Managing Type 2 Diabetes Mellitus in Older Adults
RELEASE DATE
November 1, 2022
EXPIRATION DATE
November 30, 2024
FACULTY
Joshua J. Neumiller, PharmD, CDCES, FADCES, FASCP
Vice-Chair & Professor
Department of Pharmacotherapy
College of Pharmacy and Pharmaceutical Sciences
Washington State University
Spokane, Washington
FACULTY DISCLOSURE STATEMENTS
Dr. Neumiller is a consultant for Bayer, has served on Advisory Boards for Novo Nordisk and Sanofi, and has served on the Speaker's Bureau for Dexcom.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT
 Pharmacy
Pharmacy
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-22-135-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients' conditions and possible contraindications or dangers in use, review of any applicable manufacturer's product information, and comparison with recommendations of other authorities.
GOAL
To update pharmacists on considerations related to the management of type 2 diabetes mellitus (T2DM) in older adults
OBJECTIVES
After completing this activity, the participant should be able to:
- Recall current guideline recommendations for the management of T2DM.
- List key comorbidities, functional limitations, and geriatric syndromes that should be considered when setting treatment goals and formulating treatment plans for older adults with T2DM.
- Understand strategies to individualize and simplify diabetes management in older adults, including liberalization of treatment targets, deintensification of therapy, and medication regimen simplification.
- Review considerations for use of glucose-lowering therapies in older adults with T2DM.
ABSTRACT:Older adults with type 2 diabetes mellitus often present with multiple comorbidities, functional limitations, and geriatric syndromes that may impact selection of glucose-lowering therapies and patient-specific treatment goals. Screening for common geriatric syndromes, such as cognitive impairment and depression, and avoiding inappropriate polypharmacy are important to optimize treatment outcomes, patient safety, and quality of life. Liberalization of glycemic targets, deintensification of therapy, and/or medication regimen simplification may be beneficial in older adults as their capacity for self-care and treatment goals change over time. Utilization of glucose-lowering regimens that minimize hypoglycemia and therapeutic burden is recommended whenever possible.
According to most recent estimates from the CDC, over 37 million people currently live with diabetes mellitus in the United States.1 This figure equates to over 11% of the population having diabetes.1 Of the 37 million individuals with diabetes living in the U.S., the large majority (approximately 90%-95%) have type 2 diabetes mellitus (T2DM).1 The incidence of T2DM also increases in parallel with age. Over 29% of adults aged 65 years or older in the U.S. have diabetes.1 With the population continuing to age, the total number of people living with diabetes in the U.S. and worldwide is expected to increase substantially in the coming decades.2
Considerations for the management of diabetes often change for patients over their life span. Older adults with diabetes are more likely to experience microvascular and macrovascular complications, have multiple coexisting chronic medical conditions, and present with functional limitations and a host of geriatric syndromes that may impact diabetes care and outcomes.3 While glycemic targets and treatment strategies should always be individualized in patients with diabetes, special consideration is warranted in older adults to avoid overtreatment that can overwhelm patients and potentially lead to harm.
This article will focus on considerations for the management of older adults with T2DM. First, guideline recommendations for the management of T2DM will be reviewed, followed by a discussion of key comorbidities, functional limitations, and geriatric syndromes that should be considered when establishing treatment goals and formulating treatment plans in this high-risk population. Strategies to individualize and simplify diabetes management will also be discussed, in addition to a brief overview of considerations when using currently available glucose-lowering therapies in older adults with T2DM.
