New Therapeutic Options for the Management of ADHD
RELEASE DATE:
May 1, 2018
EXPIRATION DATE:
May 31, 2020
FACULTY:
Clayton English, PharmD, BCPS, BCPP
Associate Professor of Pharmacy Practice
Albany College of Pharmacy & Health Sciences
Colchester, Vermont
FACULTY DISCLOSURE STATEMENTS:
Dr. English has no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT:
Pharmacy
 Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-18-024-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE:
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@jobson.com
DISCLAIMER:
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients' conditions and possible contraindications or dangers in use, review of any applicable manufacturer's product information, and comparison with recommendations of other authorities.
GOAL:
To give participants an overview of new treatments and dosage formulations approved by the FDA for the management of attention-deficit/ hyperactivity disorder (ADHD) in children and adults.
OBJECTIVES:
After completing this activity, the participant should be able to:
- Describe ADHD's epidemiology, diagnostic criteria, and underlying pathophysiology.
- Select appropriate first-line pharmacotherapy based on current guidelines for managing ADHD in children and adolescents.
- Identify new treatments that are FDA-approved for managing ADHD in children and adults.
- Compare the advantages and disadvantages of newly approved stimulant dosage formulations.
ABSTRACT: Attention-deficit/hyperactivity disorder (ADHD) is a common neurodevelopmental psychiatric disorder that originates in childhood and often persists into adulthood. Stimulants continue to be the cornerstone treatment for the management of ADHD in children, adolescents, and adults. Since 2012, various new methylphenidate and amphetamine formulations have been approved for the management of ADHD. The majority of these agents offer more flexibility in dosing or provide long-acting options for patients with swallowing difficulties. Given the multitude of choices available, pharmacists can assist in tailoring dosage-form selection to meet the needs of patients requiring stimulant therapy for ADHD.
Attention-deficit/hyperactivity disorder (ADHD) is a common neurodevelopmental psychiatric condition whose symptoms commence in childhood. The core symptoms of ADHD include deficits in attention, increases in motor hyperactivity, and displays of marked impulsivity that are noncongruent with age-appropriate standards. ADHD symptoms can lead to academic, occupational, and social impairment in children and adolescents, and approximately two-thirds of patients continue to display symptoms into adulthood.1,2
The etiology and pathophysiology of ADHD are intricate and multifaceted. Genetic and nongenetic causes have been implicated, including having first-degree relatives with ADHD, as well as polymorphisms in various chromosomal regions that influence monoamine transporters and receptors, primarily dopamine.2 Positron emission tomography scans have shown variations in dopamine metabolism, cerebral blood flow, and glucose metabolism in the frontal lobe of the brain.3 Currently, the primary ADHD treatments target dopamine and norepinephrine, which are the principal neurotransmitters involved in the pathophysiology of ADHD. Dopamine neurotransmission sustains attention on monotonous tasks, and norepinephrine is responsible for cortical arousal and attention control; therefore, neurotransmission deficits or impairments in either of these neurotransmitters can lead to signs and symptoms of ADHD.4
Stimulants continue to be the preferred first-line treatment for elementary-school children, adolescents, and adults without contraindications or intolerances to them.5 Over the past 5 years, the FDA has approved a number of new stimulant dosage forms for the management of ADHD. This article will review the efficacy and safety of new dosage formulations approved to treat ADHD, as well as the fundamentals of pharmacotherapeutic management of ADHD.
Epidemiology and Diagnostic Criteria
The prevalence of ADHD is roughly 5%, according to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5); however, data published in 2018 estimate that 6.1 million U.S. children aged 2 to 17 years (9.4%) have been diagnosed with ADHD.1,6 Prevalence increases with age in children and adolescents, with 2.2 million school-aged children (8.9%) and 2.9 million adolescents (11.9%) reported to have a current diagnosis of ADHD.6 Less epidemiological data exist on adult ADHD, but studies suggest a prevalence of 2.5% to 4%.7,8 The diagnosis of ADHD is more common in males than in females, with a 3-4:1 ratio.2 Hispanic children are less likely than Caucasian and African American children to receive an ADHD diagnosis.6
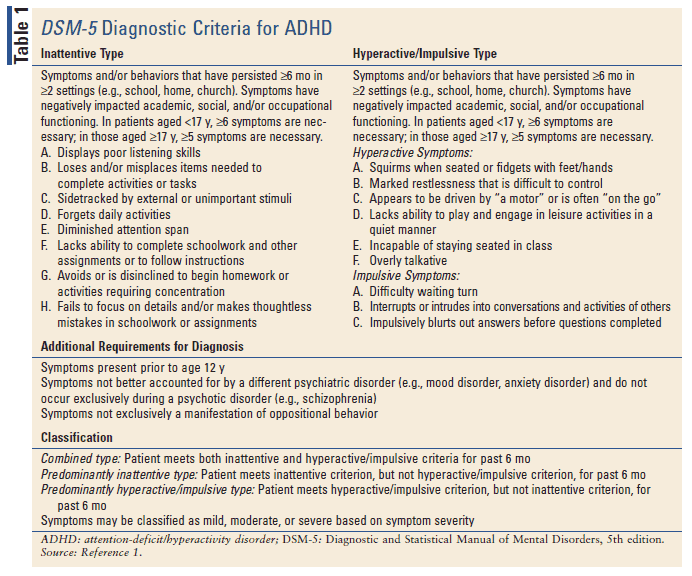
The diagnosis of ADHD is primarily based on medical evaluation and clinical-symptom presentation. To receive an ADHD diagnosis, a patient must fulfill the criteria specified by the DSM-5 (TABLE 1). In addition to considering DSM-5 criteria, the provider should conduct a full physical- and mental-status examination to rule out other causes of symptoms (e.g., untreated major depression, generalized anxiety disorder).
