Medication Use During Pregnancy: Optimizing Safety and Minimizing Risk
RELEASE DATE:
September 1, 2017
EXPIRATION DATE:
September 30, 2019
FACULTY:
Cortney M. Mospan, PharmD, BCACP, BCGP
Assistant Professor of Pharmacy
Wingate University School of Pharmacy
Wingate, North Carolina
Megan Coleman, PharmD, BCPS, CPP
Assistant Professor of Pharmacy
Wingate University School of Pharmacy
Wingate, North Carolina
FACULTY DISCLOSURE STATEMENTS:
Drs. Mospan and Coleman have no potential or actual conflicts of interest in relation to this activity. Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT:
Pharmacy
 Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-17-064-H05-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE:
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@jobson.com
DISCLAIMER:
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients' conditions and possible contraindications or dangers in use, review of any applicable manufacturer's product information, and comparison with recommendations of other authorities.
GOAL:
To provide pharmacists with knowledge of pharmacokinetic and pharmacodynamic changes during pregnancy, safety of medication use in pregnancy, associated risks of teratogenicity or adverse outcomes, and resources and counseling strategies for advising pregnant patients.
OBJECTIVES:
After completing this activity, the participant should be able to:
- Describe labeling of medications regarding use in pregnancy.
- Discuss pharmacokinetic and pharmacodynamic changes occurring during pregnancy that affect medication use and safety.
- Review existing literature regarding medication use, safety, and potential adverse outcomes during pregnancy.
- Counsel patients regarding safe and effective medication use during pregnancy to minimize adverse events and their impact on fetal growth and development.
ABSTRACT: Advising patients on medication use during pregnancy remains challenging owing to a lack of literature on which to base decisions and the limitations of pregnancy labeling. Medication use during pregnancy is common, and prevalence continues to increase as women's age at pregnancy increases. Pharmacists must carefully appraise potential risks of medication use versus risks of untreated disease during pregnancy. Pharmacists should provide patients with information regarding both benefits and risks of medication use while also discussing the limitations of available knowledge so that women are empowered to make informed decisions that are best for them and their babies.
Over 90% of women reported taking at least one OTC or prescription medication during pregnancy between 2006 and 2008.1 Seventy percent reported taking at least one prescription medication.1 Between 1976 and 2008, the number of women using four or more prescription medications during pregnancy more than doubled, and the number more than tripled during the first trimester, demonstrating that medication use during pregnancy is common and has increased over time.1 Furthermore, 6% of pregnant women are exposed to teratogenic medications; in 3%, physical or mental birth defects occur in their children as a result.2
In December 2014, the FDA published the Pregnancy and Lactation Labeling Rule (PLLR) to address the shortcomings of prescription drug labeling.3 Prior to the PLLR, drug-product labeling included Pregnancy Categories A, B, C, D, and X, with A considered safe and X considered teratogenic.3 The categories were highly relied upon by healthcare providers, but were often misinterpreted and misused because they did not accurately communicate differences in degree of risk or provide meaningful clinical information.3 Only four of the 172 (2.3%) drugs approved between 2000 and 2010 had sufficient data to determine teratogenic risk; over 70% (126/172) had no human data to determine teratogenic risk.4 The final rule, which went into effect on June 30, 2015, required the removal of the pregnancy categories from medication labeling.3 The rule does not apply to OTC medications. Medications approved before 2001 are not required to comply with the final rule; however, the letter category previously assigned must be removed by June 30, 2018.3
The PLLR incorporates additional labeling changes in order to create a consistent format for providing information about the risks and benefits of drug use during pregnancy and lactation, as well as use of drugs by females and males of reproductive potential.3 The subsections formerly labeled Labor and Delivery and Nursing Mothers have been replaced. The new subsections are entitled Lactation and Females and Males of Reproductive Potential.3 The Pregnancy subsection remains and now includes information previously found in Labor and Delivery; the Lactation subsection now incorporates the previous Nursing Mothers subsection.3
MEDICATION USE IN PREGNANCY: WHERE TO FIND INFORMATION
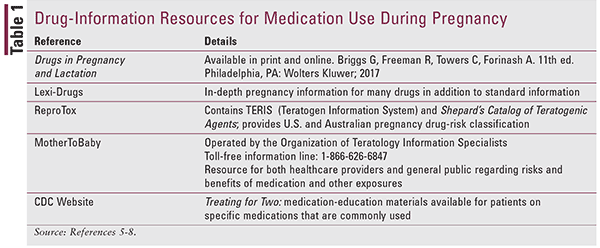
Pharmacists' knowledge of medication benefits and risks during pregnancy and how to utilize drug-information resources to counsel pregnant women is crucial. Although only 30 medications have been established as teratogens, it has been observed that since the thalidomide disaster, there is a tendency among healthcare professionals to act as though all medications are teratogenic.4,5 Pregnant patients often refuse medications, even if significant morbidity or life-threatening complications may result.5 Pharmacists must provide evidence-based counseling to patients and know where to find pertinent information. TABLE 1 provides a brief summary of valuable evidence-based drug-information resources that can be consulted.5-8 These references are intended for healthcare providers, but two of them provide patient resources as well.