 |
GUIDELINE RECOMMENDATIONS FOR THE MANAGEMENT OF T2DM
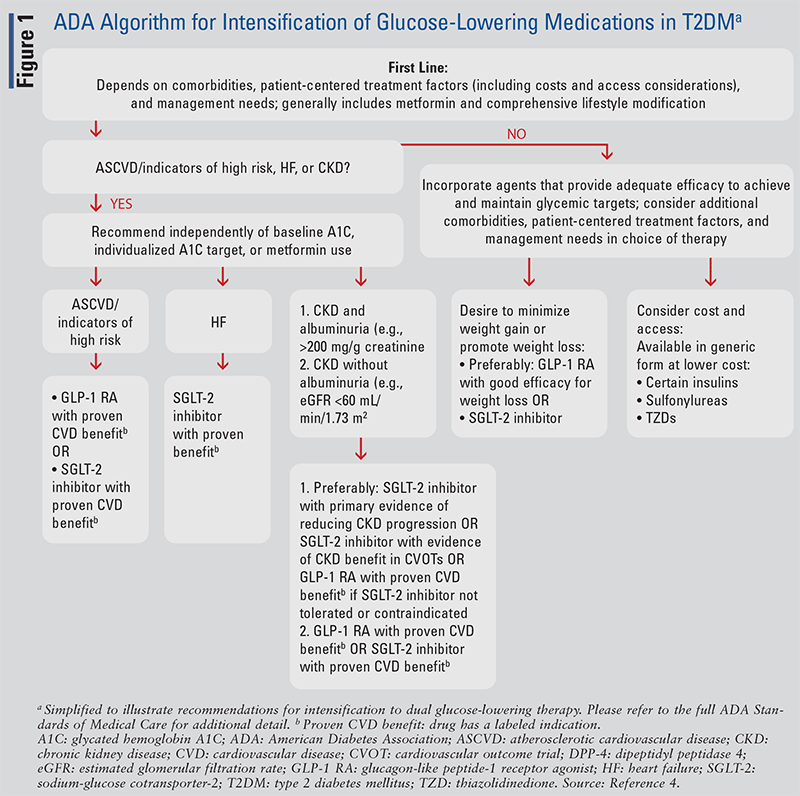
As previously noted, current guidelines for the management of T2DM recommend individualization of care based on patient- and medication-specific factors and considerations.4 The American Diabetes Association (ADA) provides recommendations for intensification of glucose-lowering therapies in patients with T2DM to meet individualized glycemic targets and to decrease atherosclerotic cardiovascular disease (ASCVD), heart failure (HF), and kidney risk.4 The ADA does not provide a separate algorithm for the management of older adults with T2DM, and it is appropriate to apply the general T2DM management algorithm in older adults with T2DM. It is important, however, to carefully consider the appropriateness of specific glucose-lowering therapies in older adults based on their kidney function, risk for hypoglycemia, and other patient-specific factors. Before discussing management considerations that are specific to the management of older adults, it is useful to review current general recommendations for the management of T2DM. The following provides an overview of recommendations from the 2022 ADA Standards of Medical Care in Diabetes.4 FIGURE 1 provides a simplified summary of the ADA's 2022 algorithm for intensification to a dual glucose-lowering medication regimen in patients with T2DM.4
First-Line Therapy in T2DM
This year, the ADA made major changes to its recommendation for initial first-line therapy in patients with T2DM.4 The ADA no longer recommends metformin as the preferred first-line agent in all people with T2DM.4 Instead, first-line therapy is now determined based on consideration of compelling comorbidities (e.g., ASCVD, HF, and/or chronic kidney disease [CKD]), patient-specific factors (such as medication cost and patient access considerations), and person-specific management needs.4 Intensification of the glucose-lowering regimen is also informed by careful consideration of patient- and medication- related factors. In the case of older adults with T2DM, such considerations may include hypoglycemia risk, kidney function, and other patient-specific factors.
THERAPIES IN PATIENTS WITH ASCVD OR INDICATORS OF HIGH RISK, HF, OR CKD
Based on cardiovascular (CV), HF, and kidney disease outcome trial data with agents from the glucagon-like peptide-1 (GLP-1) receptor agonist and sodium-glucose cotransporter 2 (SGLT-2) inhibitor classes, guideline-directed management of patients with T2DM has shifted from an approach primarily driven by the need for glucose lowering to one focused on comprehensive risk reduction. When intensifying glucose-lowering therapy in patients with T2DM, the ADA first recommends considering whether the patient has ASCVD or indicators of high risk, HF, or CKD (see FIGURE 1).4 If one or more of these comorbidities are present, use of an agent with proven ASCVD, HF, and/or CKD benefit is recommended independent of the patient's glycated hemoglobin A1C (A1C) or individualized A1C target.4 Use of agents with evidence of organ protection is recommended whenever a patient develops one of these comorbidities, regardless of the background glucoselowering regimen. Additional detail is provided below on recommended therapies in the setting of ASCVD, HF, and CKD—comorbidities often encountered in older adults with T2DM.
