Medication-Focused Overview of the 2022 AHA/ACC/HFSA Heart Failure Management Guideline
RELEASE DATE
February 1, 2023
EXPIRATION DATE
February 28, 2025
FACULTY
Donna M. Lisi, PharmD, BCPS, BCGP, BCACP, BCPP, BCMTMS
Clinical Pharmacist
Somerset, New Jersey
FACULTY DISCLOSURE STATEMENTS
Dr. Lisi has no actual or potential conflicts of interest in relation to this activity
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT
 Pharmacy
Pharmacy
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-23-015-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients' conditions and possible contraindications or dangers in use, review of any applicable manufacturer's product information, and comparison with recommendations of other authorities.
GOAL
To educate pharmacists about the most recent guideline on the management of heart failure (HF) in order to optimize guideline-directed medication therapy (GDMT).
OBJECTIVES
After completing this activity, the participant should be able to:
- Describe recent epidemiologic trends regarding HF in the United States.
- Identify HF guideline updates involving the four major classes of medications that constitute GDMT.
- Discuss how treatment options differ based on stage of HF and left ventricular ejection fraction.
- Explain the pharmacist's role in the management of HF.
ABSTRACT: In May 2022, the American Heart Association, American College of Cardiology, and Heart Failure Society of America released an updated guideline on the management of heart failure (HF). The guideline includes several major changes, including recommending the angiotensin receptor/neprilysin inhibitor sacubitril/valsartan as first-line therapy without initial dose titration of another renin-angiotensin system agent and recommending initiation of sodium-glucose cotransporter 2 inhibitor therapy in HF patients regardless of the presence of diabetes. Other changes include recognizing the emerging field of cardio-oncology and listing anticancer agents that may increase the risk of HF development; identifying disparities in HF management; focusing on patient-reported outcomes; and acknowledging knowledge gaps in HF management. This guideline is a useful resource for pharmacists to individualize care and optimize guideline-directed medication therapy in patients with HF.
In the United States, it is estimated that about 6.2 million adults have heart failure (HF).1 After age 45 years, the lifetime risk of developing HF ranges from 20% to 45%, depending on race and ethnicity.2
Hospitalizations for HF have been rising, as evidenced by data showing that the primary HF hospitalization rate per 1,000 U.S. adults, which was 4.4 in 2010 followed by a decrease to 4.1 in 2013, increased to 4.2 in 2014 and then to 4.9 in 2017.3 A similar trend was observed in postdischarge HF readmissions, with the 2010 rate of 1.0 per 1,000 U.S. adults first dropping to 0.9 in 2014 and then rising to 1.1 in 2017. This trend was also seen in all-cause 30-day readmissions, with a rate of 0.8 per 1,000 U.S. adults in 2010 that fell to 0.7 in 2014 and then increased to 0.9 in 2017.3 Pooled survival rates for all types of HF are estimated to be 95.7%, 86.5%, 72.6% , 56.7%, and 34.9% at 1 month, 1 year, 2 years, 5 years, and 10 years, respectively.4
Between 2025 and 2060, the prevalence of HF in the U.S. is expected to increase by 33.4% (from 9.7 million to 12.9 million Americans). This increase will disproportionately affect African American and Hispanic populations.5 As the most accessible healthcare professional, the pharmacist can positively affect the trajectory of this growth by helping HF patients achieve target doses of medications that have been found to decrease HF morbidity and mortality.
2022 AHA/ACC/HFSA HF GUIDELINE
In May 2022, the American Heart Association (AHA), American College of Cardiology (ACC), and Heart Failure Society of America (HFSA) published a revised guideline on the management of HF (hereinafter referred to as the 2022 HF guideline) to update and consolidate the 2013 ACCF/AHA Guideline for the Management of Heart Failure and the 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure into a new document.6,7 To accomplish this, a literature search was conducted from May 2020 to December 2020, and additional relevant studies that were published through September 2021 were considered. The recommendations in the 2022 HF guideline constitute guideline-directed medical therapy (GDMT).8
HF CLASSIFICATION BY LEFT VENTRICULAR EJECTION FRACTION
In 2021, the HFSA, the Heart Failure Association of the European Society of Cardiology, and the Japanese Heart Failure Society issued a consensus statement on the development of a universal definition and classification for HF. They defined HF as “a clinical syndrome with symptoms and or signs caused by a structural and/or functional cardiac abnormality and corroborated by elevated natriuretic peptide levels and or objective evidence of pulmonary or systemic congestion.” They also proposed the following HF classification based on left ventricular ejection fraction (LVEF): HF with reduced EF (HFrEF; EF ≤40%), HF with mildly reduced EF (HFmrEF; EF 41%-49%), HF with improved EF (HFimpEF; EF improves from <40% to >40%), and HF with preserved EF (HFpEF; EF ≥50%).9 The 2022 HF guideline defines HF as “a complex clinical syndrome with symptoms and signs that result from any structural or functional impairment of ventricular filling or ejection of blood”; in contrast to the 2021 consensus statement’s definition, it does not mention natriuretic peptide levels or congestion. HF has a range of clinical manifestations that depend on underlying pathophysiology, EF, and stage of disease. The 2021 classification for HF based on EF was incorporated into the 2022 HF guideline.8 In the 2013 guideline, HFmEF and HFimpEF were listed as subcategories of HFpEF.6