Collateral information, another essential part of diagnosis, includes data gathered from parents, teachers, and other adults who are an active part of the child's life (e.g., daycare providers, caretakers) in order to confirm symptom presentation in multiple settings. Assessment scales completed in different settings can be beneficial for diagnosis. A history of performance in academic settings may also be useful for identifying academic impairment.
Criteria for adult ADHD are similar to those in children and adolescents, but the number of symptoms required for the inattention and hyperactivity/impulsivity domains is five instead of six. Evidence of symptoms prior to age 12 years is also requisite.1 For adults lacking a childhood diagnosis, thorough collateral information should be obtained from family members, colleagues, and former providers to ensure diagnostic accuracy.
Former school reports, job-performance assessments, and employment history may also be beneficial for validating the diagnosis.9 Although adults may display symptoms similar to those in children and adolescents, they tend to exhibit more symptoms of inattentiveness (e.g., managing time appropriately, avoiding difficult and demanding work tasks, procrastinating on work and family-related activities).10 Impulsive symptoms (e.g., low frustration tolerance) may also be present. Motor hyperactivity is less common or is displayed in a different manner (e.g., inability to sit through work meetings, avoiding activities because of boredom).9,10
Pharmacotherapeutic Management
Management of ADHD often involves a multimodal approach, including pharmacotherapeutic and nonpharmacologic methods. The treatment approach often depends on symptom severity, comorbidities, and patient's age. In the 2011 American Academy of Pediatrics guideline on ADHD in children and adolescents, behavior therapy and nonpharmacologic methods are recommended as firstline treatment for children aged 4 to 5 years.5 Behavioral approaches include withholding privileges for poor behavior, incorporating positive-reinforcement models for good behavior, and using behaviormodification methods such as time-out. To improve success, family therapy and parental training may be implemented in combination with behavioral approaches. For school-aged children, classroom interventions such as behavioral classroom management, behavior plans, and academic accommodations (e.g., smaller classroom settings, longer testing periods) may also be incorporated. Methylphenidate may be attempted in preschoolers if nonpharmacologic methods are unsuccessful.5
Medications FDA-approved for ADHD are recommended as first-line treatment in elementaryschool children (ages 6-11 years) and adolescents (ages 12-18 years). Pharmacotherapy is considered the first-line approach in adults with ADHD, unless the patient prefers nonpharmacologic management (i.e., cognitive behavioral management) or has comorbidities (e.g., major depression, anxiety disorder).
Stimulants—methylphenidate and amphetamine derivatives—have the strongest evidence supporting use in elementary-school children and are the treatment of choice for adolescents and adults unless a contraindication exists or a risk of substance misuse or stimulant diversion is recognized upon assessment.11,12 Both methylphenidate and amphetamine block the reuptake of norepinephrine and dopamine, increasing concentrations in the synaptic cleft. Amphetamine also can cause the release of dopamine from presynaptic nerve terminals.4 Elementary-school children and adolescents who fail one stimulant class at an effective dose and duration should switch to a stimulant in the other class before nonstimulant medications are attempted.5 Response rates for stimulants range from 70% to 85% in patients with an accurate diagnosis.13 Along with pharmacodynamic differences between methylphenidate and amphetamine products, stimulants have differing pharmacokinetics (e.g., onset of action, duration of effect), dosage-form availability, and cost. Nonpharmacologic methods have also shown benefit in combination with pharmacotherapy, but behavior therapy used alone in elementary-school children and adolescents with ADHD is typically less effective than stimulant treatment unless comorbid illnesses are present, there is diagnostic uncertainty, or symptom presentation is mild.5
Nonstimulant treatments may be used first-line, but they are typically preferred for patients with contraindications to or intolerable adverse effects from stimulants.5 Substance abuse on the part of the patient or immediate family members may steer providers toward prescribing noncontrolled medications such as atomoxetine as first-line treatment. The presence of comorbid Tourette or other tic disorders may also preclude providers from using stimulants because motor tics can be aggravated by stimulant therapy. In several studies, exacerbation of tics was not demonstrated for stimulant therapy in patients with Tourette disorder, and methylphenidate remains the preferred treatment in this population; however, alpha2-adrengergic agonist treatment targets both ADHD symptoms and motor tics and may be used by some clinicians.14,15 Alpha2-adrenergic agonists are not well studied in adults, and they lack an adult indication.
