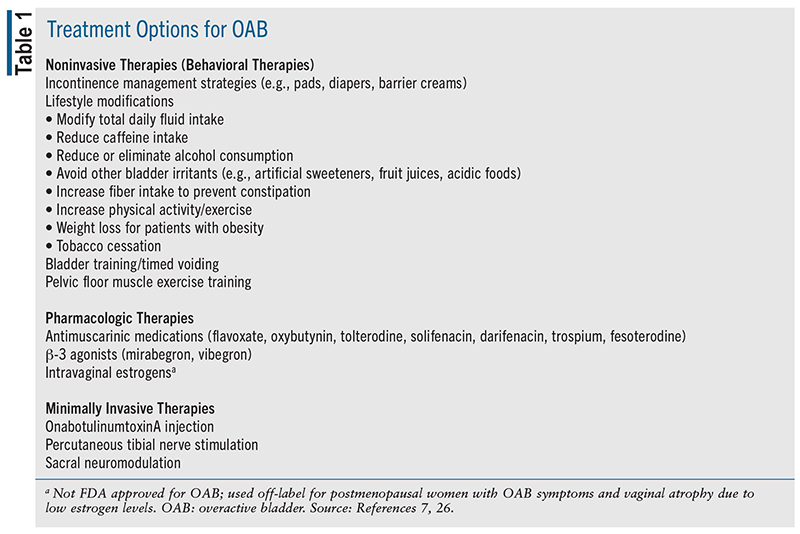
Patients should be reevaluated after 4 to 8 weeks to assess the response to initial treatment. For patients who remain symptomatic or experience intolerable side effects, pharmacologic therapies or minimally invasive therapies should be considered. The combination of pelvic floor exercises and other behavioral therapies with pharmacotherapy appears more effective than either approach alone.3
PHARMACOLOGIC THERAPIES
Medications are generally considered second line following the noninvasive, nonpharmacologic therapies described above (see TABLE 1). Two primary classes of medications are available for the treatment of OAB and include cholinergic muscarinic receptor antagonists (antimuscarinics) and β-3 adrenergic receptor agonists (β-3 agonists). Antimuscarinic medications have been the mainstay of pharmacologic therapy for OAB since the early 1970s with the introduction of flavoxate (Urispas) and oxybutynin (Ditropan). Since the mid-1990s, numerous additional antimuscarinic drugs have been introduced into the market.8 The β-3 agonists came to the market in 2012 with the introduction of mirabegron (Myrbetriq) and in 2018 with vibegron (Gemtesa). β-3 agonists have shown some advantages compared with antimuscarinic therapies for their improved side-effect profile and potential for improved efficacy.9 Since these drugs target different receptor systems on the detrusor muscle of the bladder, combination therapy is also an attractive option for patients who are not provided adequate symptom relief from single-drug therapy.
Antimuscarinic Therapy
Antimuscarinics have long been used to treat neurogenic detrusor overactivity and symptoms of OAB.8 Anticholinergic drugs block muscarinic receptor activation and inhibit the spontaneous detrusor muscle contractions found in OAB, leading to the subsequent relaxation of the bladder. By exerting a direct spasmolytic action on the smooth muscle of the bladder, antimuscarinic medications decrease symptoms associated with urge incontinence and OAB and can further increase bladder capacity and decrease or eliminate urge incontinence associated with OAB.10 For the antimuscarinic drugs, the efficacy is similar across the various drug agents in improving OAB symptoms but often with a relatively low overall magnitude of effect. This low magnitude of effect was also associated with only a modest improvement in QoL measures.10
The utility of antimuscarinics is often limited by unwanted antimuscarinic side effects, such as dry mouth, dry eyes, confusion, constipation, somnolence, blurred vision, and increased heart rate.10 Studies have shown that the available anticholinergic agents may vary in their side-effect profiles based on their chemical structure, size, and polarity, and in particular their penetration into the central nervous system (CNS).10,11 Extended-release (ER) formulations are generally preferred over immediate-release (IR) formulations due to improved compliance and decreased adverse side effects by maintaining lower and less fluctuating plasma concentrations of the medication.11 For patients who complain of a lack of therapeutic response or undesired side effects, dose adjustment or switching to another antimuscarinic agent or to a β-3 agonist are reasonable choices.