High-Risk or Established ASCVD
Per FIGURE 1, the ADA recommends use of either an SGLT-2 inhibitor or GLP-1 receptor agonist with proven ASCVD benefit in patients with a history of ASCVD or indicators of high ASCVD risk.4 A preference is not given for one class over the other in the absence of head-to-head clinical trials comparing the effects of agents from these preferred classes. The ADA operationally defines an agent as having “proven CV benefit” if it carries an FDA-approved indication for reducing CV events.4 The ADA additionally recommends considering use of an agent with evidence of benefit from both classes (SGLT-2 inhibitor plus GLP-1 receptor agonist) if the CV benefits are thought to outweigh the risks of combination therapy.5
Heart Failure
For patients with HF, the ADA recommends use of an SGLT-2 inhibitor with evidence of HF benefit.4 This recommendation is based on consistently observed benefits of SGLT-2 inhibitor therapy in dedicated HF outcome trials, with both dapagliflozin and empagliflozin carrying formal indications to improve HF outcomes.6-12 A recently published meta-analysis that included major HF outcome trials completed to date concluded that SGLT-2 inhibitor therapy reduces the risk for CV-related death and hospitalization for HF in a broad range of HF patients, including those with reduced and preserved ejection fraction HF.13
Chronic Kidney Disease
CKD is a commonly encountered diabetes-related complication in older adults with T2DM. The ADA preferentially recommends an SGLT-2 inhibitor with primary evidence of slowing CKD progression in patients with T2DM, CKD, and albuminuria (e.g., ≥200 mg/g creatinine).4 Dedicated kidney outcome trials for both canagliflozin and dapagliflozin have been published, with both agents demonstrating kidney benefit in patients with T2DM and CKD.14,15 A press release for the EMPA-Kidney trial, a dedicated kidney outcome trial with empagliflozin, has also reported benefit of empagliflozin treatment on primary kidney outcomes, further supporting a class effect for delayed CKD progression with SGLT-2 inhibitors.16
In patients who cannot take an SGLT-2 inhibitor due to a contraindication or intolerance, the ADA recommends use of a GLP-1 receptor agonist with proven CV benefit.4 In patients with T2DM and established CKD (estimated glomerular filtration rate [eGFR] <60 mL/min/1.73 m2) who do not have albuminuria, ADA recommends use of either an SGLT-2 inhibitor or GLP-1 receptor agonist with proven CV benefit. This recommendation is supported by the considerable ASCVD burden associated with CKD.17
T2DM Patients Without Evidence of ASCVD, HF, or CKD
 For patients without evidence of ASCVD, HF, or
CKD, the addition of glucose-lowering agents is
based on the need for additional glycemic efficacy. As
highlighted in FIGURE 1, selection of glucose-lowering
therapies is based on three key patient-centered treatment preferences: minimization of hypoglycemia,
minimization of weight gain or promotion of weight
loss, or a need to minimize medication costs.4 One or
more of these factors may impact therapeutic selection in older adults with T2DM. As will be discussed
later, avoidance of hypoglycemia is often desirable in
older adults—a population more susceptible to hypoglycemia-related morbidity and mortality.
For patients without evidence of ASCVD, HF, or
CKD, the addition of glucose-lowering agents is
based on the need for additional glycemic efficacy. As
highlighted in FIGURE 1, selection of glucose-lowering
therapies is based on three key patient-centered treatment preferences: minimization of hypoglycemia,
minimization of weight gain or promotion of weight
loss, or a need to minimize medication costs.4 One or
more of these factors may impact therapeutic selection in older adults with T2DM. As will be discussed
later, avoidance of hypoglycemia is often desirable in
older adults—a population more susceptible to hypoglycemia-related morbidity and mortality.
INTENSIFICATION OF INJECTABLE GLUCOSE-LOWERING AGENTS
Another algorithm within the 2022 ADA Standards of Medical Care provides guidance on intensification of injectable agents (e.g., GLP-1 receptor agonists and insulin therapies) in patients requiring injectable glucose-lowering therapies to meet individualized glycemic targets.4 Because older adults with T2DM often have a long duration of diabetes, it is not uncommon to encounter older adults receiving insulin therapies to maintain glycemic control. The appropriateness of insulin regimens should be frequently reevaluated in older adults with T2DM to ensure safety.3GERIATRIC SYNDROMES: IMPACT ON DIABETES SELF-MANAGEMENT
As previously discussed, it is generally appropriate to apply the ADA's algorithm for use of glucose-lowering therapies to older adults with T2DM. The ADA is specific in stating that all treatment decisions should be made in consideration of patient-specific factors. For older adults with T2DM, there are often multiple factors to consider that may include, but are not limited to, presence of comorbidities, capacity for selfcare, the presence of key geriatric syndromes, and other factors that may impact medication use safety (e.g., kidney function, risk for hypoglycemia-related morbidity and mortality).