PHARMACOKINETIC AND PHARMACODYNAMIC CHANGES
Drug safety has generally focused on the first trimester because there is typically an "all or none" effect (fetal loss) during the first 8 weeks, and most birth defects occur early in development.7,8 Physiological changes to maternal organ systems begin in the first trimester and peak during the second trimester. Changes in cardiovascular, pulmonary, gastrointestinal, renal, and hepatic function during pregnancy can lead to pharmacokinetic variations and alter the metabolism of medications.9,10 Additionally, changes in transport proteins and enzyme systems further alter the metabolic profile of medications during pregnancy.9,10 A recent systematic review found a significant gap between knowledge of pharmacokinetic changes in pregnancy and understanding of how these clinically impact the mother and the baby.11 Potential teratogenic effects of medications should be considered with the gestational age of the baby in mind. Potential fetal impact is most relevant when that corresponding stage of development is occurring (i.e., a medication with impact on cartilage development should be avoided when the skeleton is developing in gestation).8
Absorption: Increases in gastric pH during pregnancy may affect the absorption of weak acids and bases. Additionally, nausea, vomiting, and delayed gastric emptying may alter the absorption of medications.9,10
Distribution: Owing to an increase in body fat during pregnancy, the volume of distribution of fat-soluble drugs may increase. The volume of distribution of medications that are highly protein bound is increased due to decreasing albumin concentrations. The concentration of unbound drugs remains relatively unchanged, as they are more rapidly cleared by the liver and kidneys.9,10 Medications that are fat-soluble will have a decreased rate of elimination due to their greater volume of distribution.11
Metabolism: Alterations in the CYP450 enzyme system occur, affecting drug metabolism during pregnancy. Concentrations of both CYP3A4 and CYP2D6 are increased, while concentrations of CYP1A2 are decreased. Studies have also identified changes in uridine 5'-diphosphate glucuronosyltransferase and N-acetyltransferase. Additionally, increased levels of estrogen and progesterone alter the activity of liver enzymes, which can increase the elimination of some medications but result in the accumulation of others.9,10
Excretion: During pregnancy, maternal plasma volume, cardiac output, and glomerular filtration rate increase, potentially lowering the concentration of renally eliminated medications.9,10 In general, pregnancy results in decreased drug exposure due to increased drug elimination of both bound and unbound drugs, likely providing benefit in minimizing potential for adverse gestational outcomes.11
MEDICATION USE: SAFETY VS. HARM
Studying medication use in pregnancy may often result in a "bias against the null hypothesis," meaning that it is often assumed that medications are more likely to be teratogenic than to have no adverse fetal impact.5 Pregnant patients are more likely to report a poor outcome (i.e., birth defect) than they are to report a healthy baby. Literature has also shown that studies of medications with no increased risk of adverse effects are much less likely to get published or get picked up by the media.5 Pharmacists should be mindful of these limitations and consider informing patients about these. Medications discussed in this article focus on emerging evidence and conditions pregnant patients may experience that pharmacists may be less familiar with compared with more prevalent conditions in pregnancy, such as nausea.