Flavoxate (Urispas)
Flavoxate hydrochloride was the earliest of the muscarinic antagonist and spasmolytic medications available for the treatment of OAB. Flavoxate is an antispasmodic agent that exerts an inhibition of the phosphodiesterases, a moderate calcium antagonistic activity, and a local anesthetic effect.12 Flavoxate treatment was associated with an improvement in urinary tract symptoms, including diurnal and night frequency, urgency and urinary incontinence, suprapubic pain, dysuria, hesitancy, and burning.12 The use of flavoxate fell with the advent of oxybutynin and other antimuscarinic medications.13
Oxybutynin (Ditropan)
Oxybutynin first became available in 1975 for the treatment of OAB and was found to be better tolerated than flavoxate, with fewer side effects.13 IR formulations (IR tablets and solution) tend to be less well tolerated than longer-acting formulations (ER tablets, patch, gel) due to a higher incidence of antimuscarinic side effects, such as dry mouth and constipation; however, IR formulations may be useful as a single daily dose in patients with bothersome symptoms during limited timeframes (e.g., nocturia without daytime symptoms). Oxybutynin is a lipophilic tertiary amine and readily crosses the blood-brain barrier (BBB), causing the potential for CNS adverse effects.14,15
Tolterodine (Detrol)
Tolterodine became available in 1998. When compared with oxybutynin, the reduction in the frequency of incontinence episodes was similar in both drugs; however, tolterodine, a tertiary amine, has low lipophilicity and shows limited effects on quantitative electroencephalography activity that were comparable with trospium chloride, a quaternary amine that fails to cross the BBB.14,16
Darifenacin (Enablex)
Darifenacin is a selective muscarinic M3–receptor antagonist for patients with OAB that is available as a controlled-release formulation. Darifenacin significantly reduced the frequency of urinary incontinence, frequency of micturition, and frequency and severity of urgency versus placebo, an effect observed as early as 2 weeks after starting treatment. In patients dissatisfied with earlier antimuscarinic treatments (oxybutynin ER or tolterodine ER), satisfaction levels were found to be higher in patients treated with darifenacin.17,18 CNS tolerability appeared to be similar to that of placebo, and no adverse effects were observed on cognitive function in healthy elderly patients.17
Solifenacin (Vesicare)
Solifenacin is also an M3-receptor-selective antimuscarinic medication for the treatment of neurogenic detrusor overactivity and symptoms of OAB. Solifenacin is available as a tablet (VESIcare) and liquid suspension (VESIcare LS) for children or those who are unable to swallow tablets. A study comparing solifenacin and tolterodine ER showed a significant benefit of solifenacin treatment.19 Solifenacin is a tertiary amine that is larger than other antimuscarinic agents such as oxybutynin, producing a reduced capacity to penetrate the BBB into the CNS, with clinical studies indicating little effect on cognition.20
Trospium (Sanctura)
Unlike other antimuscarinics for OAB, trospium is a quaternary ammonium compound that has been shown to decrease the number of voids, urge incontinent episodes, total daily micturitions, and urge severity and to improve QoL.21 Trospium significantly improved both nocturnal and diurnal OAB symptoms, with improvements in sleep-related QoL benefits.22 While trospium was associated with anticholinergic adverse effects similar to those of other anticholinergic agents, trospium has a lower risk of CNS side effects due its lower ability to cross the BBB.14,23 Trospium is also metabolized by the kidneys, thereby having fewer interactions with other medications and making it beneficial for elderly patients or those on multiple medications.24 One drawback is that both the IR and ER formulations of trospium need to be taken on an empty stomach with water at least 1 hour before meals. As a result, the once-daily ER formulation may be preferred due to less frequent dosing.
