New Vaccines and Immunization Schedule Changes
RELEASE DATE:
April 1, 2018
EXPIRATION DATE:
April 30, 2020
FACULTY:
Cortney M. Mospan, PharmD, BCACP, BCGP
Assistant Professor of Pharmacy
Wingate University School of Pharmacy
Wingate, North Carolina
FACULTY DISCLOSURE STATEMENTS:
Dr. Mospan has no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT:
Pharmacy
 Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-18-018-H06-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE:
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@jobson.com
DISCLAIMER:
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients' conditions and possible contraindications or dangers in use, review of any applicable manufacturer's product information, and comparison with recommendations of other authorities.
GOAL:
To provide pharmacists with knowledge of important updates surrounding immunization schedules and recommendations for adult patients to allow pharmacists to play an active role in the immunization effort. This article will also focus on the newly approved herpes zoster vaccine, Shingrix, a recombinant, adjuvanted vaccine for prevention of shingles.
OBJECTIVES:
After completing this activity, the participant should be able to:
- Describe the role of the Advisory Committee on Immunization Practices in recommended immunizations for adult patients.
- Discuss new immunization schedule recommendations and changes for the 2018 Immunization Schedule for Adults Aged 19 Years or Older.
- Review clinical data and literature that were utilized to inform the 2018 Immunization Schedule for Adults Aged 19 Years or Older.
- Review literature regarding safety and efficacy of available herpes zoster vaccines that resulted in preferential vaccination with Shingrix.
- Identify vaccination needs based on patient disease states, exposures, and vaccination history.
ABSTRACT: Pharmacists play a major role in immunization efforts and must stay abreast of yearly changes in the Advisory Committee on Immunization Practices immunization schedule, which details optimal and appropriate patient-immunization practices. Given that a new herpes zoster vaccine, Shingrix, is entering the market and in view of evolving influenza immunization recommendations, major changes in immunization practices are forthcoming. Pharmacists must be aware of these changes in order to vaccinate patients appropriately and to ensure an adequate vaccine supply at their practice site.
For 50 years, the Advisory Committee on Immuization Practices (ACIP) has developed written recommendations on the use of vaccines licensed by the FDA for use in both children and adult populations.1 The ACIP is charged with using a systematic, science-based mechanism to establish national immunization policy for the United States, which became formal once adopted by the Director of the CDC and the Secretary of the Department of Health and Human Services.1 All ACIP recommendations are publicly available on the CDC's website and are published, once approved by the CDC Director, in the CDC's weekly publication, Morbidity and Mortality Weekly Report (MMWR).1,2 ACIP focuses on policy development for recommendations on different vaccines to protect against the same disease state (i.e., herpes zoster), vaccine formulations, vaccine use in high-risk groups (i.e., measles outbreak), whether to include a new vaccine in the routine immunization schedule (i.e., Shingrix), and use of vaccines outside of the routine schedules.2 Although ACIP recommendations do not carry any associated legal mandate, they are generally regarded as national policy and are adopted by most insurers.2
Influenza
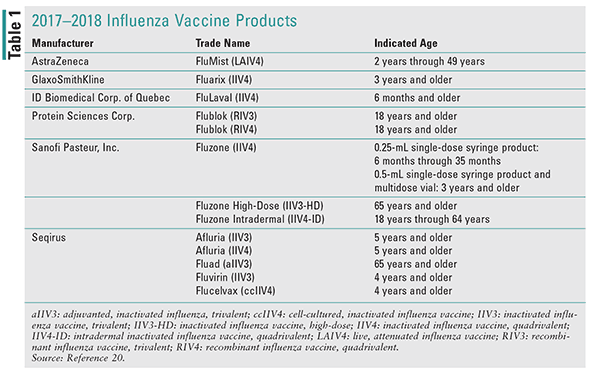
For the first time, the ACIP had made a preferential recommendation for or against a particular influenza vaccine.3,4 In previous years, the committee had avoided making any particular recommendations for specific vaccine formulations due to concern for adequate supply to support recommendations and a general desire for all individuals aged 6 months and older to be vaccinated, regardless of the product. For the 2016–2017 and 2017–2018 influenza seasons, use of live attenuated influenza vaccine (LAIV4) was not recommended based on concerns regarding its effectiveness against (H1N1)pdm09 viruses during both the 2013–2014 and 2015–2016 seasons. At the February 2018 ACIP meeting, the committee voted to reinstate LAIV4 as a recommended influenza vaccine for the 2018–2019 season. An additional consideration to preferentially recommend the inactivated influenza vaccine (IIV) and recombinant influenza vaccine (RIV) over LAIV was voted down.5 The FluMist (LAIV4) product should not be utilized for influenza vaccination until the 2018–2019 season. (See TABLE 1 for available influenza vaccines for the 2017-2018 season.)