Antibiotics
Treatment of infections is crucial for growth and development of the fetus, prevention of infection transmission, and maternal health. Previous studies have noted associations between birth defects and nitrofurantoin and sulfonamides. A retrospective study, the National Birth Defects Prevention Study, looked at antibiotic use for treatment of urinary tract infections (UTIs), comparing nitrofurantoin and sulfonamides to penicillins. The study looked at medication use the month before conceiving through the third month of pregnancy. Researchers found associations between nitrofurantoin and oral cleft; trimethoprim-sulfamethoxazole and esophageal atresia and diaphragmatic hernia; and cephalosporin and anorectal atresia/stenosis. While exposure to these antibiotics may increase birth defects, incidence is rare, and use must be compared to negative outcomes of an untreated UTI. Uncomplicated UTIs have been associated with intrauterine growth restriction and preterm labor. In 2011, the American College of Obstetrics and Gynecology (ACOG) released a committee opinion that these antibiotics may be prescribed in the first trimester if deemed appropriate.12
Anticoagulants
Pulmonary embolism is the leading cause of maternal death. Risk of venous thromboembolism (VTE) increases fivefold during pregnancy. VTE treatment guidelines for pregnancy are largely derived from algorithms in nonpregnant patients.13 Low-molecular-weight heparin is the drug of choice during pregnancy.14 Warfarin is contraindicated.13,14 Warfarin easily crosses the placenta and has been associated with nasal bone hypoplasia and stippled epiphyses if used during the first trimester. A case series evaluating warfarin use during the second and third trimesters in mechanical heart valve patients found an association with possible neurologic sequelae (e.g., seizure, developmental delay, hypotonia). The American Heart Association and American College of Cardiology allow for use of warfarin during the second and third trimesters under certain circumstances.13
Medications for Asthma and Allergic Rhinitis
Severe, uncontrolled asthma is associated with higher risks of preeclampsia, low birthweight, preterm delivery, and fetal death.15,16 A slight association has been found between congenital abnormalities and asthma-medication exposure; however, stopping or decreasing asthma medication use will likely result in significant adverse maternal-health outcomes.15,17 Studies that have discussed birth-defect associations with asthma medications have advised caution in interpreting and applying data due to the large number of confounders (i.e., asthma control).17,18 Because asthma control often changes during pregnancy, monthly evaluation of asthma symptoms and pulmonary function is recommended.15,16
Albuterol is the preferred short-acting beta2agonist (SABA) with the most human-safety data.15,16 A recent cohort study found an association between SABA use and renal dysplasia, but this finding should be carefully weighed against risks of stopping the medication.17,18 Inhaled corticosteroids (ICSs) are the preferred long-term treatment.15,16 Many studies have shown no increase in perinatal risks with ICSs, but there is some evidence of potential causation of anal atresia with higher doses.15,17 Budesonide is the preferred ICS in pregnancy, with the most safety data; however, studies do not indicate that the other ICSs are unsafe and should be avoided.15,16 Systemic corticosteroid use should be avoided, owing to an association with oral cleft and adverse gestational outcomes such as low birthweight, preterm birth, intrauterine growth restriction and preeclampsia.19
Cromolyn is considered safe to use during pregnancy but is less efficacious than ICSs. Additionally, the safety data for long-acting beta2agonists (LABAs) is limited, but these agents are expected to have a safety profile similar to that of albuterol.15,16 One cohort study found an association between ICS/LABA use and severe congenital heart defects.15,17 All potential risks of asthma medication use should be considered, keeping in mind an awareness of adverse outcomes associated with asthma itself, potential confounding, and risks of stopping asthma treatment.