When selecting therapies in older adults with T2DM, it is important to consider their capacity for self-care and the impact of any functional impairments the patients may have on their ability to follow through on a given treatment plan. Both aging and diabetes are risk factors for the development of functional impairments.18 Screening for functional limitations in older adults with diabetes is important when determining their capacity for self-care and to inform the development of treatment plans that are achievable and establishing glycemic targets that are safely attained by the patient. Assessing an older adult's ability to independently perform activities of daily living (ADLs) and instrumental ADLs (IADLs) is helpful in determining a patient's ability to engage in diabetes self-management. ADLs encompass basic self-care activities that are necessary for older adults to perform if they are to live independently in the community setting. Examples of ADLs include the ability to perform basic personal hygiene tasks (e.g., bathing, grooming), dressing oneself, eating without assistance, toileting independently, and transferring without the assistance of a caregiver. Transferring independently can include assessing the person's ability to stand independently from a seated position, get in and out of bed, and ambulate from one room to another.19 IADLs include more complex tasks that require critical thinking and organizational skills but are still critical to perform if an individual lives independently. Examples of IADLs include the ability to prepare meals, shop, manage finances, manage transportation (either driving or being able to arrange for alternative means of transportation), perform housecleaning and home maintenance tasks, communicate (e.g., using a phone, mail, and/or email), and manage medications. Medication management includes both obtaining medications from the pharmacy and being able to self-administer them correctly.19
An older adult's ability to perform ADLs and IADLs is clearly important when assessing if an individual can safely live independently, but ADL/IADL deficits can also shed light on an individual's ability to manage complex diabetes self-care tasks and safely manage complex medication regimens (e.g., insulin regimens). If ADL/IADL deficits are present, additional assessment is recommended. Important follow- up evaluations in older adults with T2DM should include an assessment of glycemic control and prompt screening for the presence of diabetes-related complications and geriatric syndromes that can be contributing to observed or suspected functional impairments.18
The ADA recommends screening for geriatric syndromes in older adults with diabetes to inform management and treatment decisions.3 Key geriatric syndromes highlighted in the literature include cognitive impairment, depression, functional disabilities, polypharmacy, falls and fractures, and urinary incontinence.3
Cognitive Impairment
Diabetes is associated with an increased risk for cognitive impairment and dementia.20 Diabetes has been linked to increased risk for development of both Alzheimer's disease and vascular dementia, yet the risk is greatest for vascular dementia.21,22 Cognitive impairment is associated with diminished capacity for diabetes self-care and increased risks for severe hypoglycemia and hospitalization.23-26 The relationship between cognitive impairment and risk for hypoglycemia and CV-related morbidity and mortality is bidirectional, with CV disease and recurrent hypoglycemia associated with increased risk for development of cognitive impairment.27 Screening for cognitive impairment is recommended in older adults with diabetes to facilitate early recognition and adjustment of the diabetes treatment plan. Cognitive impairment can complicate medication management (e.g., calculating appropriate insulin doses), glucose monitoring, and adherence to lifestyle recommendations. The ADA specifically recommends cognitive screening in adults aged 65 years or older at the initial diabetes visit, annually, and as appropriate based on patient presentation.3 Several interventions can be considered in older adults with diabetes who have cognitive deficits to tailor the treatment plan and maximize patient safety.28 First, involvement of caregivers in the development of treatment plans and when making care decisions can help establish realistic management goals. Avoiding overly aggressive glycemic targets (and use of glucose-lowering agents with a high risk for hypoglycemia) can help minimize hypoglycemia risk and undue treatment burden. Use of alarms, pill boxes, and other tools to assist with medication management can also be helpful for patients and caregivers.28
Depression
 Depression is a common comorbidity in people with
diabetes.29 Depression can adversely impact a patient's
motivation to engage in recommended self-care and
adversely impact diabetes management, including
adherence to recommended therapies.30 It is therefore
important to screen for and treat depression to improve
patient care and quality of life. The ADA offers the
following specific recommendations for depression
screening and management31:
Depression is a common comorbidity in people with
diabetes.29 Depression can adversely impact a patient's
motivation to engage in recommended self-care and
adversely impact diabetes management, including
adherence to recommended therapies.30 It is therefore
important to screen for and treat depression to improve
patient care and quality of life. The ADA offers the
following specific recommendations for depression
screening and management31:
- Providers should consider annual screening of all patients with diabetes, especially those with a self-reported history of depression, for depressive symptoms with age-appropriate depression screening measures, recognizing that further evaluation will be necessary for individuals who have a positive screen.
- Beginning at diagnosis of complications or when there are significant changes in medical status, consider assessment for depression.
- Referrals for treatment of depression should be made to mental health providers with experience using cognitive behavioral therapy, interpersonal therapy, or other evidence-based treatment approaches in conjunction with collaborative care with the patient's diabetes treatment team.
Functional Impairments
Functional impairment in older adults with diabetes can result from a host of comorbidities and diabetes-related complications. The presence of peripheral neuropathy, for example, can contribute to gait and balance problems that may limit physical activity and increase risk for falls.32 Compromised vision can increase the risk for falls and injury. Vision loss can also prevent patients from accurately reading blood glucose meter or continuous glucose monitor results and accurately preparing and administering insulin. Hearing deficits can impair communication with healthcare providers, leading to gaps in self-care knowledge and skills. Limitations in hand dexterity can impair older adults' ability to use blood glucose meters or prescribed medications (e.g., syringes, pens). Potential strategies to address functional disabilities include recommending use of assistive devices to help manage functional limitations (e.g., glasses, hearing aids, walkers) and recommending physical activities and medications manageable given the physical capabilities of the patient.28
Polypharmacy
Older adults with T2DM are frequently managed on combination glucose-lowering therapy to meet individualized glycemic targets. While focusing on the specific number of medications an older adult with diabetes takes may not be appropriate, simplification of the regimen whenever possible is recommended to avoid overtreatment and excessive treatment burden. Polypharmacy in older adults with diabetes can contribute to risk for medication errors, drug side effects, and unnecessary morbidity.33 When assessing appropriateness of therapies in older adults, a great resource is the American Geriatrics Society Beers Criteria for Potentially Inappropriate Medication Use in Older Adults.34 While the Beers Criteria offer a framework for evaluating use of potentially inappropriate medications in older adults, decisions on use of Beers Criteria medications should be made on an individual basis in consideration of potential risks and benefits of therapy. The following strategies may be considered in older adults with diabetes to minimize polypharmacy28:
- Frequently review medication use (including prescription, OTC, and herbal products).