HF CLASSIFICATION BY ACC/AHA STAGE
The ACC/AHA classifies HF into four stages: A through D. While the stages are the same as in previous guidelines, changes have been made to the terminology.8 Stage A, which was previously defined as being at high risk for HF without structural heart disease or symptoms of HF, is now defined as at risk for HF and applies to persons at risk who do not have current or previous signs or symptoms of the disease, structural or functional heart disease, or abnormal biomarkers indicative of HF. Patients in this group include those with hypertension, diabetes, or obesity, those who have been exposed to cardiotoxic agents, and those who either possess the variant for or have a family history of cardiomyopathy. This mention of exposure to cardiotoxic agents is a new consideration in the 2022 HF guideline.8
Previously, stage B included patients with structural heart disease but without signs or symptoms of HF; this group is now called pre-HF. In stage B, patients still do not have current or previous signs or symptoms of HF, but there is now evidence of one of the following: structural heart disease, increased filling pressure, and increased natriuretic peptide levels or elevated cardiac troponin levels that remain persistently elevated in the absence of another explanation for these findings.8
Stage C, which is now called symptomatic HF, was previously described as the presence of structural heart disease with prior or current symptoms of HF. At this stage, patients now demonstrate signs and/or symptoms of the disease. Based on the trajectory of symptoms and effect on functional capacity, stage C may be further classified as new-onset/de novo HF, resolution of symptoms, persistent HF, or worsening HF.8
Patients who have marked HF symptoms that interfere with daily life or who have numerous readmissions secondary to HF despite attempts at optimization of GDMT are in stage D, or advanced HF (previously referred to as refractory HF).8
The 2022 HF guideline notes that the New York Heart Association (NYHA) classification system (i.e., classes I-IV) is used to characterize the symptoms and functional capacity of patients with symptomatic (stage C) or advanced (stage D) HF.8
INTERPRETATION OF THE 2022 HF GUIDELINE
Recommendations in the 2022 HF guideline are classified both by class (i.e., strength of the recommendation) and by level (i.e., quality of the evidence).8 The classes, which are based on benefit versus risk, are as follows: class 1 (a strong recommendation; use is considered recommended, indicated, useful, effective, or beneficial), class 2a (a moderate recommendation; use is considered reasonable and the intervention may be useful, effective, or beneficial), and class 2b (a weak recommendation; use may or may not be reasonable or considered because the intervention’s usefulness or effectiveness is unclear). In class 3, there is no benefit or the benefit equals the risk (a moderate recommendation; use is not recommended, indicated, useful, effective, or beneficial), or there is evidence of harm (a strong recommendation; should be avoided).8
Levels of evidence include A, B-R, B-NR, C-LD, and C-EO. Level A is high-quality evidence based on randomized, controlled trials (RCTs) or meta-analyses of highquality RCTs, and level B-R is moderate-quality evidence based on RCTs or meta-analyses of moderate-quality RCTs. Level B-NR is moderate-quality evidence based on well-designed, well-executed, nonrandomized trials that can include observational studies or registry studies or meta-analyses of these types of studies. Level C-LD is based on limited data from randomized or nonrandomized observational or registry studies or meta-analyses of these studies, but these studies have design or methodology limitations or can include physiologic or mechanistic studies in human subjects. Level C-EO, with the lowest level of evidence, is based on expert opinion.8
PHARMACOTHERAPY FOR HF MANAGEMENT
New to the 2022 HF guideline is the inclusion of four classes of medications considered to constitute the mainstay of HF therapy: renin-angiotensin system (RAS) inhibitors (i.e., ACE inhibitors [ACEIs], angiotensin II receptor blockers [ARBs], or angiotensin receptor/neprilysin inhibitor [ARNI]), beta-blockers (BBs), mineralocorticoid receptor antagonists (MRAs), and sodiumglucose cotransporter 2 (SGLT2) inhibitors. Other agents that are used, depending on patient-specific factors, include diuretics, hydralazine and isosorbide dinitrate, ivabradine, digoxin, and soluble guanylyl cyclase (sGC) stimulators.8
Diuretics
This drug class (TABLE 1) includes loop diuretics (e.g., bumetanide, furosemide, torsemide), thiazide (e.g., chlorthalidone, hydrochlorothiazide) and thiazide-like diuretics (e.g., indapamide), and potassium-sparing diuretics (e.g., spironolactone). Loop diuretics are preferred in HF because of their more potent effects on diuresis. Ethacrynic acid, one of the loop diuretics, is not included in the 2022 HF guideline. Thiazides are used for milder fluid retention. The 2022 HF guideline recommends that diuretics be utilized in HF patients with fluid retention to relieve congestion, improve symptoms, and prevent worsening HF (class 1, level B-NR). Metolazone or chlorothiazide should be added only in patients not responsive to moderate or high doses of loop diuretics because of concerns about electrolyte imbalances (class 1, level B-NR). These agents are first used in stage C HF, although thiazides or thiazide-related medications may be used earlier in patients with comorbid hypertension.8