Fesoterodine (Toviaz)
Fesoterodine is one of the newer antimuscarinic agents approved for the management of OAB. It is unique in that it is converted to the same active metabolite as tolterodine, 5-hydroxymethyl tolterodine (5-HMT), by plasma esterases, which provide a fast and efficient conversion to 5-HMT. Clinical trials have established efficacy, as well as improvements in QoL.25
Antimuscarinic Adverse Drug Reactions
In general, the incidence of CNS side effects is low and similar to that with placebo; however, any patient showing signs of CNS dysfunction should be evaluated and a less lipophilic medication considered, such as trospium.14 Other antimuscarinic side effects may include urinary retention, agitation, drowsiness, blurred vision, decreased sweating, indigestion, urinary tract infections, and dry or itchy eyes. Antimuscarinic side effects may contribute to limited long-term adherence. Antimuscarinics are contraindicated or should be used with caution in patients with uncontrolled tachyarrhythmias, myasthenia gravis, gastric retention, or narrow angleclosure glaucoma. They should also be used with caution in older adults or those with cognitive impairment.26 One other concern with antimuscarinic medications is their possible interaction with other commonly prescribed drugs that have anticholinergic effects, increasing the anticholinergic “load” in patients with OAB who are being managed with antimuscarinic agents, potentially increasing the frequency and severity of anticholinergic side effects.8,10
β-3 Agonists
Mirabegron and vibegron are β-3 agonists used to treat symptoms of OAB. β-3 agonists work by binding the β-3 adrenergic receptor, causing direct relaxation of the detrusor muscle, increasing bladder capacity during bladder filling, and reducing symptoms of OAB.9,27 The β-3 agonists have quickly become a recommended first-line treatment for OAB and reducing symptoms such as urgency, UUI, and increased urinary frequency.3,9 Both mirabegron and vibegron improve OAB symptoms compared with placebo. Similar improvements for both drugs have been reported for number of incontinence episodes, number of micturition episodes, and number of UUI episodes.27-29
Comparison With Anticholinergic Medications
The clinical effectiveness of both mirabegron and vibegron appears to be at least similar to that of the antimuscarinics, although β-adrenergic medications may be more effective at reducing nocturia episodes.30 In clinical trials, there was a reduced discontinuation rate and fewer treatment-related adverse effects compared with antimuscarinics, favoring the use of β-3 adrenergic agonists.30,31
Mirabegron (Myrbetriq)
The FDA approved mirabegron for clinical use in the United States in June 2012 and as granules in 2021. In May 2018, the FDA approved mirabegron in combination with solifenacin for the treatment of OAB. The compound is highly lipophilic, metabolized in the liver, and eliminated in the urine and feces, mainly unchanged. Mirabegron shows minimal interactions with other frequently prescribed drugs, including warfarin, metformin, and oral contraceptives.32 However, as a moderate inhibitor of CYP2D6, it may increase levels of drugs that are metabolized by CYP2D6 (e.g., digoxin). Mirabegron was superior to placebo for reducing frequency, number, and severity of urgency episodes and number of incontinence episodes, as well as a reduction in nocturia episodes. A majority of studies have documented both reduction of symptom severity and improvement in treatment satisfaction and QoL.33
Vibegron (Gemtesa)
Vibegron was approved in the U.S. in December 2020. It is excreted in feces and urine unchanged. The plasma concentration of the drug increases in patients aged ≥65 years and those with moderate-to-severe renal impairment. Vibegron does not induce or inhibit the activity of CYP2D6 and CYP3A4 enzymes and thus reduces drug-drug interactions with most prescribed agents. Vibegron was shown to be superior when compared with placebo with respect to decreased frequency, urgency-incontinence episodes, incontinence episodes, and urgency episodes per day.34 This study also documented the additive effect of the administration of vibegron plus tolterodine once daily.35 The incidence and severity of drug-related side effects are comparable with placebo.36
β-3 Agonists Adverse Drug Reactions
The most frequently reported side effects of mirabegron were nasopharyngitis, dry mouth, hypertension, constipation, headache, dyspepsia, urinary tract infection, dizziness, blurred vision, nausea, cardiovascular events, influenza, prolonged QT interval, and upper respiratory tract infection; however, with the exception of nasopharyngitis, no significant difference was found in the incidence of side effects between mirabegron and placebo.27 In addition, no increased risk of cognitive impairment was found in elderly patients receiving mirabegron.37 Mirabegron can increase blood pressure (BP) and is not recommended for patients with severe uncontrolled hypertension, defined as a systolic BP of 180 mmHg or higher and/or a diastolic BP of 110 mmHg or higher. Mirabegron should also be used with caution in patients with stage 2 hypertension. Patients with preexisting hypertension should routinely have their BP monitored while taking mirabegron. In rare cases, mirabegron has been reported to worsen preexisting hypertension. Those with well-controlled BP on antihypertensive (BP lowering) medication can safely use mirabegron.38 Although rare, serious angioedema reactions have occurred with mirabegron.