- Consider discontinuation of medications that are no longer providing benefit and/or when the risk of use outweighs potential benefits of continuation.
Falls and Fractures
The ADA recommends assessing fracture history and risk factors for falls in older adults with diabetes.31 In older adults with diabetes who experience a fall, it is important to assess for medications that may have been contributory, environmental factors such as loose rugs or extension cords in the home, and health or physical changes that may have contributed to the fall. The potential impact of glucose-lowering medications on bone health should also be considered. The ADA recommends caution with use of thiazolidinediones and SGLT-2 inhibitors in patients with a history of fracture.31 When possible, minimizing use of sedative hypnotic medications, agents associated with causing orthostasis, and hypoglycemic medications is recommended in high-risk patients.18 Referral to physical therapy for balance training and fall prevention education can be helpful for many patients.
Urinary Incontinence
Urinary incontinence is common in older adults with diabetes but often remains unreported.35 Urinary incontinence can contribute to depressive symptoms and risk for falls and fractures. It is recommended that older adults be questioned about urinary symptoms at least annually.36 In older adults with urinary incontinence, SGLT-2 inhibitor therapy may exacerbate symptoms.
GLYCEMIC TARGETS
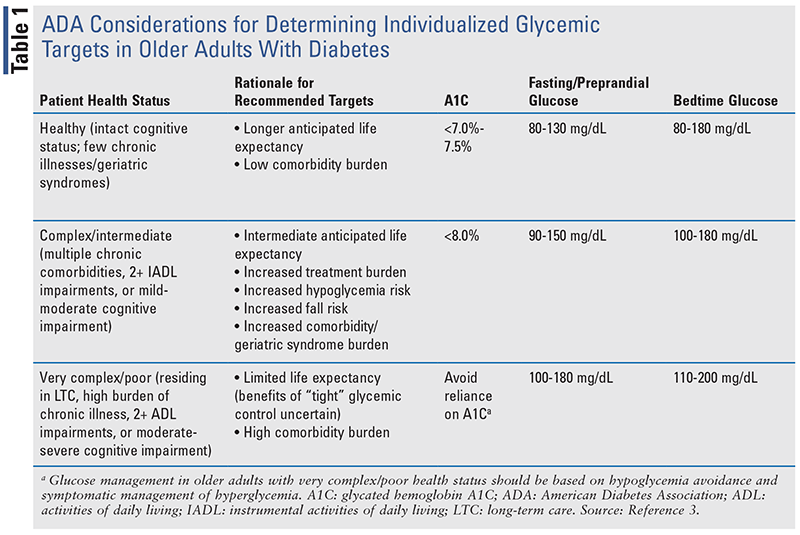
Current guidelines from the ADA and the Endocrine Society recommend setting individualized glycemic goals for older adults with diabetes and recommend diabetes regimens that minimize hypoglycemia risk whenever feasible.3 TABLE 1 provides a summary of current recommendations from the ADA for individualizing glycemic targets in older adults based on their overall health status and other patient-specific factors.3
 |
GLUCOSE-LOWERING MEDICATION USE IN OLDER ADULTS WITH T2DM
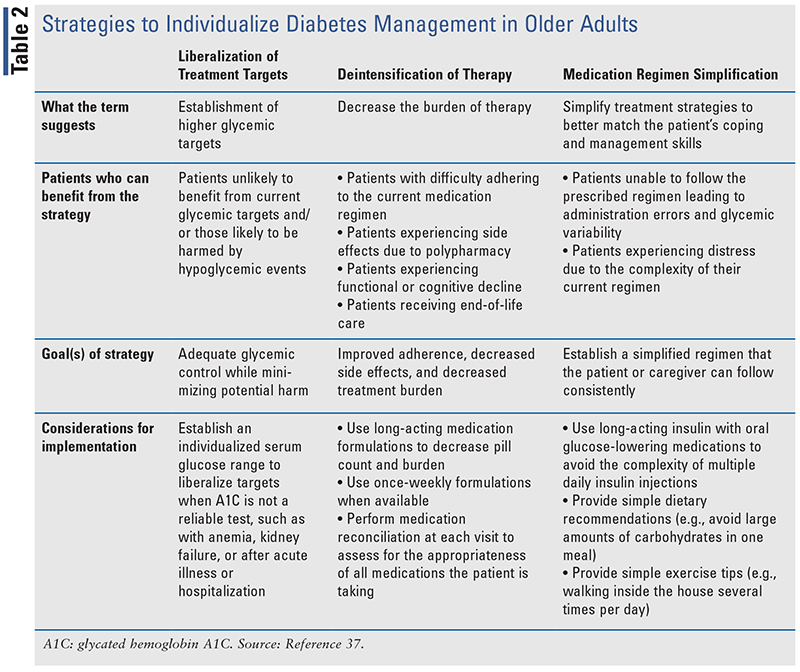
The ADA stresses that selection and use of glucose-lowering agents in older adults with T2DM require special care.3 Considerations for use of specific agents include, but are not limited to, the desire to prevent CV events and slow CKD progression, cost and access considerations, hypoglycemia risk, and other safety considerations. The ADA additionally notes that it is important to match the complexity of the medication regimen to the self-management ability of the older adult with diabetes.3 Strategies to simplify or deintensify the regimen and/or treatment goals may be necessary in older adults as their health status and goals change over time (see TABLE 2).37 Medication-specific considerations for commonly used glucose-lowering medication classes are provided below.
 |
Metformin