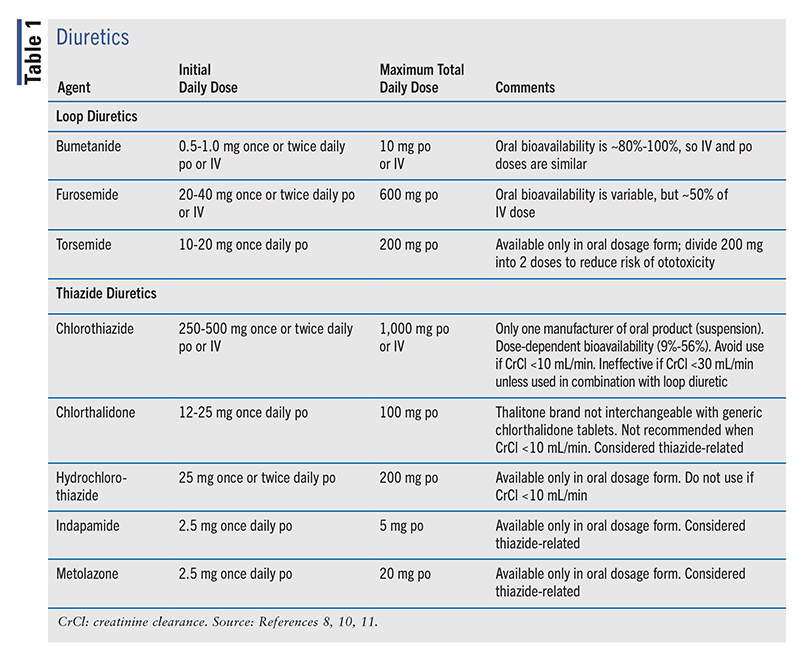 |
The oral dose equivalency between the loop diuretics is: bumetanide 1 mg is equal to furosemide 40 mg, which is equal to 10 mg to 20 mg torsemide.10,11
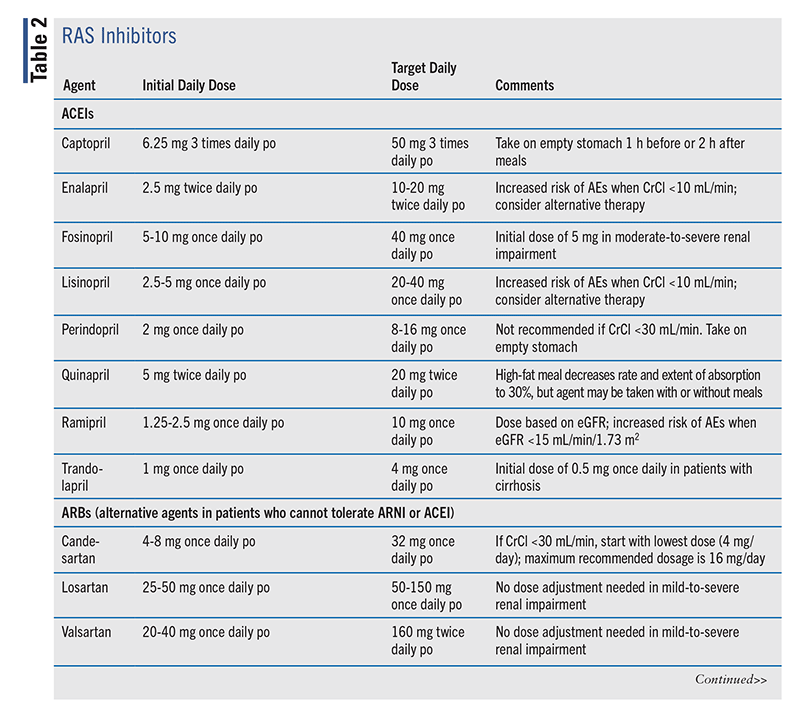
RAS Inhibitors
Inhibition of the RAS has been shown to reduce morbidity and mortality in patients with HFrEF.8 RAS inhibitors include ACEIs, ARBs, and ARNI therapy (TABLE 2). Among the ACEIs, benazepril and moexipril are not indicated for HFrEF. Among the ARBs, azilsartan, eprosartan, irbesartan, olmesartan, and temisartan are not approved for HFrEF.10 Sacubitril/valsartan is the only ARNI on the U.S. market.10
 |
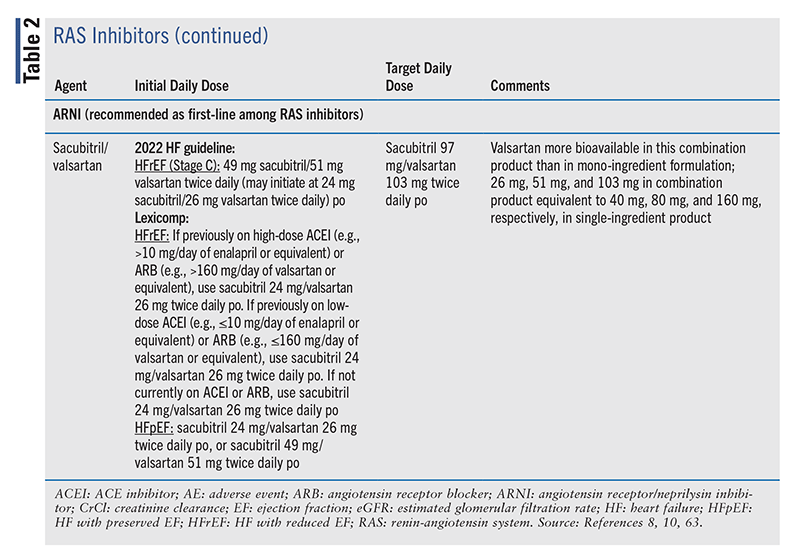 |
Generally used in stage C or higher, ACEIs (or, if not tolerated, ARBs) may be used in stage B when LVEF is ≤40% with or without recent myocardial infarction (MI). They may also be used early on in patients with hypertension. New recommendations include ARNI use over ACEIs and ARBs in patients with HFrEF and NYHA class II-III symptoms, and ARNI initiation de novo in hospitalized patients with acute HF prior to discharge.8 For HFrEF, the 2017 HF guideline had recommended that patients demonstrate tolerance to an ACEI or ARB prior to transitioning to ARNI therapy.6 However, ARNI therapy in the absence of a trial of an ACEI or ARB has been demonstrated to have beneficial effects on cardiac remodeling and on reducing cardiac biomarkers.12
If ARNI therapy is unavailable and the patient has previous or current symptoms of chronic HFrEF, use of an ACEI is recommended over an ARB unless the patient is experiencing intolerable cough. In HFrEF, all of the RAS inhibitors reduce morbidity and mortality (all are class 1, level A). A class 1, level B-R recommendation is made for replacing an ACEI or ARB with ARNI therapy in patients with chronic symptomatic HFrEF NYHA class II-III who tolerate either an ACEI or an ARB. The guideline states that in patients with chronic symptomatic HF, the use of ARNI therapy instead of an ACEI provides high economic value despite the higher cost of the ARNI. The guideline warns (class 3—harm) that neither ARNI therapy nor an ACEI should be administered to patients with a history of angioedema (level C-LD) and that the ARNI should not be given concomitantly or within 36 hours of the last dose of an ACEI (level B-R). While the occurrence of angioedema may be similar between ACEIs and ARNI therapy, hypotension is more pronounced in the combination agent.8
The number needed to treat (NNT) for all-cause mortality at 36 months is 26 for ACEIs or ARBs and 27 for ARNI therapy. The benefit increases with time since the NNT for all-cause mortality at 12 months is 77 and 80, respectively.8
Beta-Blockers
The BBs indicated for the management of HFrEF include bisoprolol, carvedilol, and extended-release metoprolol succinate (TABLE 3). BBs reduce the risk of death, decrease the combined risk of death or hospitalization, improve LVEF, lessen HF symptomatology, and enhance clinical status in patients with HFrEF. Beneficial effects from BBs are seen in patients with diabetes, in older adults, among women, in patients with or without coronary artery disease, and across racial and ethnic groups; beneficial effects have not been observed in patients with atrial fibrillation.8
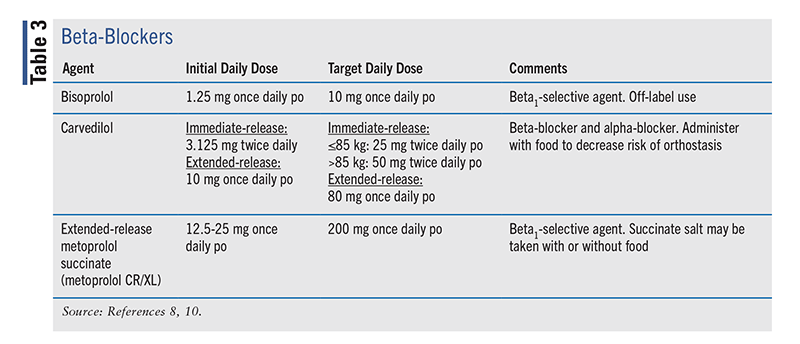 |
The 2022 HF guideline recommends that BBs—specifically, bisoprolol, carvedilol, and sustained-release metoprolol succinate—be administered to all HFrEF patients (i.e., those with current or previous symptoms; class 1, level A) to reduce mortality and hospitalizations, even while they are still hospitalized for HF, unless contraindications or intolerance is present. The guideline also states that BB therapy provides high economic value in HFrEF patients with current or previous symptoms.8
The NNT for BBs for all-cause mortality at 12 months and 36 months is 28 and 9, respectively.8
Mineralocorticoid Receptor Antagonists
The MRAs (also known as aldosterone antagonists or antimineralocorticoids) include spironolactone, eplerenone, and finerenone (TABLE 4). Spironolactone and eplerenone are associated with reductions in all-cause mortality, HF hospitalization, and sudden cardiac death in patients with HFrEF.8 Finerenone is not indicated for the management of HFrEF.
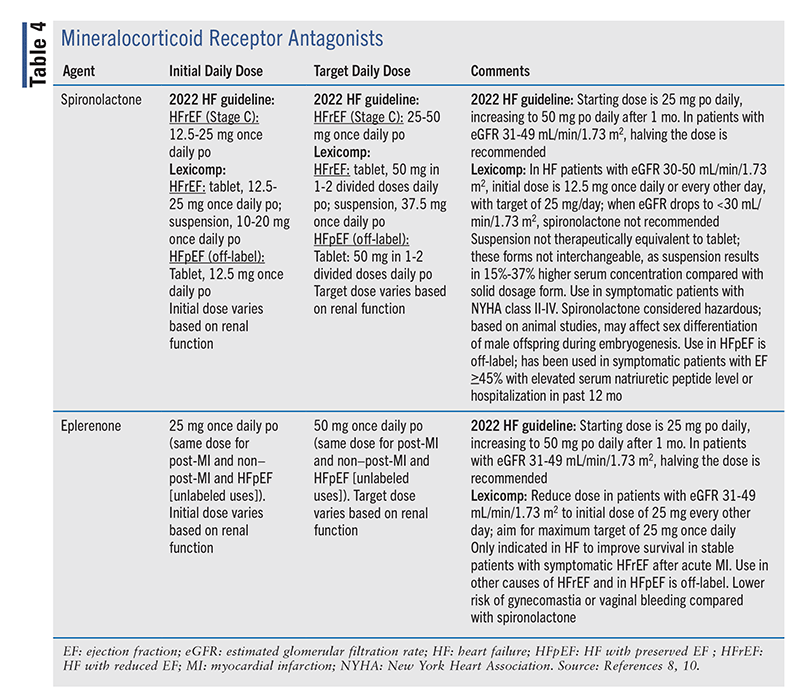 |
The 2022 HF guideline recommends that either spironolactone or eplerenone be prescribed in patients with HFrEF and NYHA class II-IV symptoms with both an estimated glomerular filtration rate (eGFR) level >30 mL/ min/1.73 m2 and a serum potassium level <5.0 mEq/L. These drugs are associated with the development of hyperkalemia, especially in patients with renal impairment. Serum potassium and renal function should be monitored at baseline and throughout therapy, and the dosing of diuretics should be adjusted as needed to minimize hyperkalemia and renal dysfunction (class 1, level A). The use of MRAs in these HF patients is considered to have high economic value. If serum potassium levels cannot be maintained at <5.5 mEq/L, the MRA should be discontinued (class 3—harm, level B-NR). MRAs are contraindicated in patients with an eGFR ≤30 mL/ min/1.73 m2 and in those with a serum potassium level ≥5 mEq/L.8 HF patients with serum creatinine levels ≥2.5 mg/dL were excluded from clinical trials. For both MRAs, the serum creatinine should be >2.5 mg/dL in men and >2 mg/dL in women prior to the initiation of therapy.10