In comorbid allergic rhinitis, intranasal corticosteroids are preferred. Loratadine and cetirizine are the recommended second-generation antihistamines.15,16 Antihistamines have generally been found to be safe; however, some risks exist and media reports have overstated these.5 Fetal safety with antihistamine use in pregnancy has been documented in several studies and metaanalyses, but there is less information available regarding use of second-generation antihistamines.5,20 The National Birth Defects Prevention Study evaluated maternal use of 14 antihistamines and 26 specific birth defects. Some associations were found, but they were generally weak-to-moderate and had no statistical association; however, they likely warrant further investigation.20 Epidemiologic data over 40 years provides little teratogenicity support for first-generation antihistamines, and chlorpheniramine is the antihistamine of choice during pregnancy, owing to available safety data.20 Decongestants are associated with birth defects and should not be used during pregnancy.19
Epilepsy Medications
Maternal seizures increase the risk of fetal hypoxia and bradycardia, stillbirth, perinatal death, and cognitive impairment. Rate of seizures remains unchanged for the majority of pregnant women. Changes to drug therapy should occur before conception, utilizing monotherapy at the lowest effective dose.
Carbamazepine, lamotrigine, levetiracetam, and phenytoin have the lowest risk of major fetal malformations; however, there have still been associations with some congenital malformations.21,22 Valproate is clearly associated with impaired cognitive development and increased risk for autism and autism spectrum disorders.23 More data are needed, but exposure to carbamazepine, lamotrigine, levetiracetam, and phenytoin are associated with more favorable cognitive and behavioral outcomes compared with valproate.24 Borgelt and colleagues provide a robust summary of congenital malformations and other outcomes associated with antiepileptics.24 Phenobarbital is teratogenic in the first trimester, and oxcarbazepine has been associated with developmental delays, congenital malformations, and fetal death. Topiramate has been associated with cleft palate and lithium carbonate has been associated with cardiac malformations.24 Folic acid 4 to 5 mg daily is recommended prior to conception and is continued during pregnancy in women taking antiepileptic drug therapy because many antiepileptic drugs lower folic acid levels and carry a risk of neural tube defects.21,22
Mental Health Medications
Untreated or inadequately treated maternal mental health conditions are associated with poor prenatal care, inadequate nutrition, exposure to other drugs or herbal products, increased alcohol and tobacco use, impaired mother-infant bonding, and negative fetal outcomes. Optimal treatment includes monotherapy at higher doses, and the use of medications with fewer metabolites, higher levels of protein binding, and fewer drug interactions.25
Depression: Perinatal depression may be more harmful than depression that occurs at other times of life; it can result in reduced prenatal care, self-neglect, substance abuse, and lower birthweights.26 Sixty-eight percent of women who discontinue antidepressants during pregnancy experience relapse.26 In 2006, the FDA released health advisory warnings concerning selective serotonin reuptake inhibitors (SSRIs) that were removed in 2011 because of conflicting evidence regarding complications of SSRI use during pregnancy.26 SSRIs and tricyclic antidepressants have not been associated with structural malformations. Symptoms of a transient neonatal abstinence syndrome (tachypnea, hypoglycemia, temperature instability, irritability, weak or absent cry, and seizures) have been reported and are associated with exposure to SSRIs taken late in pregnancy. These symptoms typically resolve within 2 weeks of delivery. Late exposure to SSRIs may also be associated with an increased risk of neonatal pulmonary hypertension. Studies with atypical antidepressants (bupropion, mirtazapine, nefazodone) and serotonin-norepinephrine reuptake inhibitors (SNRIs) do not suggest an increased risk of congenital malformations; however, exposure to SNRIs late in pregnancy resulted in an increased risk of neonatal abstinence syndrome symptoms, as was the case with SSRIs.25,27
While malformations are rare, potential behavioral associations are emerging regarding antidepressant use in pregnancy. A Quebec study found that antidepressant use in the second or third trimester, particularly SSRIs, was associated with a significant increase in autism spectrum disorders.28 The ACOG has developed guidelines on how to diagnose and treat perinatal depression that should be utilized for antidepressant management or cessation during pregnancy.26 A Norwegian study of discordant siblings found that in utero exposure to antidepressants resulted in increased anxiety at age 3 years compared with nonexposed siblings.29 There is a need for a greater understanding of long-term effects on childhood development that are associated with antidepressant use during pregnancy.