Metformin is often utilized as first-line therapy in older adults with T2DM. Metformin is affordable, is effective at lowering A1C, and has a low risk for contributing to hypoglycemia.3 Metformin can be used safely in patients with an eGFR ≥30 mL/min/1.73 m2 but is contraindicated once the eGFR falls below 30 mL/min/1.73 m2. Older adults may be more sensitive to gastrointestinal (GI) side effects with metformin, which can contribute to reduced appetite in some patients. Metformin discontinuation may be necessary in older adults with persistent and/or severe GI symptoms. Because long-term metformin use has been associated with vitamin B12 deficiency, monitoring should be considered.38
Sulfonylureas
Sulfonylureas are insulin secretagogue medications that place older adults at increased risk for hypoglycemia. The ADA recommends cautious use of sulfonylureas in older adults.3 If a sulfonylurea is used, glipizide and glimepiride are preferred. Glyburide is a longer-acting sulfonylurea that is not recommended for use in older adults or in patients with CKD.39
Thiazolidinediones
Thiazolidinediones (TZDs) are effective glucose-lowering therapies with a low risk of contributing to hypoglycemia. The ADA recommends cautious use of TZDs in older adults on insulin therapy (due to additive fluid retention) and in those with comorbid HF, osteoporosis, falls or fractures, and/or macular edema.3 As noted within the ADA's algorithm for intensification of glucose-lowering therapy in T2DM (see FIGURE 1), however, TZDs are generically available, making them more affordable for low-income patients and/or those without insurance. Use of lower doses of pioglitazone in combination with other glucose-lowering agents may help mitigate problematic side effects.3
Dipeptidyl Peptidase 4 Inhibitors
Dipeptidyl peptidase 4 (DPP-4) inhibitors are orally administered medications that are generally well tolerated and have a low risk for contributing to hypoglycemia.3 DPP-4 inhibitors are currently available only as branded products, which can pose a financial barrier for some patients. While once-daily oral administration and good tolerability make DPP-4 inhibitors appealing options for older adults with T2DM, DPP-4 inhibitors do not provide CV or kidney benefit and have relatively modest glycemic efficacy when compared to other branded glucose-lowering therapies. It is also important to remember that DPP-4 inhibitors require dose adjustment based on kidney function, with the exception of linagliptin which does not require renal dose adjustment.40
GLP-1 Receptor Agonists
As previously discussed, GLP-1 receptor agonists are preferentially recommended by the ADA to improve CV outcomes in at-risk patients with T2DM.4 The benefits of GLP-1 receptor agonists on major adverse CV events, CV-related death, stroke, and myocardial infarction appear to be similar in patients older and younger than age 65 years.41 With the exception of oral semaglutide, GLP-1 receptor agonists are administered via subcutaneous injection and do require intact visual, motor, and cognitive skills for appropriate and successful administration.3 GLP-1 receptor agonist therapy is additionally associated with GI side effects and weight loss. Careful consideration of the benefits versus risks of GLP-1 receptor agonist therapy is recommended in frail and/or underweight older adults where additional weight loss may be undesirable.