The NNT for MRAs for all-cause mortality at 18 months and 6 months is 28 and 9, respectively.8
SGLT2 Inhibitors
Of the four commercially available SGLT2 inhibitors—canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin—only empagliflozin and dapagliflozin (TABLE 5) are FDA approved for use in HF.10 One of the most significant additions to the 2022 HF guideline is the recommendation that SGLT inhibitors be utilized across all HF categories, even in the absence of a concomitant diagnosis of diabetes. For patients with symptomatic HFrEF, this is a class 1, level A recommendation; however, it is only a class 2a, level B-R recommendation for HFmrEF and HFpEF. HFimpEF has a class 1, level B-R recommendation that GDMT (including SGLT2 inhibitors) be continued to prevent relapse of HF and LV dysfunction, including in patients who have become asymptomatic.8 Empagliflozin is approved for both HFrEF and HFpEF; it is indicated to reduce the risk of cardiovascular (CV) death and hospitalization from HF in adults with or without diabetes, as the beneficial CV effects of SGTL2 inhibitors appear to be independent of their effect in diabetes. Dapagliflozin is indicated for HFrEF but is also used off-label for HFpEF.10
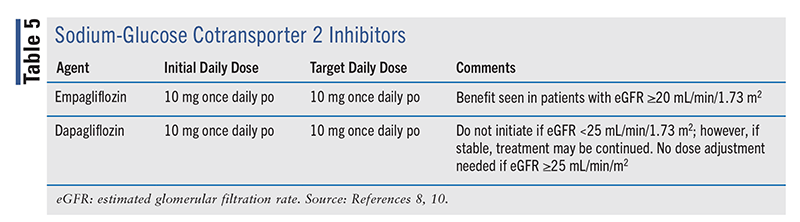 |
Patients who are receiving SGLT2 inhibitor therapy need to be monitored for the development of euglycemic ketoacidosis, genital mycotic infections, volume depletion, urosepsis, necrotizing fasciitis of the perineum, acute kidney injury, and bone fractures. Concomitant diuretic therapy must be adjusted accordingly.10 Empagliflozin and dapagliflozin should be administered in the morning.10
The NNT for the SGLT2 inhibitors for all-cause mortality at 63 months and 22 months is 28 and 9, respectively.8
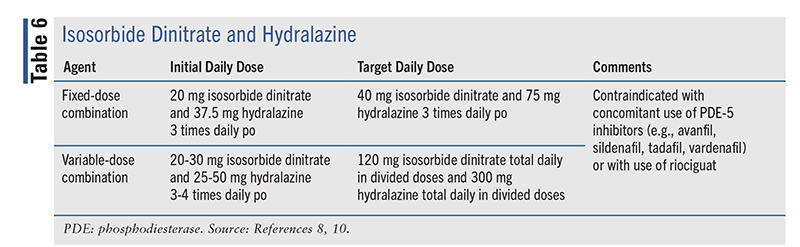
Hydralazine and Isosorbide Dinitrate
In addition to the four aforementioned mainstay HF therapies, medications that are useful in the African American population include the combination of hydralazine and isosorbide dinitrate. The 2022 HF guideline recommends that in African American patients with NYHA class III-IV who are on optimal GDMT, the hydralazine/isosorbide dinitrate combination should be added to the drug regimen to improve symptoms and reduce morbidity and mortality (class 1, level A). This combination may also be utilized in patients with current or previous symptomatic HFrEF in whom first-line agents (e.g., RAS agents) cannot be administered because of drug intolerance or renal insufficiency (class 2b, level C-LD). Hydralazine/isosorbide dinitrate was found to provide high economic value in African American patients with NYHA class III-IV HFrEF who are on optimal GDTM with the four mainstay HF medications. It has been suggested that the combination regimen may reduce mortality in HF patients already on digoxin and diuretics.8
The NNT for isosorbide dinitrate/hydralazine in African American patients for all-cause mortality at 12 months and 36 months is 21 and 7, respectively (TABLE 6).8
 |
Ivabradine
Ivabradine (TABLE 7) is indicated as an adjunctive agent in HFrEF to reduce the risk of hospitalization for worsening HF in adult patients with stable, symptomatic chronic HFrEF and for the treatment of stable symptomatic HF due to dilated cardiomyopathy in pediatric patients aged 6 months and older.13 It works by selectively and specifically inhibiting the hyperpolarization-activated cyclic nucleotide-gated channel, which is responsible for the cardiac pacemaker current (If); If regulates heart rate. Ivabradine’s effects are most pronounced in the sinoatrial node. This medication has no effect on ventricular repolarization or on myocardial contractility.13
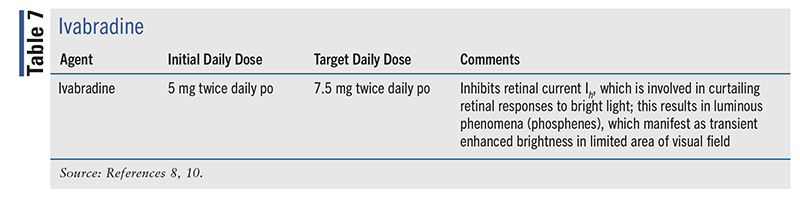 |
Ivabradine is contraindicated in patients with acute decompensated HF, clinically significant hypotension, sick sinus syndrome, sinoatrial block or third-degree atrioventricular block (unless patient has a pacemaker), clinically significant bradycardia, or severe hepatic impairment. It is also contraindicated when the patient’s heart rate is maintained exclusively by a pacemaker, and in patients receiving concomitant strong CYP3A4 inhibitors, such as ritonavir.13
The 2022 HF guideline recommends that ivabradine may be useful in reducing HF hospitalization and CV death in patients with symptomatic NYHA class II-III stable chronic HFrEF (LVEF ≤35%) who are on GDMT, including a BB at maximally tolerated dose, and who are in sinus rhythm with a heart rate of ≥70 beats/minute (class 2a, level B-R).8
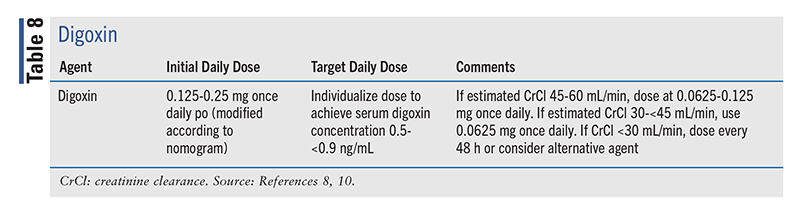
Digoxin
While the use of digoxin (TABLE 8) has not been associated with decreased mortality in HFrEF, the 2022 HF guideline recommends that in patients with symptomatic HFrEF despite GDMT or in patients who cannot tolerate GDMT, the use of digoxin might be considered to reduce hospitalizations due to HF (class 2b, level B-R).8 However, this is one of the weaker recommendations.