Off-label treatments for adult ADHD may be considered for patients with comorbid substance abuse or for those who failed stimulants. Off-label options for adults include bupropion, desipramine, and venlafaxine.12
New Stimulant Formulations
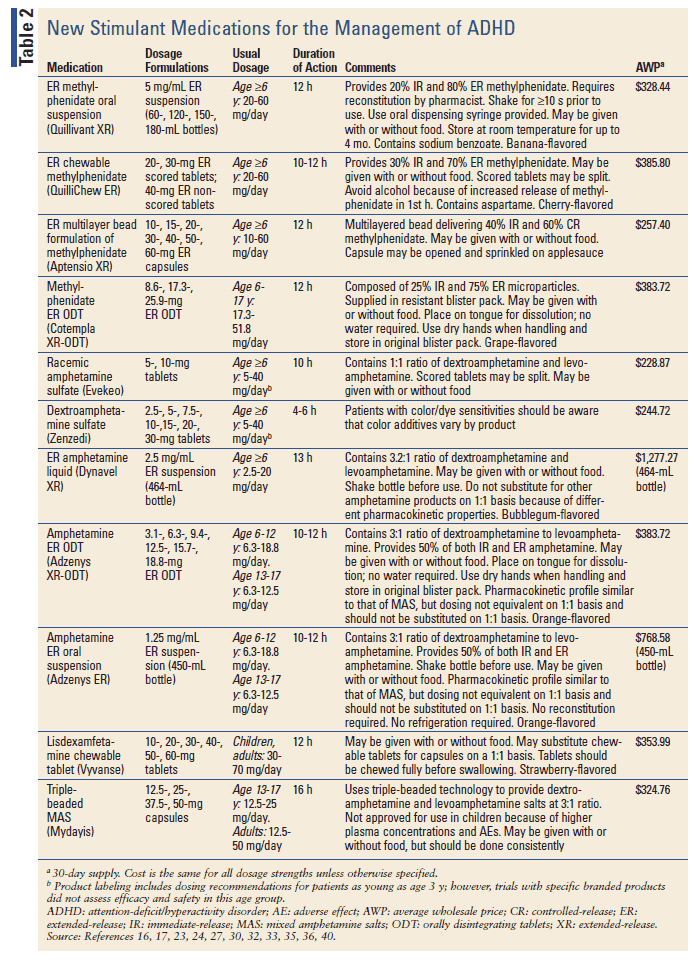
Given that stimulants are the treatment of choice for most patients with ADHD, it is important to have dosage formulations that fit the needs of individuals across age groups. Since 2012, a number of products have gained FDA approval for ADHD management in children and adults. The majority of dosage forms on the market provide more flexible options for children with swallowing difficulties and include extended-release (ER) properties to reduce dosing frequency. Even in the newly available stimulants, adverse effects including appetite suppression, abdominal pain, insomnia, and irritability remain common. Changes in heart rate and blood pressure are infrequent but should be monitored periodically. ECG is unnecessary unless physical examination and history indicate possible heart disease. Although their occurrence is rare, psychiatric reactions including psychotic symptoms have been noted with stimulants, and abnormal behaviors should be reported to providers immediately. TABLE 2 provides dosing, pharmacokinetic, and cost information for the new stimulants.
ER Methylphenidate Oral Suspension: ER methylphenidate oral suspension (ERMOS; Quillivant XR) was approved in 2012 for the treatment of ADHD in patients aged 6 years and older. The suspension is supplied as a powder composed of cationic polymer matrix particles. The particles bind the racemic mixture of methylphenidate through an ion-exchange mechanism. Reconstitution with water is required prior to dispensing, and after reconstitution the suspension should be kept in its original container for up to 4 months. After reconstitution, the suspension consists of coated particles that are approximately 80% ER and uncoated particles that are approximately 20% immediate-release (IR). The relative bioavailability of a single 60-mg dose of ERMOS is 95% compared with two 30-mg doses of methylphenidate IR oral solution given 6 hours apart.16,17
The efficacy of ERMOS was demonstrated in a randomized, double-blind, crossover, placebo-controlled trial that enrolled 45 subjects in a laboratoryclassroom environment.18 Patients were aged 6 to 12 years, had a diagnosis of ADHD requiring medication therapy, had safety and tolerability problems with the current regimen, had suboptimal efficacy with other therapies, or desired treatment with a long-acting liquid formulation. Notable exclusion criteria included presence of Tourette disorder, family history of Tourette or other tic disorder, and other serious chronic medical and psychiatric illnesses (e.g., serious cardiac condition, seizure disorder, major depression).