SGLT-2 Inhibitors
SGLT-2 inhibitors are recommended by the ADA in patients with T2DM to reduce ASCVD, HF, and CKD risk.4 SGLT-2 inhibitors are orally administered medications which may be more convenient and manageable for older adults when compared to injectable agents. While the glucose-lowering effects of SGLT-2 inhibitors are blunted as kidney function declines, their heart and kidney benefits are maintained at lower eGFRs.42 Similar to GLP-1 receptor agonists, the benefits of SGLT-2 inhibitor therapy appears to be preserved in older adults.3 SGLT-2 inhibitors are associated with symptoms of volume depletion, urinary tract infections, genital mycotic infections, and worsening urinary incontinence—side effects that may be more prominent in older adults.3
Insulin
Insulin therapy is often utilized in older adults with T2DM to meet individualized glycemic targets. Safe and effective use of insulin requires visual acuity, manual dexterity, and cognitive abilities to safely dose and administer. Insulin carries significant risk for hypoglycemia, with prandial insulins carrying the greatest risk. Multiple daily injection regimens may be overly complex for older adults. Similarly, use of slidingscale insulin regimens can contribute to confusion and unnecessarily complex instructions that may place older adults at increased risk for hypoglycemic events. The Beers Criteria recommend avoiding use of sliding-scale insulin regimens in older adults.34 The ADA encourages frequent reassessment of the insulin regimen, with consideration of insulin regimen simplification in patients with advanced diabetes complications, life-limiting chronic conditions, or limited functional status.3
SUMMARY OF KEY ADA RECOMMENDATIONS
In summary, the management of T2DM in older adults should be individualized, with treatment approaches informed by the patient's overall health status, compelling comorbidities, and capacity for self-care. The following is a list of select recommendations from the ADA regarding the care of older adults with diabetes3:
General Recommendations:
- Consider the assessment of medical, psychological, functional (self-management abilities), and social domains in older adults to provide a framework to determine targets and therapeutic approaches for diabetes management.
- Screen for geriatric syndromes (i.e., polypharmacy, cognitive impairment, depression, urinary incontinence, falls, persistent pain, and frailty) in older adults, as they may affect diabetes self-management and diminish quality of life.
Neurocognitive Function: Screening for early detection of mild cognitive impairment or dementia should be performed for adults 65 years of age or older at the initial visit, annually, and as appropriate.
Hypoglycemia: Because older adults with diabetes have a greater risk of hypoglycemia than younger adults, episodes of hypoglycemia should be ascertained and addressed at routine visits.
Treatment Goals:
- Older adults who are otherwise healthy with few coexisting chronic illnesses and intact cognitive function and functional status should have lower glycemic goals (such as A1C <7.0%-7.5%), while those with multiple coexisting chronic illnesses, cognitive impairment, or functional dependence should have less stringent glycemic goals (such as A1C <8.0%).
- Glycemic goals for some older adults might reasonably be relaxed as part of individualized care, but hyperglycemia leading to symptoms or risk of acute hyperglycemia complications should be avoided in all patients.
- Screening for diabetes complications should be individualized in older adults. Particular attention should be paid to complications that would lead to functional impairment.
Pharmacologic Therapy:
- In older adults with T2DM at increased risk for hypoglycemia, medication classes with low risk of hypoglycemia are preferred.
- Overtreatment of diabetes is common in older adults and should be avoided.
- Deintensification (or simplification) of complex regimens is recommended to reduce the risk of hypoglycemia and polypharmacy if it can be achieved within the individualized A1C target.
- Consider costs of care and insurance coverage rules when developing treatment plans in order to reduce risk of cost-related nonadherence.
CONCLUSION
Management of older adults with T2DM should be individualized. Glycemic targets and the diabetes care plan should be informed by comorbid conditions, the presence of key geriatric syndromes, and the patient's self-management capabilities. Avoidance of hypoglycemia is key to preventing hypoglycemia-related morbidity and mortality, with use of agents with a low risk of contributing to hypoglycemia generally preferred when clinically appropriate.
REFERENCES
- CDC. National diabetes statistics report. www.cdc.gov/diabetes/ data/statistics-report/index.html. Accessed September 13, 2022.
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed. Brussels, Belgium: 2021. www.diabetesatlas.org/. Accessed September 12, 2022.
- American Diabetes Association Professional Practice Committee; Draznin B, Aroda VR, Bakris G, et al. 13. Older adults: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S195-S207.
- American Diabetes Association Professional Practice Committee. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S125-S143.
- American Diabetes Association Professional Practice Committee.
10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S144-S174. - McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995-2008.
- Solomon SD, McMurray JJV, Claggett B, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387:1089-1098.
- Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413-1424.
- Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with preserved ejection fraction. N Engl J Med. 2021;385:1451-1461.
- Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384:117-128.
- Farxiga (dapagliflozin) product information. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2022.
- Jardiance (empagliflozin) product information. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; 2022.
- Vaduganathan M, Docherty KF, Claggett BL, et al. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet. 2022;400:757-767.
- Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295-2306.