 |
Soluble Guanylate Cyclase Stimulators
Nitric oxide (NO) binds to sGC (an important enzyme in the NO signaling pathway), resulting in the synthesis of intracellular cyclic guanosine monophosphate (cGMP), a second messenger that plays a role in the regulation of vascular tone, cardiac contractility, and cardiac remodeling. In HF, there is impaired NO synthesis and decreased sGC activity, which may contribute to myocardial and vascular dysfunction. Stimulation of sGC results in the augmentation of intracellular cGMP levels, which produces smooth-muscle relaxation and vasodilation.14
Two sGC stimulators, vericiguat and riociguat, are on the market; however, riociguat is approved only for chronic thromboembolic pulmonary hypertension or pulmonary arterial hypertension. Vericiguat (TABLE 9), approved in January 2021, is indicated as adjunctive therapy in adults with symptomatic chronic HF and EF <45% to reduce the risk of CV death and HF hospitalization following a hospitalization for HF or need for outpatient IV diuretics.10 Vericiguat has a boxed warning regarding the risk of embyro-fetal toxicity and is contraindicated in pregnancy.14
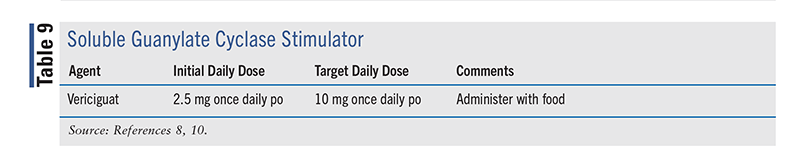 |
The 2022 HF guideline recommends that the use of vericiguat be considered in selected high-risk patients with HFrEF and recent worsening of HF despite being on GDMT to reduce HF hospitalizations and CV death (class 2b, level B-R). As with digoxin use in HFrEF, this is one of the weaker recommendations.8
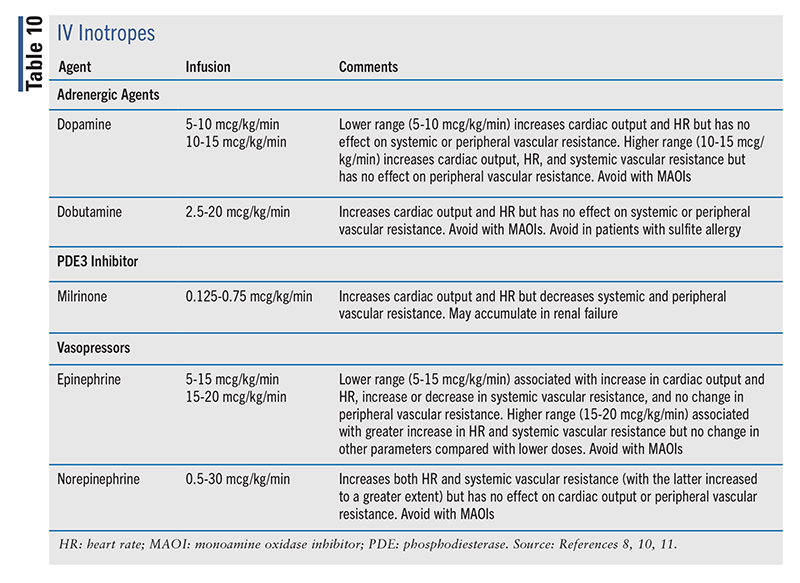
Inotropes
These medications are considered for use in stage D either as palliation or as a bridge to cardiac transplantation. Examples of inotropes (TABLE 10) are adrenergic agonists (e.g., dopamine, dobutamine), phosphodiesterase (PDE) 3 inhibitors (e.g., milrinone), and vasopressors (e.g., epinephrine, norepinephrine). Despite their use, none of these drugs have been shown to improve survival in advanced HF. They may benefit patients who are refractory to other therapies and those in whom end-organ hypoperfusion is affecting function. There is limited evidence to support the use of one agent over another. These medications may be useful in the short term in hospitalized patients with severe systolic dysfunction, hypotension, and a low cardiac index. Tachyphylaxis may develop with these agents. All of them increase heart rate and cardiac output (except for norepinephrine, which does not affect the latter parameter). Only milrinone decreases peripheral vascular resistance; the rest have a neutral effect. The effect of inotropes on systemic vascular resistance is variable.8
 |
Other Drugs
Other drugs that have been used in the management of HF include omega-3-polyunsaturated fatty acids (PUFA), potassium binders, and anticoagulation.8
Omega-3 PUFA: The 2022 HF guideline indicates that PUFA supplementation may be utilized as adjunctive therapy in patients with NYHA class II-IV symptoms to reduce mortality and CV hospitalizations, as it has been demonstrated to decrease fatal and nonfatal CV events by 10% to 20%.8
Potassium Binders: Several classes of medications used in the management of HF, including RAS agents and MRAs, can increase the risk of hyperkalemia development, especially in patients with reduced renal function. However, the 2022 HF guideline states that in HF patients who experience hyperkalemia—defined as a serum potassium level ≥5.5 mEq/L while on RAS therapy—the role of potassium binders (e.g., patiromer, sodium zirconium cyclosilicate) is unclear (class 2b, level B-R).8
Anticoagulation: The 2022 HF guideline recommends against anticoagulation in patients with HFrEF unless there is a clear indication for it, such as venous thromboembolism, atrial fibrillation, a previous thromboembolic event, or a cardioembolic event (class 3—no benefit, level B-R).8
ACHIEVING TARGET DOSES OF GDMT
The value of GDMT in HF is evidenced by the decompensation that occurs upon withdrawal of these therapies. In patients with HF and asymptomatic recovered dilated cardiomyopathy whose LVEF improved from <40% to ≥50%, 44% relapsed upon withdrawal of their cardiac medications.15
Most HF patients are not achieving the target doses of GDMT. CHAMP-HF was a prospective, observational, nonrandomized study of adult outpatients with HFrEF (LVEF ≤40% on most recent imaging within 12 months of enrollment) who were receiving ≥1 oral medication for HF at enrollment.16 At baseline, 25.4%, 20.3%, 11.1%, and 1.7% of patients were receiving target doses of MRA, BB, ACEI/ARB, and ARNI therapy, respectively, whereas 0.7% of patients were simultaneously treated with target doses of ACEI/ARB/ARNI, BB, and MRA at both baseline and 12 months.16 Pharmacists can play a key role in assisting patients in achieving their target goals by optimizing their HF medication regimens.
PHARMACOLOGIC MANAGEMENT BY HF STAGE
Primary prevention is indicated in those patients who are in HF stages A and B, in order to help prevent disease progression.8
Stage A
In stage A, the goal is to target modifiable risk factors by controlling blood pressure via GDMT (class 1, level A); prescribing an SGLT2 inhibitor in patients with type 2 diabetes and established or high risk for CV disease (class 1, level A); encouraging healthy lifestyle (e.g., physical activity, weight control, proper diet, avoiding or stopping smoking; class 1, level B-NR); employing a team-based approach to assess natriuretic peptide biomarkers and adjust GDMT accordingly (class 2a, level B-R); and using validated multivariable risk scores (e.g., Framingham Heart Failure Risk Score, Health ABC Heart Failure Score, ARIC Risk Score, Pooled Cohort Equations to Prevent HF) to determine further HF risk (class 2a, level B-NR).17-20 Reductions in brain natriuretic peptide (BNP) and N-terminal prohormone BNP levels predict improved long-term outcomes compared with patients who have persistently elevated levels despite appropriate treatment. Assessing risks resulting from the use of cardiotoxic agents is a focus of the growing field of cardiooncology.8
Stage B
In stage B, interventions should focus on treating risk and structural heart disease to prevent progression to overt HF. In patients with LVEF ≤40%, ACEI therapy should be initiated to prevent symptomatic HF and reduce the risk of death (class 1, level A). Statins should be utilized to prevent HF symptomatology in patients with a remote history of MI or with acute coronary syndrome (ACS; class 1, level A). If a patient is intolerant to ACEIs and has a history of recent MI or LVEF ≤40%, an ARB should be substituted (class 1, level B-R) for its benefits in preventing symptoms and decreasing mortality. BBs should be employed to reduce mortality in patients with a recent or remote history of MI or ACS and LVEF ≤40% (class 1, level B-R). BBs may also be used to prevent symptomatic disease in patients who have LVEF ≤40%, although the level of evidence is not as strong (class 1, level C-LD).