Common side effects of vibegron are similar to those observed with mirabegron and include headache, urinary tract infection, nasopharyngitis, diarrhea, nausea, and upper respiratory tract infection. Mirabegron and vibegron carry a caution for urinary retention, noting that patients with bladder outlet obstruction and those on concurrent antimuscarinic therapy are at higher risk. Vibegron does not carry any notices regarding its use in patients with hypertension.36
Initial Drug Choice
From a practical perspective, side-effect profile, tolerability, medical comorbidities, and insurance coverage have a significant impact on the choice of medications. Orally administered antimuscarinics or β-3 agonists are considered the standard pharmacologic treatment in both men and women for the treatment of OAB. If monotherapy is not effective, due to their differing mechanisms of action, combination therapy with antimuscarinics and β-3 agonists may provide improved symptom relief and a higher likelihood of achieving continence than using monotherapy.39 Since β-3 agonists provide similar efficacy as the antimuscarinics, but with an improved side-effect profile treatment of OAB with a β-3 adrenergic agonist is favored in patients at higher risk of anticholinergic adverse events.40
When selecting antimuscarinic therapy, trospium, solifenacin, or darifenacin may be preferable choices compared with the other antimuscarinic agents due to their lower incidence of adverse events, relative inability to cross the BBB, and efficacy rates comparable with that of tolterodine and oxybutynin.13 The potential risk for developing dementia and cognitive impairment with antimuscarinic medications should be discussed. All antimuscarinic medications should be started with the lowest dosage and patients assessed regularly for response and side effects. In patients with an insufficient response, an increase in the dosage can be considered, as long as the patient is tolerating the medication. In patients with intolerable side effects or inadequate improvement with medications, offer a different medication in the same class or from a different class. In patients with inadequate improvement with a single medication, offer combination therapy with a medication from a different class.
The most common adverse events associated with oral antimuscarinics are dry mouth and constipation, which may be managed with sugar-free hard candies and stool softeners. Constipation and dry mouth should be managed before abandoning effective antimuscarinic therapy. Caution should be used in prescribing antimuscarinics in patients who are using other medications with anticholinergic properties.
MINIMALLY INVASIVE THERAPIES
Minimally invasive therapies (see TABLE 1) are generally reserved as third line or in situations where patients have an inadequate response to, have intolerable side effects from, or are unwilling or incapable of using noninvasive and/or pharmacologic therapies. Choice of therapy should be determined in the context of shared decision-making. Patients do not need to undergo any particular sequence of therapies before changing or combining therapies, including minimally invasive therapies. Follow-up after initiation of any therapy is important to assess symptom control, adherence to therapy, and side effects, and if needed, to discuss alternative management options or referral to a specialist. Four to 8 weeks of behavioral modification or oral medication treatment should be allowed to establish response.
SPECIAL CONSIDERATIONS IN OLDER WOMEN
Older women have many unique issues, including frailty, functional and cognitive impairment, comorbidities, polypharmacy, estrogen deficiency, or remaining life expectancy, that may have a significant impact on symptom management and the optimal choice of therapy for OAB. Age alone is not a good indicator of functionality and overall health status. Frailty, functional impairment, and cognitive impairment all increase the risk of falls. Functional impairment interferes with a person’s ability to perform basic activities of daily living, including the ability to go to the bathroom safely to void. Older individuals have a higher prevalence of visual and hearing impairment, which can affect the patient’s ability to utilize some behavioral therapies effectively and may make it difficult to take medications correctly. Urogenital atrophy due to low estrogen levels is associated with urinary urgency, frequency, nocturia, and incontinence in postmenopausal women. Low-dose vaginal estrogen therapy is sometimes used off-label in combination with other therapies in women with urogenital atrophy and OAB to help with symptoms of OAB; however, it is currently not included in the AUA/SUFU treatment guidelines.3,26
Minimally invasive therapies (e.g., scheduled/ prompted voiding, PFMT) are usually preferred first line in older women due to their low risk of harm. However, a patient’s level of frailty, functionality, and cognitive status should be considered when recommending behavioral therapies. Timed/ prompted voiding and PFMT require effort on the part of the patient and/or caregiver and a level of comprehension that may be limited in patients with cognitive deficits.
Antimuscarinic medications should be avoided in patients aged ≥65 years, those at increased risk of falls, and those with cognitive impairments. Oral oxybutynin carries the highest risk of cognitive impairment. Trospium may be preferred over other antimuscarinic formulations in older women, as trospium is the least likely to cross the BBB. β-3 agonists may be preferred over antimuscarinic medications in older women. However, both classes of pharmacologic therapies may be inappropriate for some patients due to functional and cognitive factors affecting adherence.