Prior to the double-blind phase, patients took part in a dose-optimization open-label phase, after which they were randomized to 1 week of treatment with the active optimal dose of ERMOS or placebo.18 After the week of treatment, patients in each group were crossed over and received the opposite product. The primary efficacy outcome was change in the Swanson, Kotkin, Agler, M-Flynn, and Pelham (SKAMP) Rating Scale-Combined score at 4 hours post administration of ERMOS. (The SKAMPCombined score is a 13-item rating scale that identifies impairment in the classroom setting. The scale is validated, and each item is ranked on a seven-point scale, with a 0 rating indicating normal behavior and a score of 6 indicating greatest impairment.)
SKAMP scores were significantly improved at 4 hours with ERMOS (least squares mean [LSM] score, 7.12; 95% CI: 4.85-9.39) versus placebo (LSM score, 19.58; 95% CI: 17.31-21.86). Additionally, SKAMP-Combined scores were significantly lower at all other time points compared with placebo. Common adverse effects reported during the open-label dose-optimization phase were similar to those seen within the psychostimulant class, including decrease in appetite (55.6%), abdominal pain (42.2%), lability in affect (26.7%), insomnia (17.8%), headache (17.8%), irritability (13.3%), and vomiting (11.1%).18
ER Chewable Methylphenidate: ER chewable methylphenidate (ERCM, QuilliChew ER) is a daily tablet approved in 2015 for management of ADHD in patients older than age 6 years. The tablets contain 15% methylphenidate hydrochloride and 85% methylphenidate attached to sodium polystyrene sulfonate particles, with 30% of the dose composed of IR methylphenidate and 70% composed of ER methylphenidate. A 40-mg dose of ERCM is bioequivalent to two 20-mg doses administered 6 hours apart; however, the AUC and mean peak concentration were about 11% and 20% lower, respectively, for ERCM.19
The efficacy of ERCM was assessed in a laboratory-classroom study of 90 subjects who underwent a 6-week open-label, dose-optimization period followed by a 1-week placebo-controlled, doubleblind trial.20 Notable exclusion criteria were similar to those in the ERMOS trial. SKAMP-Combined scores were assessed at 0.75, 2, 4, 8, 10, 12, and 13 hours post administration. The primary outcome was the average of all postdose SKAMP-Combined scores on the classroom study day. The average SKAMP-Combined score was significantly less in patients who received ERCM versus placebo (12.1 vs. 19.1, P <.001). Common adverse effects were similar to those for other stimulants (e.g., appetite suppression, insomnia).20
ER Multilayer Bead Formulation of Methylphenidate: A new ER multilayer bead formulation of methylphenidate (MPH-MLR) in capsules (Aptensio XR) was approved in 2015 for the treatment of ADHD in patients aged 6 years and older. The multilayer beads contain 60% controlled-release methylphenidate and 40% IR methylphenidate. The duration of effect for MPH-MLR is approximately 12 hours, similar to that of other long-acting methylphenidate products (i.e., methylphenidate osmotic-release delivery, ERMOS).21
The clinical effect of MPH-MLR was evaluated in two clinical studies. The first one, a 2-week double-blind, randomized, placebo-controlled, crossover clinical trial, included 26 children aged 6 to 12 years with ADHD.22 An optimal dose of MPHMLR was obtained during a 2- to 4-week open-label phase; patients then received the active treatment or placebo for 1 week before crossing over to the opposite product for an additional week. The primary clinical efficacy outcome was mean SKAMP-Combined score determined in a laboratory-classroom setting after 1 week of treatment at nine time points between 1 and 12 hours. The LSM postdose SKAMP-Combined score was lower for MPH-MLR compared with placebo (2.18 vs. 1.32, respectively; P = .0001).22
The second study, which included 221 children and adolescents aged 6 to 18 years with ADHD, was a parallel, double-blind, randomized, placebocontrolled, fixed-dose trial. Patients were randomized to receive 10 mg, 15 mg, 20 mg, 40 mg, or placebo for 1 week. The primary efficacy endpoint was change in total score at 1 week for the ADHD Rating Scale Questionnaire (18-item tool that rates ADHD symptoms on a scale of 0-3, with higher scores indicating higher levels of symptoms). The double-blind phase was followed by an 11-week open-label phase for further dose optimization. At the end of the double-blind phase, all treatment groups showed improvements; however, only the 20-mg and 40-mg groups differed statistically from placebo. Symptom scores continued to improve during the open-label phase of treatment. Common adverse effects with MPH-MLR were abdominal pain, nausea, vomiting, decreased appetite, dizziness, headache, and insomnia.23
MPH-MLR offers another effective long-acting methylphenidate option for clinicians and patients; however, no distinct clinical advantage for MPHMLR over other long-acting methylphenidate products has been ascertained in trials, and comparative data are needed to determine whether it has an advantage over older formulations.21
ER Methylphenidate Orally Disintegrating Tablet: The first ER methylphenidate orally disintegrating tablet (ERM-ODT), Cotempla XR-ODT, was released in 2017 for the treatment of ADHD in children aged 6 to 17 years. The ODT formulation is composed of 75% ER and 25% IR methylphenidate particles. A pharmacokinetic-equivalent study was conducted in adults taking ERM-ODT 60 mg versus ER methylphenidate 60 mg (Metadate CD). The drugs displayed similar pharmacokinetic parameters, except for maximal concentrations, which were 25% higher with ERM-ODT.24
The efficacy and safety of ERM-ODT were evaluated in 87 children with ADHD aged 6 to 12 years in a randomized, double-blind, placebo-controlled, parallel, laboratory-classroom study. A 4-week open-label, dose-optimization phase was implemented, with patients remaining on the same dose for at least 1 week. After the open-label phase, patients were randomized to continue treatment or receive placebo for 1 week. The primary outcome was difference in SKAMP-Combined score on the classroom testing day at the end of the 1-week double-blind phase. The average SKAMP-Combined score was significantly less in patients treated with ERM-ODT compared with placebo patients (14.3 vs. 25.3, P <.0001). During the dose-optimization phase, reported adverse effects were those typical of psychostimulants (i.e., appetite suppression, insomnia).25 ERM-ODT offers an easily administered dosage form for patients with swallowing difficulties, and its pharmacokinetic properties are similar to those of ER methylphenidate (Metadate CD).