Anxiety Disorders: SSRIs are often used for the treatment of anxiety disorders. Other agents used in the treatment of anxiety include buspirone and hydroxyzine. Data for these agents show no association with congenital malformations. Benzodiazepines, when used immediately before delivery or chronically near term, have been associated with floppy infant syndrome, characterized by hypothermia, lethargy, poor respiratory effort, and feeding difficulties. Neonatal withdrawal syndromes have also been reported with maternal benzodiazepine use, with symptoms lasting as long as 3 months after delivery.25
Mood Disorders: Management of medication use in mood disorders remains challenging because many medications used for mood disorders have been associated with adverse perinatal outcomes and congenital malformations; however, medication management during pregnancy is critical. Relapse risk is 86% when medications are discontinued compared with 37% when continued.30 Valproate, carbamazepine, lamotrigine, and lithium carbonate have all been associated with risk of malformations and/or perinatal complications. Valproate and polypharmacy demonstrate greater evidence of adverse neurodevelopmental outcomes compared with other mood stabilizers.30
Pain and Rheumatologic Medications
Use of OTC pain medications is common during pregnancy. Ibuprofen and other nonsteroidal antiinflammatory drugs (NSAIDs) should generally be avoided during pregnancy. There are studies showing increased birth-defect prevalence (i.e., gastrochisis), although evidence is often inconsistent.31 It is critical to avoid NSAIDs during the third trimester due to risk of premature closure of the ductus arteriosus. As a result, acetaminophen has become the drug of choice and is thought to have a substantially greater safety profile than NSAIDs. Emerging evidence shows an association between acetaminophen and hyperkinetic diagnoses in children, use of attention-deficit/hyperactivity disorder (ADHD) medications, and ADHD-like behaviors.32
A potential association between acetaminophen use and autism spectrum disorders has been found, but only when accompanied by a hyperkinetic diagnosis. When confounders have been controlled for, there is no association at age 4 years between acetaminophen use during pregnancy and the child's IQ. Risks may be dose-dependent, although no maximum safe dose has been identified. Greater frequency of use is also thought to be associated. The association is with the drug itself, not an inflammatory process; however, fever in pregnancy is associated with adverse gestational outcomes.32 With uncertainty about the causal link between acetaminophen and neurodevelopmental conditions, acetaminophen use should not be denied or advised against when the patient has a clear indication. However, patients should be aware that there may be an association between acetaminophen use during pregnancy and the conditions discussed, and should limit use if possible.
The European League Against Rheumatism has developed guidelines for antirheumatic drug use before and during pregnancy; many of the medications used to treat these conditions can have adverse gestational outcomes, but pregnancy often worsens disease control. It is critical that family planning be discussed with all females of childbearing age who are taking antirheumatic drugs; adjustment of regimen before pregnancy is optimal. Methotrexate, mycophenolate mofetil, and cyclophosphamide should be withdrawn before pregnancy because they are teratogenic. NSAIDs may be used during the first and second trimesters, if needed, to control active disease symptoms.33
Thyroid-Disorder Medications
Untreated hypothyroidism increases the risk of low birthweight and impaired fetal neurologic development. Because of these risks, women should receive thyroid replacement with levothyroxine therapy. The starting dose of levothyroxine is 1 to 2 mcg/kg daily (~100 mcg) titrated according to thyroid-stimulating hormone (TSH) levels obtained every 4 to 6 weeks. TSH goals differ based on trimester: 0.1 to 2.5 mIU/L during the first trimester; 0.2 to 3.0 mIU/L during the second; and 0.3 to 3.0 mIU/L during the third.34 Women taking levothyroxine therapy prior to pregnancy may require a higher dosage due to elevated estrogen levels.34,35
Hyperthyroidism during pregnancy is associated with low birthweight, very preterm deliveries, and increased infant mortality; however, there is no increased risk for congenital abnormalities.36 The preferred treatment during the first trimester is propylthiouracil because of the higher risk of congenital malformations associated with methimazole. Due to risk of hepatotoxicity with propylthiouracil, it is recommended that therapy be switched to methimazole in the second trimester. Free T4 and TSH levels should be monitored every 2 to 6 weeks, and therapy should be titrated to maintain free T4 concentrations at or slightly above the normal reference range to minimize the risk of fetal or neonatal hypothyroidism.34,35