Due to the complex nature of OAB treatment in older women, the standard AUA/SUFU treatment algorithm may not always be applicable. Minimally invasive therapies may be preferable over noninvasive and pharmacologic therapies in some older women.26
CONCLUSION
While the treatment of hypogonadism still has several unknowns, a growing development of novel therapies gives the potential of having even more effective choices. In the future, successful novel treatments for this disease state will be of low cost, easily administered by the patient, and a low risk versus benefit. Pharmacists are at the forefront of counseling patients effectively with this disease state and must be attentive to individualized needs to manage this disease state well.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
- Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29:4-20.
- Reynolds WS, Fowke J, Dmochowski R. The burden of overactive bladder on US public health. Curr Bladder Dysfunct Rep. 2016;11(1):8-13.
- Cameron AP, Chung DE, Dielubanza EJ, et al. The AUA/SUFU guideline on the diagnosis and treatment of idiopathic overactive bladder. J Urol. 2024;212(1):11-20.
- Kwon J, Kim DY, Cho KJ, et al. Pathophysiology of overactive bladder and pharmacologic treatments including β3-adrenoceptor agonists—basic research perspectives. Int Neurourol J. 2024;28(Suppl 1):12-33.
- Hegde SS, Eglen RM. Muscarinic receptor subtypes modulating smooth muscle contractility in the urinary bladder. Life Sci. 1999;64(6-7):419-428.
- Yamaguchi O, Chapple CR. Beta3-adrenoceptors in urinary bladder. Neurourol Urodyn. 2007;26(6):752-756.
- Lukacz ES, Santiago-Lastra Y, Albo ME, Brubaker L. Urinary incontinence in women: a review. JAMA. 2017;318(16):1592-1604.
- Herbison P, Hay-Smith J, Ellis G, Moore K. Effectiveness of anticholinergic drugs compared with placebo in the treatment of overactive bladder: systematic review. BMJ. 2003;326(7394):841-844.
- Krhut J, Skugarevská B, Míka D, et al. Clinical utility of β3-adrenoreceptor agonists for the treatment of overactive bladder: a review of the evidence and current recommendations. Res Rep Urol. 2022;14:167-175.
- Stoniute A, Madhuvrata P, Still M, et al. Oral anticholinergic drugs versus placebo or no treatment for managing overactive bladder syndrome in adults. Cochrane Database Syst Rev. 2023;5(5):CD003781.
- Shamliyan T, Wyman JF, Ramakrishnan R, et al. Benefits and harms of pharmacologic treatment for urinary incontinence in women: a systematic review. Ann Intern Med. 2012;156(12):861-874, W301-W310.
- Arcaniolo D, Conquy S, Tarcan T. Flavoxate: present and future. Eur Rev Med Pharmacol Sci. 2015;19(5):719-731.
- Hesch K. Agents for treatment of overactive bladder: a therapeutic class review. Proc (Bayl Univ Med Cent). 2007;20(3):307-314.
- Todorova A, Vonderheid-Guth B, Dimpfel W. Effects of tolterodine, trospium chloride, and oxybutynin on the central nervous system. J Clin Pharmacol. 2001;41(6):636-644.
- Chancellor M, Boone T. Anticholinergics for overactive bladder therapy: central nervous system effects. CNS Neurosci Ther 2012;18(2):167-174.
- Diokno AC, Appell RA, Sand PK, et al; OPERA Study Group. Prospective, randomized, double-blind study of the efficacy and tolerability of the extended-release formulations of oxybutynin and tolterodine for overactive bladder: results of the OPERA trial. Mayo Clin Proc. 2003;78(6):687-695.
- Croom KF, Keating GM. Darifenacin: in the treatment of overactive bladder. Drugs Aging. 2004;21(13):885-894.
- Zinner N, Kobashi KC, Ebinger U, et al. Darifenacin treatment for overactive bladder in patients who expressed dissatisfaction with prior extended-release antimuscarinic therapy. Int J Clin Pract. 2008;62(11):1664-1674.
- Basra R, Kelleher C. A review of solifenacin in the treatment of urinary incontinence. Ther Clin Risk Manag. 2008;4(1):117-128.
- Dantas LP, Forte ARCC, Lima BC, et al. Treatment of bladder dysfunction with solifenacin: is there a risk of dementia or cognitive impairment? Braz J Med Biol Res. 2022;55:e11721.