Thiazolidinediones should be avoided in patients with pre-HF, as these agents increase the risk of overt disease (class 3—harm, level B-R). Nondihydropyridine calcium channel blockers (i.e., diltiazem, verapamil) should also be avoided because of their negative inotropic effect (class 3—harm, level C-LD). An implantable cardioverter defibrillator is recommended to prevent sudden death in patients who have had an MI within at least the past 40 days, have reduced LVEF (i.e., ≤30%), are experiencing NYHA class I HF symptoms, are optimized on GDMT, and are expected to survive for ≥1 year (class 1, level B-R).8
When the classification of HF is based on stage, if a patient who is in stage C HF becomes asymptomatic, he or she is still considered to have stage C HF. However, when the classification is based on the LVEF, the 2022 HF guideline recognizes that improvements in function can occur. The current classification system is indicative of the prognosis and the response to therapy, although these improvements do not indicate a full myocardial recovery or the normalization of LV function.8
Stage C
Several strategies to promote patient self-care should be initiated at this stage, including vaccination against respiratory infections (class 2a, level B-NR); screening for depression, social isolation, frailty, and low health literacy (class 2a, level B-NR); HF-specific patient education (class 1, level B-R); sodium restriction (class 2a, level C-LD); exercise training or regular physical activity (class 1, level A); and cardiac rehabilitation (class 2a, level B-NR).8
Recommendations for GDMT Sequencing and Uptitration
GDMT is estimated to reduce the risk of CV death or HF hospitalization in patients with HFrEF by up to 62% compared with conventional treatment.2 The 2022 HF guideline includes recommendations on sequencing and uptitrating doses of pharmacologic agents that are part of GDMT. In HFrEF patients, it is recommended to achieve target doses of medications that have been shown in RCTs to be efficacious in reducing CV mortality and HF hospitalizations (class 1, level A). Uptitration may be performed as frequently as every 1 to 2 weeks based on the patient’s symptoms, vital signs, and laboratory data (class 2a, level C-EO).8
The 2022 HF guideline provides a six-step algorithm for the treatment of HFrEF stages C and D.8
Step 1: Following the establishment of a diagnosis of HFrEF and the addressing of any fluid overflow/congestion that may be present, GDMT should be initiated. Therapy with all four mainstay pharmacologic drug classes—RAS inhibitors (ARNI in NYHA class II-III, ACEI/ARB in NYHA class II-IV), BBs, MRAs, and SGLT2 inhibitors—should be initiated simultaneously at initial low doses recommended for HFrEF. Diuretics may be used as needed. An alternative approach to simultaneous initiation of all four medication classes is to start them sequentially without achieving target doses before the next drug is added to the regimen. The goal is to reach target for all four classes of medications.8
Step 2: Doses of mainstay medications should be titrated to target dosing as tolerated, laboratory testing should be ordered as needed to assess fluid and electrolyte status, health status should be monitored for adverse events, and LVEF should be reassessed. This step applies to patients in stage C with LVEF ≤40% with persistent HFrEF and to those with LVEF >40% with HFimp EF.8
Step 3: Individual patient scenarios should be considered, such as African-American race, survival expectations, and electrographic findings of more severe disease.8
Step 4: Additional non–first-line GDMT may be implemented, as well as the use of implantable devices. For African-American patients with NYHA class III-IV, hydralazine and nitrates should be added. For patients with NYHA class I-III, those with LVEF ≤35%, or those with an expected survival of >1 year, an implantable cardioverter-defibrillator should be considered. In patients with NYHA class II-III, those on ambulatory IV cardiac medications, those with LVEF ≤35%, or those who are in normal sinus rhythm and have a QRS ≥150 ms with a left bundle branch block, cardiac resynchronization therapy should be considered.8
Step 5: Symptoms should be reassessed, laboratory values should be checked, health status should be reviewed, and it should be determined whether the patient benefited from the previous steps (e.g., if symptoms have not improved, in which case the patient has refractory HF).8
Step 6: The patient should be referred to an HF specialist to explore additional options, which may include mechanical circulatory support, cardiac transplant, or palliative care.8
Stage D (Advanced)
As HF progresses, management becomes more complex. The role of fluid restriction in patients with advanced HF and hyponatremia has been questioned, as it is of uncertain benefit in reducing congestive symptoms (class 2b, level C-LD). The 2022 HF guideline also calls for the management of advanced HF patients by a specialized HF team, which includes a pharmacist.8
For patients with advanced HF, inotropic support is indicated. The continuous use of IV inotropes is recommended as bridge therapy in patients with advanced HF who are refractory to GDMT and device therapy and who are eligible for and awaiting mechanical circulatory support or cardiac transplantation (class 2a, level B-NR). Similarly, inotropic therapy should also be considered for these patients if they are not eligible for mechanical circulatory support or transplantation; in this case, inotropic therapy is considered palliative and designed for symptom control and improvement of functional status (class 2b, level B-NR). Inotropic therapy is considered harmful when utilized long-term for continuous or intermittent therapy if the goal is not palliation or bridge therapy (class 3—harm, level B-R).8
Too often, GDMT is scaled back or abandoned during an acute admission. It is essential to maintain or optimize GDMT and fluid status prior to discharge and to advance GDMT toward targets for outpatient therapy in patients with decompensated HF (class 1, level C-LD). Precipitating factors that may contribute to an HF hospitalization for decompensated HF include nonadherence to the medication regimen, medications that increase sodium retention (e.g., nonsteroidal anti-inflammatory drugs), and medications with negative inotropic effects (e.g., verapamil).8
Recommendations for GDMT maintenance or optimization during hospitalization in patients with HFrEF include continuing and optimizing preexisting GDMT to improve outcomes, unless contraindicated; not discontinuing diuresis or other GDMT in patients experiencing mild decreases in renal function or asymptomatic decreases in blood pressure; initiating GDMT during hospitalization once clinical stability has been achieved; and reinstituting and optimizing GDMT as soon as possible if it was deemed necessary to discontinue it during hospitalization. All of these recommendations are class 1, level B-NR.8
In patients hospitalized for decompensated HF and fluid overload, diuretics are a mainstay of therapy to improve symptoms and decrease morbidity. Diuretics and GDMT should be titrated with a goal of resolving clinical evidence of congestion to prevent rehospitalization and improve symptoms. Following an HF hospitalization, patients should be discharged with a plan for adjusting their diuretic therapy in order to prevent rehospitalization. These are all class 1, level B-BR recommendations. If patients do not adequately respond to diuretic therapy, it is recommended to intensify the diuretic regimen by either using higher doses of IV loop diuretics or adding a second diuretic (class 2a, level B-NR).9
In patients with decompensated HF who are experiencing dyspnea but not systemic hypotension, IV nitroglycerin or nitroprusside may be considered in addition to diuretic therapy (class 2b, level B-NR). Prophylaxis against venous thromboembolism is recommended in patients hospitalized with decompensated HF (class 1, level B-R). The risk of thromboembolism is greatest during the first 30 days following hospitalization for decompensated HF.8
In patients with decompensated HF who develop cardiogenic shock, the use of IV inotropics is necessary to maintain systemic perfusion and preserve end-organ function (class 1, level B-NR). If pharmacologic therapy fails to maintain end-organ function, temporary use of a mechanical circulatory-support device may be needed to support cardiac function. These patients should be managed by a multidisciplinary team experienced in managing patients in shock (both recommendations: class 2a, level B-NR).8
PHARMACOLOGIC MANAGEMENT BY LVEF
HFmrEF
HFmrEF refers to HF with LVEF of 41% to 49%; it has also been called HF with mid-range EF or borderline EF.21 No prospective RCTs have been specifically designed to study pharmacologic agents in HFmrEF; therefore, recommendations are based on subset analyses of major clinical trials. Among the drugs for HFmrEF, SGLT2 inhibitors have a class 2a, level B-R recommendation for reducing HF hospitalizations and CV mortality; recommendations for other medications (including ARNIs, ACEIs, ARBs, MRAs, and BBs) are ranked as class 2b, level B-R, so these agents may be considered especially in patients whose LVEF is at the lower end of the range. Diuretics should be used as needed.8
HFimpEF
In HFimpEF, the patient has an improved HF with EF increased to >40%. Pharmacologic management is similar to that for patients with HFrEF (class 1, level B-R).8 GDMT for HFrEF should be continued to prevent relapse, even if the patient is asymptomatic, as HFimpEF does not imply normal LVEF or recovery from heart damage (class 1, level B-R).8 Caution is advised, as many medications can precipitate or exacerbate HF.