Diabetes Medications
Uncontrolled diabetes has been associated with increased risk of spontaneous abortion, fetal malformations, preeclampsia, macrosomia, and neonatal hypoglycemia and hyperbilirubinemia. Insulin is the preferred treatment option for the management of women with preexisting type 1 or type 2 diabetes because it does not cross the placenta.37,38
The mainstays of therapy for gestational diabetes are medical nutrition therapy, physical activity, and weight management. Medication therapy may be initiated if glycemic targets are not being met with lifestyle modifications alone. Insulin is recommended first-line, but metformin and glyburide may also be used. Although data support the efficacy and safety of metformin and glyburide use during pregnancy, both agents cross the placenta and lack long-term safety data. Studies indicate that metformin may cross the placenta to a greater extent than glyburide, but glyburide may result in higher rates of neonatal hypoglycemia and macrosomia than metformin and insulin.37,38
Lower risks of neonatal hypoglycemia and less maternal weight gain are potential benefits of metformin compared with insulin. Although metformin and glyburide are oral options, some women may not achieve glycemic targets and may require insulin.37,38
Antihypertensives
Chronic and gestational hypertension can increase the risk of fetal growth restriction, deficiencies in amniotic fluid, placental abruption, premature birth, and stillbirth.39 A cohort study found that women with chronic hypertension were at an increased risk for congenital cardiac malformation, but this is in both treated and untreated women.40 Therefore, hypertension is likely the causative factor, not the blood pressure medication. Antihypertensive medications are recommended for women with chronic hypertension who have persistent readings >160/105 mmHg and for women who have severe hypertension with blood pressure >160/110 mmHg and preeclampsia. The recommended treatment options are labetalol, extended-release nifedipine, and methyldopa. Although these medications have established safety data in pregnancy, adverse effects, contraindications, and cost impact the selection of the most appropriate treatment. Following treatment initiation, medication therapy should be titrated to achieve a blood pressure goal of 120 to 160/80 to 105 mmHg.39
MEDICATION-USE COUNSELING
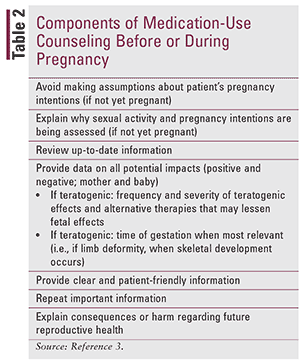
Literature has shown that pregnant women often take medications without adequate knowledge, which can have a significant impact on maternal and fetal health.41 A survey of pregnant patients demonstrated knowledge about their condition, but less than 50% were aware that there may be adverse effects associated with their medications.41 Pharmacists should provide counseling (TABLE 2) regarding potential teratogenic effects to all women of child-bearing potential, regardless of sexual activity, sexual orientation, or intention to become pregnant. Patients desire knowledge of all potential fetal outcomes as a result of medication use to treat chronic conditions.2 Patients reported a desire for adequate time to discuss these risks and a private location, and also noted that they felt information previously received was not comprehensive.2
In general, there is a lack of information regarding long-term outcomes of medication use in pregnancy, and it is challenging to provide information about risks beyond the perinatal period.7 Teratogenic information focuses on physical alterations, with a decreased emphasis on functional alterations (e.g., neurodevelopment, metabolic function) that may not be apparent at birth.41 Further, developing robust data is challenging because of study design flaws, follow-up loss, access to data, and challenges of data matching across multiple data sets.42 Pharmacists should inform their patient if use of a medication is "off-label" during pregnancy; this applies to the use of most medications owing to the lack of evidence from clinical trials or postmarketing surveillance studies.43 Research in this area is challenging because pregnant patients are considered a vulnerable population; however, pharmacists should counsel patients with available information highlighted in TABLE 2. As "pregnant women become ill and sick women become pregnant," medication use, and the necessity of use, during pregnancy will become more prevalent.44
CONCLUSION
With a changing prevalence of chronic disease and age at which women are having children, pharmacists must be well equipped to assess the safety of medication use during pregnancy. Providing well-informed advice regarding medication use can be challenging due to a lack of available literature. Pharmacists must balance potential risks of medication harm against adverse health outcomes that may result from not treating a condition.