- Heerspink HJL, Stefansson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436-1446.
- EMPA-KIDNEY. The study of heart and kidney protection with empagliflozin. www.empakidney.org. Accessed September 10, 2022.
- Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032-2045.
- LeRoith D, Biessels GJ, Braithwaite SS, et al. Treatment of diabetes in older adults: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2019;104:1520-1574.
- Kernisan L. What are activities of daily living (ADLs) & instrumental activities of daily living (IADLs)? Better Health While Aging. www.betterhealthwhileaging.net/what-are-adls-and-iadls. Accessed September 11, 2022.
- Biessels GJ, Staekenborg S, Brunner E, et al. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64-74.
- Gudala K, Bansal D, Schifano F, Bhansali A. Diabetes mellitus and risk of dementia: a meta-analysis of prospective observational studies. J Diabetes Investig. 2013;4(6):640-650.
- Abner EL, Nelson PT, Kryscio RJ, et al. Diabetes is associated with cerebrovascular but not Alzheimer's disease neuropathology. Alzheimers Dement. 2016;12(8):882-889.
- Yaffe K, Falvey CM, Hamilton N, et al; Health ABC Study. Association between hypoglycemia and dementia in a biracial cohort of older adults with diabetes mellitus. JAMA Intern Med. 2013;173(14):1300-1306.
- Punthakee Z, Miller ME, Launer LJ, et al; ACCORD Group of Investigators; ACCORD-MIND Investigators. Poor cognitive function and risk of severe hypoglycemia in type 2 diabetes: post hoc epidemiologic analysis of the ACCORD trial. Diabetes Care. 2012;35(4):787-793.
- Munshi M, Grande L, Hayes M, et al. Cognitive dysfunction is associated with poor diabetes control in older adults. Diabetes Care. 2006;29(8):1794-1799.
- Feil DG, Zhu CW, Sultzer DL. The relationship between cognitive impairment and diabetes self-management in population-based com¬munity sample of older adults with type 2 diabetes. J Behav Med. 2012;35(2):190-199.
- Geijselaers SL, Sep SJS, Stehouwer CDA, Biessels GJ. Glucose regulation, cognition, and brain MRI in type 2 diabetes: a systematic review. Lancet Diabetes Endocrinol. 2015;3(1):75-89.
- Leung E, Wongrakpanich S, Munshi MN. Diabetes management in the elderly. Diabetes Spectr. 2018;31(3):245-253.
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24(6):1069-1078.
- Lin EHB, Katon W, Von Korff M, et al. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care. 2004;27:2154-2160.
- American Diabetes Association Professional Practice Committee. 4. Comprehensive medical evaluation and assessment of comorbidities: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S46-S59.
- Menz HB, Lord SR, St George R, Fitzpatrick RC. Walking stability and sensorimotor function in older people with diabetic peripheral neuropathy. Arch Phys Med Rehabil. 2004;85:245-252.
- Geller AI, Shehab N, Lovegrove MC, et al. National estimates of insulin-related hypoglycemia and errors leading to emergency visits and hospitalizations. JAMA Intern Med. 2014;174:678-686.
- American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
- Hsu A, Conell-Price J, Cenzer IS, et al. Predictors of urinary incontinence in community-dwelling frail older adults with diabetes mellitus in a cross-sectional study. BMC Geriatr. 2014;14:137.
- American Geriatrics Society Expert Panel on Care of Older Adults with Diabetes Mellitus; Moreno G, Mangione CM, Kimbro L, Vaisberg E. Guidelines abstracted from the American Geriatrics Society Guidelines for improving care of older adults with diabetes mellitus: 2013 update. J Am Geriatr Soc. 2013;61(11):2020-2026.
- Munshi M, Neumiller JJ. Liberalisation, deintensification, and simplification in diabetes management: words matter. Lancet Diabetes Endocrinol. 2020;8(2):95-97.
- Aroda VR, Edelstein SL, Goldberg RB, et al; Diabetes Prevention Program Research Group. Long-term metformin use and vitamin B12 deficiency in the Diabetes Prevention Program Outcomes Study. J Clin Endocrinol Metab. 2016;101:1754-1761.
- Neumiller JJ, Alicic RZ, Tuttle KR. Therapeutic considerations for antihyperglycemic agents in diabetic kidney disease. J Am Soc Nephrol. 2017;28(8):2263-2274.
- Alicic RZ, Cox EJ, Neumiller JJ, Tuttle KR. Incretin drugs in diabetic kidney disease: biological mechanisms and clinical evidence. Nat Rev Nephrol. 2021;17(4):227-244.
- Karagiannis T, Tsapas A, Athanasiadou E, et al. GLP-1 receptor agonists and SGLT2 inhibitors for older people with type 2 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2021;174:108737.
- Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020;98(4S):S1-S115.