Racemic Amphetamine Sulfate: Evekeo is a 1:1 racemic mixture of levoamphetamine and dextroamphetamine that was approved in 2012 for treatment of ADHD in children aged 3 years and older. It is currently the only amphetamine tablet available that is a 1:1 racemic mixture.26 Notable differences exist between the dextro and levo isomers and their pharmacologic activity. The dextro isomer is more potent and has greater effects on dopamine neurotransmission relative to norepinephrine; the levo isomer is less potent and less stimulating, and it tends to have analogous effects on dopamine and norepinephrine reuptake. Although both isomers cause cardiovascular side effects, these effects tend to be more pronounced with the levo isomer. Despite these pharmacologic differences, the clinical impact and significance of using a 1:1 ratio of racemic amphetamine over other stimulants have not been investigated.27
Racemic amphetamine's efficacy and safety were evaluated in a phase IV, double-blind, randomized, placebo-controlled, crossover clinical trial of children aged 6 to 12 years who had a diagnosis of ADHD.27 Subjects with significant comorbid psychiatric and medical conditions, including Tourette disorder, were excluded, as were those who took an investigational drug for up to 60 days prior to trial entry. Subjects were first treated with racemic amphetamine in an 8-week open-label, dose-optimization phase before entering a 2-week double-blind crossover treatment phase. Racemic amphetamine or placebo was administered for 1 week, after which subjects were assessed in a laboratory classroom. Subjects then received the opposite product and were reevaluated in a laboratory classroom 1 week later. The primary efficacy outcome was the difference in SKAMP-Combined scores at 2 hours post dose on the full-length laboratory-classroom days. Subjects treated with racemic amphetamine achieved a significantly lower SKAMP-Combined score at 2 hours post dose compared with placebo subjects (LSM score 10.3, 95% CI: 8.1-12.4 vs. LCM score 18.1, 95% CI: 16-20.2, P <.0001). Adverse effects in the open-label phase included decreased appetite (27.6%), abdominal pain (14.3%), irritability (14.3%), and headache (13.3%).27
Dextroamphetamine Sulfate: Zenzedi, a brand of dextroamphetamine sulfate tablets, was released in 2013. Similar to other dextroamphetamine products, Zenzedi is approved to treat ADHD in patients aged 3 to 16 years. The brand offers additional tablet strengths, including 2.5 mg, 7.5 mg, 15 mg, 20 mg, and 30 mg. Zenzedi comes in strengths of 5 mg and 10 mg as well, but it should be noted that other manufacturers also produce these strengths. Each strength is manufactured in a different color and shape to reduce confusion and enable differentiation. No prominent clinical studies have evaluated this product line.28 Except for the increased number of dosage strengths, these dextroamphetamine sulfate tablets offer no distinct clinical advantage over other brand and generic stimulant products.
ER Amphetamine Oral Suspension: Dyanavel XR is a racemic ER amphetamine oral suspension (ERAOS) approved for treatment of ADHD in children aged 6 years and older. A patented technology is used that incorporates several coated particles of various thicknesses into a suspension to allow variation in IR and ER activity. The suspension contains a 3.2:1 ratio of dextroamphetamineto levoamphetaminecoated particles. Within the coated particles, amphetamine is bound to an ion-exchange resin. As the suspension passes through the gastrointestinal tract, amphetamine is displaced from the resin and is absorbed. The duration of action is approximately 13 hours.29
The efficacy and safety of ERAOS were evaluated in a randomized, double-blind, placebo-controlled trial in 99 children aged 6 to 12 years with ADHD.30 A 5-week dose-optimization phase was followed by a 1-week double-blind phase; on the last day of the 1-week phase, efficacy was assessed in a laboratory-classroom environment. The primary efficacy endpoint was the difference in SKAMP-Combined score at 4 hours post dose on the classroom assessment day. The change in SKAMP-Combined scores was significantly greater in the active-treatment group compared with the placebo group (–9.1 vs. 5.6, P <.0001). Common adverse effects in the open-label phase included decreased appetite (26.2%), insomnia (13.1%), and mood lability (9.3%).30
ERAOS represents an additional dosage form for patients who have swallowing difficulties or require requiring extended coverage for symptoms, but the price of the drug may limit its use.