REFERENCES
- Mitchell AA, Gilboa SM, Werler MM, et al. Medication use during pregnancy, with particular focus on prescription drugs: 1976-2008. Am J Obstet Gynecol. 2011;205:51.e1-51.e8.
- Santucki AK, Gold MA, Akers AY, et al. Women's perspectives on counseling about medication-induced birth defects. Birth Defects Res A Clin Mol Teratol. 2010;88(1):64-69.
- FDA. Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling (final rule). www.federalregister.gov/documents/2014/12/04/2014-28241/content-and-format-of-labeling-forhuman-prescription-drug-and-biological-products-requirements-for. Accessed June 15, 2017.
- Adam MP, Polifka JE, Friedman JM. Evolving knowledge of the teratogenicity of medications in human pregnancy. Am J Med Genet Part C Semin Med Genet. 2011;176:175-182.
- Koren G, Madjunkova S, Maltepe C. Bias against the null hypothesis: scaring pregnant women about drugs in pregnancy. Can Fam Phys. 2014;60:441-442.
- Campbell SC, Kast TT, Kamyar M, et al. Calls to a teratogen information service regarding potential exposures in pregnancy and breastfeeding. BMC Pharmacol Toxicol. 2016;17:33.
- Riley LE, Cahill AG, Beigi R, et al. Improving safe and effective use of drugs in pregnancy and lactation: workshop summary. Am J Perinatol. 2017;34(8):826-832.
- Burkey BW, Holmes AP. Evaluating medication use in pregnancy and lactation: what every pharmacist should know. J Pediatr Pharmcol Ther. 2013;18(3):247-258.
- Ward KE. Pregnancy and lactation: therapeutic considerations. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 10th ed. New York, NY: McGraw-Hill; 2017.
- Feghali MN, Mattison DR. Clinical therapeutics in pregnancy. J Biomed Biotechnol. 2011;2011:783528. Epub July 6, 2011.
- Pariente G, Leibson T, Carls A, et al. Pregnancy-associated changes in pharmacokinetics: a systematic review. PLOS Med. 2016;13(11):e1002160.
- Ailes EC, Gilboa SM, Gill SK, et al. Association between antibiotic use among pregnant women with urinary tract infections in the first trimester and birth defects, National Birth Defects Prevention Study 1997 to 2011. Birth Defects Research (Part A). 2016;106:940-949.
- Yarrington CD, Valente AM, Economy KE. Cardiovascular management in pregnancy: antithrombotic agents and antiplatelet agents. Circulation. 2015;132:1354-1364.
- Dresang LT, Fontaine P, Leeman L, King VJ. Venous thromboembolism during pregnancy. Am Fam Physician. 2008;77(2):1709-1716.
- National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–S138.
- National Heart, Lung, and Blood Institute (NHLBI). National Asthma Education and Prevention Program expert panel report: managing asthma during pregnancy—recommendations for pharmacologic treatment—2004 update. J Allergy Clin Immunol. 2005;1:34-46.
- Garne E, Hansen AV, Morris J, et al. Risk of congenital anomalies after exposure to asthma medication in the first trimester of pregnancy—a cohort linkage study. BJOG. 2016;123:1609-1618.
- Garne E, Hansen AV, Morris J, et al. Use of asthma medication during pregnancy and risk of specific congential anomalies—a European case-malformed control study. J Allergy Clin Immunol. 2015;136:1496-1502.
- Namazy JA, Schatz M. The treatment of allergic respiratory disease during pregnancy. J Investig Allergol Clin Immunol. 2016;26(1):1-7.
- Gilboa SM, Strickland MJ, Olshan AF, et al. The National Birth Defects Prevention Study. Use of antihistamine medications during early pregnancy and isolated major malformations. Birth Defects Res A Clin Mol Teratol. 2009;85(2):137-150.