HFpEF
New recommendations for HFpEF include using SGLT2 inhibitors to decrease HF hospitalizations and prevent CV mortality (class 2a, level B-R); titrating antihypertensive therapy to attain blood pressure targets according to published guidelines (class 1, level C-LD); managing symptoms of atrial fibrillation (class 2a, level C-EO); and, in selected patients—especially those with LVEF on the lower end of the spectrum—using MRAs, ARBs, or ARNI therapy to decrease hospitalizations (level 2b, level B-R). The guideline advises against routine use of nitrates or PDE-5 inhibitors, as they are ineffective in improving activity or quality of life (class 3—no benefit, level B-R).8
The 2022 HF guideline addresses the emerging specialty of cardio-oncology. It identifies chemotherapeutic agents as associated with cardiomyopathy.8 Recently, the European Society of Cardiology published guidelines identifying cardiotoxic chemotherapeutic agents and offering a plan of care for managing patients on these agents.22
RECOGNIZING DISPARITIES IN HF MANAGEMENT
One of the additions to the 2022 HF guideline is the recognition of disparities in the management of HF and identifying the needs of vulnerable populations, including women (including pregnant women), older adults, those in lower socioeconomic brackets, African Americans, Hispanics, Asians and Pacific Islanders, and Native American and Alaskan Native populations. The 2022 HF guideline calls for HF risk assessments and multidisciplinary management strategies to target known risks for CV disease and social determinants of health in vulnerable populations in order to eliminate disparities in HF outcomes (class 1, level C-LD). It also recommends that evidence of health disparities be monitored and addressed at both the clinical-practice level and the healthcare-system level (class 1, level C-LD).8
PATIENT-REPORTED OUTCOMES
HF outcomes should be reported by the patient. A number of HF-specific health-status assessment tools are available for use.8,23-25
GAPS IN EVIDENCE
Gaps in evidence in HF management include the evolving role of future therapies targeting novel pathways and endophenotypes and those with multiple CV, cardiometabolic, renovascular, and pathobiologic mechanisms.8
THE PHARMACIST'S ROLE
Pharmacists have been playing an integral role in managing HF drug therapy for decades.26 In 2013, the HFSA and the American College of Clinical Pharmacy Cardiology Practice and Research Network issued an opinion paper in which they identified positive outcomes associated with clinical pharmacist activities when clinical pharmacists are part of interdisciplinary HF teams.27 In 2019, an expert consensus document was published that highlighted the minimum competencies necessary for clinical pharmacists to deliver appropriate care to HF patients.28
Numerous studies have demonstrated the value of pharmacists’ involvement in managing HF patients’ drug therapy, including reductions in all-cause mortality; increases in prescribing of GDMT to goal; decreases in HF hospitalizations; improvements in medication adherence; increases in knowledge, symptom control, and quality of life among HF patients; prevention of medication-related problems such as adverse drug events, medication errors, and drug-drug interactions; decreases in HF and/or all-cause readmission; improvements in selfmanagement, self-maintenance, and self-confidence among HF patients; improvements in clinical outcomes, health literacy, in-home medication reconciliation, and patient education; and reductions in time to follow-up in bridge patients.29-57
Transitions of Care
Appropriately managed transitions of care are particularly important for HF patients, and it is essential for the pharmacist to ensure that the patient’s medication regimen is accurate and reflects the current treatment plan.58,59 A review published in 2018 helped define the role of the pharmacist during this critical period.60 The 2021 update to the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment provides guidance and recommendations on the clinical care of patients with HFrEF.61
During the transition of care, the pharmacist should also assess the impact that medication cost may have on adherence. While ideally patients with HFrEF should be on quadruple GDMT, it is also important to consider cost and whether ARNI therapy is covered by insurance, as lack of this coverage can lead to nonadherence.62 Pharmacists can help patients apply for pharmaceutical-assistance programs. HF medications that are the mainstay of management and are tolerated and affordable should be uptitrated to goal.
CONCLUSION
The 2022 HF guideline is a useful resource for pharmacists to assist patients in achieving target goals for GDMT. Pharmacists possess the necessary skills to help patients achieve these therapeutic goals; reduce HF hospitalizations and readmissions; prevent medication-related problems; and enhance patients’ medication knowledge, adherence, functional ability, and quality of life.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
1. CDC. Heart failure. www.cdc.gov/heartdisease/heart_failure.htm. Accessed November 10, 2022.
2. Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation. 2022;145(8):e153-e639.
3. Agarwal MA, Fonarow GC, Ziaeian B. National trends in heart failure hospitalizations and readmissions from 2010 to 2017. JAMA Cardiol. 2021;6(8):952-956.
4. Jones NR, Roalfe AK, Adoki I, et al. Survival of patients with chronic heart failure in the community: a systematic review and metaanalysis. Eur J Heart Fail. 2019;21(11):1306-1325.
5. Mohebi R, Chen C, Ibrahim NE, et al. Cardiovascular disease projections in the United States based on the 2020 census estimates. J Am Coll Cardiol. 2022;80(6):565-578.
6. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-e239.
7. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136(6):e137-e161.
8. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e895-e1032.
9. Bozkurt B, Coats AJ, Tsutsui H, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail. 2021;27(4):387-413.
10. Lexicomp drug information [app]. Hudson, OH: Lexicomp, Inc; December 2022.
11. Drugs.com. Furosemide dosage. www.drugs.com/dosage/furosemide.html#Usual_Adult_Dose_for_Congestive_Heart_Failure. Accessed November 10, 2022.
12. Belkin MN, Cifu AS, Pinney S. Management of heart failure. JAMA. 2022;328(13):1346-1347.
13. Corlanor (ivabradine) product information. Thousand Oaks, CA: Amgen Inc; August 2021.
14. Verquvo (vericiguat) product information. Rahway, NJ: Merck Sharp & Dohme LLC; May 2022.
15. Halliday BP, Wassall R, Lota AS, et al. Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (TRED-HF): an open-label, pilot, randomised trial. Lancet. 2019;393(10166):61-73.
16. Greene SJ, Fonarow GC, DeVore AD, et al. Titration of medical therapy for heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019;73(19):2365-2383.
17. Kannel WB, D’Agostino RB, Silbershatz H, et al. Profile for estimating risk of heart failure. Arch Intern Med. 1999;159(11):1197-1204.
18. Butler J, Kalogeropoulos A, Georgiopoulou V, et al. Incident heart failure prediction in the elderly: the Health ABC Heart Failure Score. Circ Heart Fail. 2008;1(8):125-133.
19. Agarwal SK, Chambless LE, Ballantyne CM, et al. Prediction of incident heart failure in general practice: the Atherosclerosis Risk in Communities (ARIC) Study. Circ Heart Fail. 2012;5(4):422-429.
20. Khan SS, Ning H, Shah SJ, et al. 10-year risk equations for incident heart failure in the general population. J Am Coll Cardiol. 2019;73(19):2388-2397.
21. Clarify new heart failure terms and management. Pharmacist’s Letter. June 2022. No. 380607.
22. von Kemp B, Halvorsen S, Nohria A. The new 2022 ESC Guidelines on Cardio-oncology and their impact on the Acute Cardiovascular Care Society. Eur Heart J Acute Cardiovasc Care. 2022;11(11):844-849.
23. Cardiomyopathy questionnaire (Kansas City). https://aci.health.nsw.gov.au/__data/assets/pdf_file/0007/632851/Kansas-City-Cardiomyopathy-Questionnaire.pdf. Accessed November 10, 2022.
24. Minnesota LIVING WITH HEART FAILURE® questionnaire. https://license.umn.edu/product/minnesota-living-with-heart-failurequestionnaire-mlhfq. Accessed November 10, 2022.
25. Ahmad RS, Kallen MA, Schifferdecker KE, et al. Development and initial validation of the PROMIS®-Plus-HF profile measure. Circ Heart Fail. 2019;12(6):e005751.
26. Schneider PJ, Larrimer JN, Visconti JA, Miller WA. Role effectiveness of a pharmacist in the maintenance of patients with hypertension and congestive heart failure. Contemp Pharm Pract. 1982;5(2):74-79.