Adzenys ER and Adzenys XR-ODT: Adzenys ER and Adzenys XR-ODT are two new amphetamine products; Adzenys ER is a long-acting suspension and Adzenys XR-ODT is an ER ODT. Both are approved in ADHD patients aged 6 years and older. Each formulation has pharmacokinetic plasma properties similar to those of ER mixed amphetamine salts (ER-MAS) and provides a 3:1 ratio of dextroamphetamine to levoamphetamine, with 50% of IR and 50% of ER amphetamine contained in each. No new clinical studies have been published to date, and the efficacy and safety of each product are expected to be similar to those of ER-MAS.31,32 Both products offer additional long-acting amphetamine dosage forms for patients with swallowing difficulties.
Lisdexamfetamine Chewable Tablet: In 2017, lisdexamfetamine (Vyvanse) chewable tablets were FDAapproved for ADHD treatment in patients aged older than 6 years. Lisdexamfetamine is a prodrug for dextroamphetamine and must be bioactivated in the gastrointestinal tract, which potentially reduces its abuse via commonly employed routes of administration (e.g., injection, insufflation).33 The chewable tablet was formulated as an alternative dosage form for patients who have difficulty swallowing lisdexamfetamine capsules or for those who find it burdensome to open and mix the capsule's contents in water.34
Only pharmacokinetic studies have assessed the chewable tablet, and no new efficacy studies have been conducted. Although lisdexamfetamine capsules and chewable tablets were not bioequivalent based on the average exposure of the prodrug, dextroamphetamine concentrations were bioequivalent.35 Based on these data, the capsules and chewable tablets may be interchanged on 1:1 basis, and no clinical difference is expected with therapeutic interchange.35 To reduce confusion and errors, each tablet strength comes in a different shape. Current dosage strengths for the chewable tablet include all strengths available for the capsule except the 70-mg formulation.34
Triple-Beaded MAS: In addition to ER-MAS (Adderall XR), which has been on the market since 2001, other single-entity amphetamine products are being released. Mydayis, a triple-beaded MAS (TB-MAS) product, is a branded amphetamine stimulant released in 2017 for ADHD treatment in patients aged 13 years and older. The product contains equal amounts of four salts: dextroamphetamine sulfate, amphetamine sulfate, dextroamphetamine saccharate, and amphetamine aspartate monohydrate. This composition is similar to that of ER-MAS; however, there are differences between the two products. The primary differences include indication, dosage-form availability, and technology used for release. TB-MAS uses three types of drug-releasing beads: an IR bead and two different types of delayed-release (DR) beads; ER-MAS employs one IR and one DR bead.36 The first DR bead releases amphetamine at pH 5.5, and the other DR beads release it at pH 7.0. A 37.5-mg dose of TB-MAS is bioequivalent to 25 mg of ERMAS augmented with a 12.5-mg IR dose of MAS given 8 hours later.36 The effects of TB-MAS may last up to 16 hours, and similar elimination half-lives are reported for dextroamphetamine and levoamphetamine when TB-MAS and ER-MAS are used in adults (10 hours for dextroamphetamine, 13 hours for levoamphetamine). Dosage formulations for TB-MAS are also supplied and are dosed differently from MAS; see TABLE 2.