- American College of Obstetricians and Gynecologists (ACOG). Educational bulletin. Seizure disorders in pregnancy. Number 231, December 1996. Committee on Educational Bulletins of the American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 1997;3:279-286.
- Harden CL, Meador KJ, Pennell PB, et al. Management issues for women with epilepsy—focus on pregnancy (an evidence-based review): II. teratogenesis and perinatal outcomes: report of the Quality Standards Subcommittee and Therapeutics and Technology Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsia. 2009;50:1237-1246.
- Gerard EE, Meador KJ. An update on maternal use of antiepileptic medications in pregnancy and neurodevelopmental outcomes. J Pediatr Genet. 2015;4:94-110.
- Borgelt LM, Hart FM, Bainbridge JL. Epilepsy during pregnancy: focus on management strategies. Int J Wom Health. 2016;8:505-517.
- ACOG Practice Bulletin: Clinical Management Guidelines for Obstetrician-Gynecologists. Number 92, April 2008 (replaces practice bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111:1001-1020.
- Kalfoglou AL. Ethical and clinical dilemmas in using psychotropic medications during pregnancy. AMA J Ethics. 2016;18(6):614-623.
- Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Obstet Gynecol. 2009;114:703-713.
- Boukhris T, Sheehy O, Mottron L, Bérard A. Antidepressant use during pregnancy and the risk of autism spectrum disorder in children. JAMA Pediatr. 2016;170(2):117-124.
- Brandlistuen RE, Ystrom E. Eberhard-Gran M, et al. Behavioural effects of fetal antidepressant exposure in a Norwegian cohort of discordant siblings. Int J Epidemiol. 2015;44:1397-1407.
- Galbally M, Roberts M, Buist A. Mood stabilizers in pregnancy: a systematic review. Aust N Z J Psychiatry. 2010;44:967-977.
- Ibuprofen. Best use of medication during pregnancy. http://medicinesinpregnancy.org/Medicine--pregnancy/Ibuprofen. Accessed June 15, 2017.
- Andrade C. Use of acetaminophen (paracetamol) during pregnancy and the risk of attention-deficit/hyperactivity disorder in the offspring. J Clin Psychiatry. 2016;77(3):e312-e314.
- Götesta Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75:795-810.
- Stagnaro-Green A, Abalovich M, Alexander E, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. American Thyroid Association Taskforce on Thyroid Disease During Pregnancy and Postpartum. Thyroid. 2011;21:1081-1125.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 148: thyroid disease in pregnancy. Obstet Gynecol. 2015;125:996-1005.
- Schurmann L, Hansen AV, Garne E. Pregnancy outcomes after fetal exposure to antithyroid medications or levothyroxine. Early Hum Dev. 2016;101:73-77.
- American Diabetes Association. Management of diabetes in pregnancy. Sec. 13. In: Standards of Medical Care in Diabetes 2017. Diabetes Care. 2017;40(Suppl 1):S114-S119.
- Committeee on Practice Bulletins—Obstetrics. Practice Bulletin No. 137: gestational diabetes mellitus. Obstet Gynecol. 2013;122(2 Pt 1):406-416.
- American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122-1131.
- Bateman BT, Huybrechts KR, Fischer MA, et al. Chronic hypertension in pregnancy and the risk of congenital malformations: a cohort study. Am J Obstet Gynecol. 2015;212(3):337.e1-e14.
- Devkota R, Khan GM, Alam K, et al. Impacts of counseling on knowledge, attitude and practice of medication use during pregnancy. BMC Pregnancy Childbirth. 2017;17:131.
- Grzeskowiak LE, Gilbert AL, Morrison JL. Methodological challenges in using routinely collected health data to investigate long-term effects of medication use during pregnancy. Ther Adv Drug Saf. 2013;4(1):27-37.
- Scaffidi J, Mol BW, Keelan JA. The pregnant women as a drug orphan: a global survey of registered clinical trials of pharmacological interventions in pregnancy. BJOG. 2017;124:132-140.
- Parisi MA, Spong CY, Zajicek A, Guttmacher AE. We don't know what we don't study: the case for research on medication effects in pregnancy. Am J Med Genet C Semin Med Genet. 2011;157(3):247-250.