For 2017–2018, the three-component (trivalent) influenza vaccines contain the following three viruses: A/Michigan/45/2015 (H1N1)pdm-9-like virus (updated from previous year), A/Hong Kong/4801/2014 (H3N2)-like virus, and B/ Brisbane/60/2008-like (B/Victoria lineage). Quadrivalent (four-component) vaccines have an additional lineage of B viruses in addition to the trivalent components. The additional strain for 2017–2018 quadrivalent vaccines is the B/ Phuket/3073/2013-like (B/Yamagata lineage) virus.6
The CDC recommends influenza vaccination for all persons aged 6 months and older in which the vaccine product is licensed for use through the end of the influenza season, although it is ideal to receive the vaccination before flu activity begins because it takes approximately 2 weeks for immunity to develop. The CDC recommends vaccination before the end of October.7 Influenza vaccination coverage, indicated by patients who receive the vaccine, typically increases by 10% from the start to the end of an influenza season, so pharmacists should still be assessing for vaccine coverage and administering the vaccine to those who have not received it throughout the entirety of the flu season.8 The effectiveness of the 2017–2018 vaccine is estimated to be low, as it was just 10% effective during Australia's influenza season.9 During the 2016–2017 influenza season, the estimated efficacy was 32%, and it is not uncommon for an influenza vaccine to have effectiveness of ~30% against H3N2 strains.10-12 Vaccine effectiveness for the 2017–2018 season—released in February 2018—was lower for the H3N2 strain than for others. Vaccine efficacy was 25% for H3N2, 67% for H1N1 pdm-9, and 42% for influenza B.13
The 2017–2018 influenza season is the strongest since the H1N1 pandemic of 2009.11 H3N2-predominant seasons are generally more severe, especially in young children and older adults. Through the end of January 2018, influenza A (H3N2) viruses were predominant, with 76.4% of positive cases caused by an Influenza A virus. From October 1, 2017, through February 17, 2018, there were 21,279 laboratory-confirmed influenza-associated hospitalizations, resulting in 97 pediatric deaths. Hospitalizations were greatest in the following three populations: patients aged >65 years, patients aged 50 to 64 years, and children aged 0 to 4 years.14 Although vaccination effectiveness is low, influenza vaccination confers a critical benefit. In elderly patients aged 65 years and older, vaccination during the current season and at least one other season out of the previous three influenza seasons was found to have significant impact on health outcomes. Vaccination was found to be twice as effective at preventing severe influenza illness (ICU admission and prevention of death) in patients admitted to the hospital.15 In pediatric patients, vaccination has been associated with a reduced risk of laboratoryconfirmed influenza-associated pediatric death.16
Vaccination rates have room to improve in all populations, particularly in pregnant patients. Influenza vaccination during the second and third trimesters of pregnancy is recommended because late-term pregnancy is a risk factor for both severe illness and hospitalization; since 2004, the ACIP has recommended influenza vaccination for women who will be pregnant during an influenza season.3,16,17 Pregnant women may receive any licensed, recommended, age-appropriate vaccine; the only product that should not be used is LAIV4.3,16,17 In November 2017, only 35.6% of pregnant patients had received the influenza vaccine, which is comparable to the general population, in which two out of five children and adults are vaccinated against influenza illness.16,18 Coverage is better in healthcare professionals (67.6%) but is still considerably below the 100% recommended to be vaccinated.19
Herpes Zoster
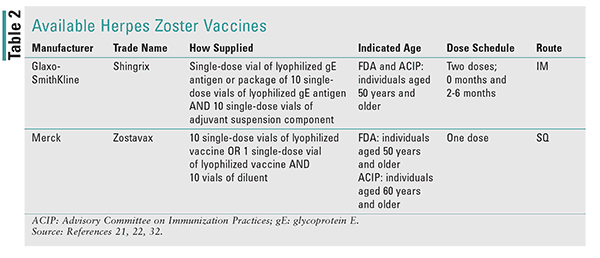
In October 2017, a new herpes zoster vaccine was approved by the FDA, with quick incorporation into ACIP guidelines for herpes zoster vaccination.21 The new vaccine, Shingrix, is a recombinant lyophilized varicella zoster virus (RZV) glycoprotein E (gE) antigen vaccine. It has its own novel adjuvant that it must be mixed with: AS01B.21,22 This vaccine possesses substantial differences from the existing live zoster vaccine (ZVL) Zostavax, which has been on the market since 2006. (See TABLE 2 for available herpes zoster vaccines, including dosing schedules, indication, etc.)