The efficacy and safety of TB-MAS were established in five double-blind, placebo-controlled trials; three short-term trials in adults aged 18 to 55 years; and two short-term trials in children aged 13 to 17 years with ADHD. TB-MAS was significantly more effective than placebo in reducing ADHD symptoms in all clinical trials. The most common adverse effects associated with TB-MAS in adults were insomnia (31%), decreased appetite (30%), dry mouth (23%), and increased heart rate (9%). Decreased appetite (22%), nausea (8%), and insomnia (8%) were the most prominent adverse effects in adolescents. TBMAS should not be used in children aged younger than 13 years; insomnia and decreased appetite occurred more frequently and systemic exposure was higher in this population.36 The extensive duration of action may be beneficial for patients with breakthrough symptoms in the evening; however, because of the extensive duration of action, patients should be monitored for insomnia and encouraged to take their dose soon after waking.36
Phosphatidylserine and Omega-3 Fatty Acids Combination
In 2013, Vayarin, a medical food consisting of phosphatidylserine and omega-3 fatty acids, was released for treatment of ADHD. Medical foods require a prescription, must be taken under the guidance of a physician, and are typically developed to help manage a disease state that has specific dietary needs. Vayarin contains a patented blend registered under the name Lipirinen. Each dose of Lipirinen contains 75 mg of the patented blend, which is composed of phosphatidylserine conjugated to omega-3 fatty acids. The omega-3 fatty acids are enhanced with eicosapentaenoic acid. The recommended daily dosage is two capsules per day.37
The mechanism of dietary foods and the role these products play in ADHD are uncertain. Phosphatidylserine, a constituent of the phospholipid bilayer of cell membranes, is found in high concentrations in the central nervous system. Phosphatidylserine plays a role in multiple homeostatic processes in the brain, including cellular-environment regulation, neuronal communication, brain-glucose regulation, and secretory-vesicle release of neurotransmitters.37
Phosphatidylserine interacts with the Raf-1 protein kinase pathway, which may be involved in preventing apoptosis of neural cells. In animal studies, phosphatidylserine increased concentrations of monoamines and acetylcholine, possibly contributing to a theoretical mechanism for increasing attention span and working memory in ADHD.37,38 Omega-3 fatty acids are known to have potential benefit in several disease states affecting the central nervous system (e.g., major depression, schizophrenia), and they are recognized as a critical component of proper brain development in children and adolescents. Blood concentrations of omega-3 fatty acids are lower in children with ADHD compared with healthy controls, and lower concentrations of omega-3 fatty acids have been linked to lower concentrations of phosphatidylserine.37-39
The efficacy and safety of the combination of phosphatidylserine and omega-3 fatty acids were evaluated in a single-center, 15-week, placebocontrolled, double-blind trial involving 200 children aged 6 to 13 years who had ADHD.39 Notable exclusion criteria included history of or a current serious medical condition, including psychotic disorders and pervasive developmental disorders; current use of medications with psychotropic properties, including ADHD treatments; and history of two or more stimulant therapy failures. The primary efficacy endpoint was change in the Conners' Teacher Rating Scale (CRS-T). The Conners' Parent Rating Scale (CRS-P) and the Child Health Questionnaire (CHQ) were used as secondary assessments.
No significant change was found on any component of the CRS-T with phosphatidylserine and omega-3 fatty acids treatment versus placebo.39 The combination significantly reduced scores for global restlessness/impulsive behavior on the CRS-P and emotional impact on the parent on the CHQ, but no other assessment subscales had significant differences versus placebo. Treatment was well tolerated, with the most common reported adverse effect being gastrointestinal distress (4.4%).39 The phosphatidylserine and omega-3 fatty acids combination is well tolerated and generally considered safe for consumption; however, based on the failed clinical trial, additional clinical studies demonstrating efficacy are needed before it can be routinely recommended for use in ADHD.
Conclusion
The market for ADHD medications has expanded rapidly. The past decade has seen the release of a variety of branded products, the majority of which are different dosage formulations of methylphenidate or amphetamine. Although these products offer some advantages, including much-needed liquid and ODT preparations, comparative trials of generic extended-release stimulants are lacking, and the cost of these agents may not be justified given the plentiful number of alternatives.40 Pharmacists remain well situated to assist providers and patients in tailoring dosage-formulation selection of ADHD medications to meet their specific needs.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Arlington, VA: American Psychiatric Publishing; 2013.
- Thapar A, Cooper M. Attention deficit hyperactivity disorder. Lancet. 2016;387:1240-1250.
- Zimmer L. Positron emission tomography neuroimaging for a better understanding of the biology of ADHD. Neuropharmacology. 2009;57:601-607.
- Stahl SM. Stahl's Essential Psychopharmacology: Neuroscientific Basis and Practical Applications. 4th ed. Cambridge, UK: Cambridge University Press; 2013.
- Subcommittee on Attention-Deficit/Hyperactivity Disorder, Steering Committee on Quality Improvement and Management. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128:1007-1022.
- Danielson ML, Bitsko RH, Ghandour RM, et al. Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. children and adolescents, 2016. J Clin Child Adolesc Psychol. 2018;47:1-14.
- Simon V, Czobor P, Bálint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194:204-211.
- Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163:716-723.
- Haavik J, Halmoy A, Lundervold AJ, Fasmer OB. Clinical assessment and diagnosis of adults with attention-deficit/hyperactivity disorder. Expert Rev Neurother. 2010;10:1569-1580.
- Adler LA. From childhood into adulthood: the changing face of ADHD. CNS Spectr. 2007;12(12 suppl 23):6-9.
- Rostain AL. Attention-deficit/hyperactivity disorder in adults: evidence-based recommendations for management. Postgrad Med. 2008;120:27-38.
- Young JL, Goodman DW. Adult attention-deficit/hyperactivity disorder diagnosis, management, and treatment in the DSM-5 era. Prim Care Companion CNS Disord. 2016;18(6).
- Shier AC, Reichenbacher T, Ghuman HS, Ghuman JK. Pharmacological treatment of attention deficit hyperactivity disorder in children and adolescents: clinical strategies. J Cent Nerv Syst Dis. 2012;5: 1-17.
- Tourette's Syndrome Study Group. Treatment of ADHD in children with tics: a randomized controlled trial. Neurology. 2002;58:527-536.