27. Milfred-Laforest SK, Chow SL, Didomenico RJ, et al. Clinical pharmacy services in heart failure: an opinion paper from the Heart Failure Society of America and American College of Clinical Pharmacy Cardiology Practice and Research Network. J Card Fail. 2013;19(5):354-369.
28. Forsyth P, Warren A, Thomson C, et al. A competency framework for clinical pharmacists and heart failure. Int J Pharm Pract.2019;27(5):424-435.
29. Patil T, Ali S, Kaur A, et al. Impact of pharmacist-led heart failure clinic on optimization of guideline-directed medical therapy (PHARMHF). J Cardiovasc Transl Res. 2022;15(6):1424-1435.
30. Shah SP, Dixit NM, Mendoza K, et al. Integration of clinical pharmacists into a heart failure clinic within a safety-net hospital. J Am Pharm Assoc (2003). 2022;62(2):575-579.e2.
31. Slade J, Lee M, Park J, et al. Harnessing the potential of primary care pharmacists to improve heart failure management. Jt Comm J Qual Patient Saf. 2022;48(1):25-32.
32. Davis LE, Pogge EK. Outcome benefits seen with 1 year of optimized sacubitril/valsartan for the treatment of systolic heart failure managed by pharmacists in a cardiology practice. Ann Pharmacother. 2022;56(5):548-555.
33. Hernández-Prats C, López-Pintor E, Lumbreras B. Pharmacist-led intervention on the reduction of inappropriate medication use in patients with heart failure: a systematic review of randomized trials and non-randomized intervention studies. Res Social Adm Pharm.2022;18(5):2748-2756.
34. Schumacher PM, Becker N, Tsuyuki RT, et al. The evidence for pharmacist care in outpatients with heart failure: a systematic review and meta-analysis. ESC Heart Fail. 2021;8(5):3566-3576.
35. Gallo-Bernal S, Calixto CA, Molano-González N, et al. Impact of a pharmacist-based multidimensional intervention aimed at decreasing the risk of hyperkalemia in heart failure patients: a Latin-American experience. Int J Cardiol. 2021;329:136-143.
36. Neu R, Leonard MA, Dehoorne ML, et al. Impact of pharmacist involvement in heart failure transition of care. Ann Pharmacother. 2020;54(3):239-246.
37. Georgiev KD, Hvarchanova N, Georgieva M, Kanazirev B. The role of the clinical pharmacist in the prevention of potential drug interactions in geriatric heart failure patients. Int J Clin Pharm. 2019;41(6):1555-1561.
38. Pape ZA, Hale G, Joseph T, et al. Impact of pharmacist-led heart failure tool kits on patient-reported self-care behaviors in a primary care-based accountable care organization. J Am Pharm Assoc (2003). 2019;59(6):891-895.e3.
39. Parajuli DR, Kourbelis C, Franzon J, et al. Effectiveness of the pharmacist-involved multidisciplinary management of heart failure to improve hospitalizations and mortality rates in 4630 patients: a systematic review and meta-analysis of randomized controlled trials. J Card Fail. 2019;25(9):744-756.
40. McKay C, Park C, Chang J, et al. Systematic review and metaanalysis of pharmacist-led transitions of care services on the 30-day all-cause readmission rate of patients with congestive heart failure. Clin Drug Investig. 2019;39(8):703-712.
41. Yates L, Valente M, Wadsworth C. Evaluation of pharmacist medication review service in an outpatient heart failure clinic. J Pharm Pract. 2020;33(6):820-826.
42. McKinley D, Moye-Dickerson P, Davis S, Akil A. Impact of a pharmacist-led intervention on 30-day readmission and assessment of factors predictive of readmission in African American men with heart failure. Am J Mens Health. 2019;13(1):1557988318814295.
43. Bonderski V, Morrow DG, Chin J, Murray MD. Pharmacy-based approach to improving heart failure medication use by older adults with limited health literacy: learning from interdisciplinary experience. Drugs Aging. 2018;35(11):951-957.
44. Schumacher C, Moaddab G, Colbert M, Kliethermes MA. The effect of clinical pharmacists on readmission rates of heart failure patients in the accountable care environment. J Manag Care Spec Pharm. 2018;24(8):795-799.
45. Bhat S, Kansal M, Kondos GT, Groo V. Outcomes of a pharmacist-managed heart failure medication titration assistance clinic. Ann Pharmacother. 2018;52(8):724-732.
46. Moye PM, Chu PS, Pounds T, Thurston MM. Impact of a pharmacy team-led intervention program on the readmission rate of elderly patients with heart failure. Am J Health Syst Pharm.2018;75(4):183-190.
47. Hale GM, Hassan SL, Hummel SL, et al. Impact of a pharmacist managed heart failure postdischarge (bridge) clinic for veterans. Ann Pharmacother. 2017;51(7):555-562.
48. Parajuli DR, Franzon J, McKinnon RA, et al. Role of the pharmacist for improving self-care and outcomes in heart failure. Curr Heart Fail Rep. 2017;14(2):78-86.
49. Dempsey JT, Matta LS, Carter DM, et al. Assessment of drug therapy-related issues in an outpatient heart failure population and the potential impact of pharmacist-driven intervention. J Pharm Pract. 2017;30(3):318-323.
50. Kang JE, Han NY, Oh JM, et al. Pharmacist-involved care for patients with heart failure and acute coronary syndrome: a systematic review with qualitative and quantitative meta-analysis. J Clin Pharm Ther. 2016;41(2):145-157.
51. Roblek T, Deticek A, Leskovar B, et al. Clinical-pharmacist intervention reduces clinically relevant drug-drug interactions in patients with heart failure: a randomized, double-blind, controlled trial. Int J Cardiol. 2016;203:647-652.
52. Kalista T, Lemay V, Cohen L. Postdischarge community pharmacist-provided home services for patients after hospitalization for heart failure. J Am Pharm Assoc (2003). 2015;55(4):438-442.
53. Warden BA, Pryor Freels J, Furuno JP, Mackay J. Pharmacy-managed program for providing education and discharge instructions for patients with heart failure. Am J Health Syst Pharm. 2014;71(2):134-139.
54. Martinez AS, Saef J, Paszczuk A, Bhatt-Chugani H. Implementation of a pharmacist-managed heart failure medication titration clinic. Am J Health Syst Pharm. 2013;70(12):1070-1076.
55. Murray MD, Ritchey ME, Wu J, Tu W. Effect of a pharmacist on adverse drug events and medication errors in outpatients with cardiovascular disease. Arch Intern Med. 2009;169(8):757-763.
56. Koshman SL, Charrois TL, Simpson SH, et al. Pharmacist care of patients with heart failure: a systematic review of randomized trials. Arch Intern Med. 2008;168(7):687-694.
57. Gattis WA, Hasselblad V, Whellan DJ, O’Connor CM. Reduction in heart failure events by the addition of a clinical pharmacist to the heart failure management team: results of the Pharmacist in Heart Failure Assessment Recommendation and Monitoring (PHARM) Study. Arch Intern Med. 1999;159(16):1939-1945.
58. McNeely EB. Treatment considerations and the role of the clinical pharmacist throughout transitions of care for patients with acute heart failure. J Pharm Pract. 2017;30(4):441-450.
59. Kitts NK, Reeve AR, Tsu L. Care transitions in elderly heart failure patients: current practices and the pharmacist’s role. Consult Pharm. 2014;29(3):179-190.
60. Anderson SL, Marrs JC. A review of the role of the pharmacist in heart failure transition of care. Adv Ther. 2018;35(3):311-323.
61. Maddox TM, Januzzi JL, Allen LA, et al. 2021 update to the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol.2021;77(6):772-810.
62. Put new heart failure guidelines in perspective. Pharmacist’s Letter. May 2022. No. 38051.
63. Tandan N, Cassagnol M. Quinapril. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2022 Jan-.