- Rovner ES. Trospium chloride in the management of overactive bladder. Drugs. 2004;64(21):2433-2446.
- Ginsberg DA, Oefelein MG, Ellsworth PI. Once-daily administration of trospium chloride extended release provides 24-hr coverage of nocturnal and diurnal symptoms of overactive bladder: an integrated analysis of two phase III trials. Neurourol Urodyn. 2011;30(4):563-567.
- Singh-Franco D, Machado C, Tuteja S, Zapantis A. Trospium chloride for the treatment of overactive bladder with urge incontinence. Clin Ther. 2005;27(5):511-530.
- Chughtai B, Levin R, De E. Choice of antimuscarinic agents for overactive bladder in the older patient: focus on darifenacin. Clin Interv Aging. 2008;3(3):503-509.
- Ellsworth P. Fesoterodine for the treatment of urinary incontinence and overactive bladder. Ther Clin Risk Manag. 2009;5:869-876.
- Pratt TS, Suskind AM. Management of overactive bladder in older women. Curr Urol Rep. 2018;19(11):92.
- Elkhashab MM, Alqahtani AM, Kim MH, et al. Safety and efficacy of β3 adrenergic agonists in treating neurogenic lower urinary tract dysfunction: a systematic review and meta-analysis. Investig Clin Urol. 2024;65(3):217-229.
- Cui Y, Zong H, Yang C, et al. The efficacy and safety of mirabegron in treating OAB: a systematic review and meta-analysis of phase III trials. Int Urol Nephrol. 2014;46:275-284.
- Shi H, Chen H, Zhang Y, Cui Y. The efficacy and safety of vibegron in treating overactive bladder: a systematic review and pooled analysis of randomized controlled trials. Neurourol Urodyn. 2020;39:1255-1263.
- Farag F, Sakalis VI, Arteaga SM, et al. What are the short-term benefits and potential harms of therapeutic modalities for the management of overactive bladder syndrome in women? A review of evidence under the auspices of the European Association of Urology, Female Non-neurogenic Lower Urinary Tract Symptoms Guidelines Panel. Eur Urol. 2023;84:302-312.
- Su S, Liang L, Lin J, et al. Systematic review and meta-analysis of the efficacy and safety of vibegron vs antimuscarinic monotherapy for overactive bladder. Medicine (Baltimore). 2021;100:e23171.
- Krauwinkel W, van Dijk J, Schaddelee M, et al. Pharmacokinetic properties of mirabegron, a β3-adrenoceptor agonist: results from two phase I, randomized, multiple-dose studies in healthy young and elderly men and women. Clin Ther. 2012;34:2144-2160.
- Deeks ED. Mirabegron: a review in overactive bladder syndrome. Drugs. 2018;78:833-844.
- Staskin D, Frankel J, Varano S, et al. International phase III, randomized, double-blind, placebo and active controlled study to evaluate the safety and efficacy of vibegron in patients with symptoms of overactive bladder: EMPOWUR. J Urol. 2020;204:316-324.
- Mitcheson HD, Samanta S, Muldowney K, et al. Vibegron (RVT- 901/MK-4618/KRP-114V) administered once daily as monotherapy or concomitantly with tolterodine in patients with an overactive bladder: a multicenter, phase IIb, randomized, double-blind, controlled trial. Eur Urol. 2019;75:274-282.
- Frankel J, Staskin D, Varano S, et al. An evaluation of the efficacy and safety of vibegron in the treatment of overactive bladder. Ther Clin Risk Manag. 2022;18:171-182.
- Griebling TL, Campbell NL, Mangel J, et al. Effect of mirabegron on cognitive function in elderly patients with overactive bladder: MoCA results from a phase 4 randomized, placebo-controlled study (PILLAR). BMC Geriatr. 2020;20(1):109.
- Ito H, Matsuo T, Mitsunari K, et al. Impact of mirabegron administration on the blood pressure and pulse rate in patients with overactive bladder. Medicina (Kaunas). 2022;58(6):825.
- Gibson W, MacDiarmid S, Huang M, et al. Treating overactive bladder in older patients with a combination of mirabegron and solifenacin: a prespecified analysis from the BESIDE study. Eur Urol Focus. 2017;3(6):629-638.
- Wani MM, Sheikh MI, Bhat T, et al. Comparison of antimuscarinic drugs to beta adrenergic agonists in overactive bladder: a literary review. Curr Urol. 2021;15(3):153-160.