- Bloch MH, Panza KE, Landeros-Weisenberger A, Leckman JF. Meta-analysis: treatment of attention-deficit/hyperactivity disorder in children with comorbid tic disorders. J Am Acad Child Adolesc Psychiatry. 2009;48:884-893.
- Quillivant XR—an extended release oral suspension of methylphenidate. Med Lett Drugs Ther. 2013;55:10-11.
- Quillivant XR (extended-release methylphenidate oral suspension) package insert. New York, NY: Pfizer Inc; June 2017.
- Wigal SB, Childress AC, Belden HW, Berry SA. NWP06, an extended-release oral suspension of methylphenidate, improved attention-deficit/hyperactivity disorder symptoms compared with placebo in a laboratory classroom study. J Child Adolesc Psychopharmacol. 2013;23:3-10.
- QuilliChew ER—extended-release chewable methylphenidate tablets. Med Lett Drugs Ther. 2016;58:68-69.
- Wigal SB, Childress A, Berry SA, et al. Efficacy and safety of a chewable methylphenidate extended-release tablet in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2017;27:690-699.
- Aptensio XR—another long-acting methylphenidate. Med Lett Drugs Ther. 2015;57:101-103.
- Wigal SB, Greenhill LL, Nordbrock E, et al. A randomized placebo-controlled double-blind study evaluating the time course of response to methylphenidate hydrochloride extended-release capsules in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2014;24:562-569.
- Wigal SB, Nordbrock E, Adjei AL, et al. Efficacy of methylphenidate hydrochloride extended-release capsules (Aptensio XR™) in children and adolescents with attention-deficit/hyperactivity disorder: a phase III, randomized, double-blind study. CNS Drugs. 2015;29: 331-340.
- Cotempla XR-ODT—another long-acting methylphenidate for ADHD. Med Lett Drugs Ther. 2017;59:183-185.
- Childress AC, Kollins SH, Cutler AJ, et al. Efficacy, safety, and tolerability of an extended-release orally disintegrating methylphenidate tablet in children 6-12 years of age with attention-deficit/hyperactivity disorder in the laboratory classroom setting. J Child Adolesc Psychopharmacol. 2017;27:66-74.
- Racemic amphetamine sulfate (Evekeo) for ADHD. Med Lett Drugs Ther. 2015;57:137-138.
- Childress AC, Brams M, Cutler AJ, et al. The efficacy and safety of Evekeo, racemic amphetamine sulfate, for treatment of attentiondeficit/hyperactivity disorder symptoms: a multicenter, dose-optimized, double-blind, randomized, placebo-controlled crossover laboratory classroom study. J Child Adolesc Psychopharmacol. 2015;25:402-414.
- Zenzedi (dextroamphetamine sulfate) package insert. Atlanta, GA: Arbor Pharmaceuticals LLC; February 2017.
- Dyanavel XR (amphetamine extended-release oral suspension) package insert. Monmouth Junction, NJ: Tris Pharma, Inc; January 2017.
- Childress AC, Wigal SB, Brams MN, et al. Efficacy and safety of amphetamine extended-release oral suspension in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2017 Dec 6; Epub ahead of print.
- Adzenys XR-ODT (amphetamine) package insert. Grand Prairie, TX: Neos Therapeutics Brands, LLC; December 2017.
- Adzenys ER (amphetamine) package insert. Grand Prairie, TX: Neos Therapeutics Brands, LLC; September 2017.
- Balogh NJ. Dextroamphetamine (Dexedrine, Dextrostat). In: Goldstein S, Naglieri JA, eds. Encyclopedia of Child Behavior and Development. 2011 ed. Boston, MA: Springer; 2011:498-499.
- Vyvanse (lisdexamfetamine) package insert. Lexington, MA: Shire US Inc; January 2017.
- Center for Drug Evaluation and Research. Application number: 208510Orig1s000 summary review. www.accessdata.fda.gov/drugsatfda_docs/nda/2017/208510Orig1s000SumR.pdf. Accessed March 4, 2018.
- Mydayis (triple-beaded mixed-amphetamine salts) package insert. Lexington, MA: Shire US Inc; June 2017.
- Vayarin (phosphatidylserine/omega-3 fatty acids) package insert. Columbia, MD: VAYA Pharma Inc; March 2018.
- Phosphatidylserine. Natural Medicines Comprehensive Database [subscription required]. Stockton, CA: Therapeutic Research Faculty; 2017. http://naturaldatabase.therapeuticresearch.com/home.aspx. Accessed March 5, 2018.
- Manor I, Magen A, Keidar D, et al. The effect of phosphatidylserine containing Omega3 fatty-acids on attention-deficit hyperactivity disorder symptoms in children: a double-blind placebo-controlled trial, followed by an open-label extension. Eur Psychiatry. 2012;27:335-342.
- Red Book Online. www.micromedexsolutions.com. Ann Arbor, MI: Truven Health Analytics; 2018. Accessed March 6, 2018.