Zostavax was approved based on a reduced burden of illness (61%), reduced incidence of postherpetic neuralgia (66%), and reduced incidence of herpes zoster (51%).23 However, the study that earned Zostavax its approval had only a median surveillance duration of 3.12 years, and concerns for Zostavax's long-term efficacy have existed for several years. The efficacy decreases with time, and incidence of herpes zoster has returned to baseline levels at approximately 8 years following vaccination; however, Zostavax has been found to decrease the incidence and complications associated with herpes zoster.24 For example, the efficacy of prevention of postherpetic neuralgia decreases from 61% at Year 4 postvaccination to 12% at Year 11 postvaccination, necessitating a better vaccine with improved long-term efficacy.24,25 Zostavax's efficacy has also been found to be lower overall in patients aged >70 years (38%) compared to patients between the ages of 50 and 59 years (70%).23,24,26
Shingrix was developed in response to long-term efficacy issues and lower efficacy in patients aged 70 years and older. This is particularly important because herpes zoster incidence increases with advancing age, with the rate significantly increasing above age 50 years.27,28 The incidence is highest in patients aged 80 years and older.27 Shingrix was found to significantly reduce the risk of herpes zoster in both the ZOE-50 (adults aged 50 years and older) and ZOE-70 (adults aged 70 years and older) trials and also reduced the risk of postherpetic neuralgia in ZOE-70.29,30 Vaccine efficacy was comparable in both patient age groups. Shingrix has been found to have greater efficacy in all age groups compared to Zostavax, with the greatest increase in efficacy found in patients aged 70 years and older.21
Shingrix is a two-dose vaccine series, with the first dose administered at 0 months and the second dose 2 to 6 months later.21,22 The vaccination series does not need to be restarted if the second dose is received more than 6 months after the first, but alterative dosing schedules have not been studied. If the second dose is received 4 or fewer weeks after the first dose, the second dose should be given again at 2 to 6 months. If patients have been previously vaccinated with Zostavax, both doses should still be administered.21 Like its predecessor, Shingrix must be mixed prior to administration. Only the diluent that comes with the vaccine, AS01B, should be used to dissolve the antigen powder. Once mixed, the vaccine should be opalescent and colorless to a pale brownish liquid. The adjuvant has a blue-green cap and should be withdrawn from its vial and inserted into the lyophilized gE antigen vial, which has a brown cap. Once dissolved, the volume should be 0.5 mL. The pharmacist should gently shake and mix the vaccine until all of the powder is completely dissolved. Unlike Zostavax, the diluent should be refrigerated along with the antigen prior to reconstitution. Both vials should be protected from light while stored.22
This vaccine does not contain preservative and should not be reconstituted until it is ready to be administered. Once reconstituted, Shingrix can be refrigerated (36-46°F) for up to 6 hours if not used immediately. After 6 hours, the vaccine should be discarded. Neither component of the Shingrix vaccine should be frozen, and if either is ever frozen, it should be discarded. The vaccine is administered intramuscularly (IM), with the preferred region being the deltoid area of the upper arm. The most prevalent localized adverse effects (in order of occurrence) were pain, local redness, and swelling. The most prevalent systemic adverse effects were myalgia, fatigue, headache, shivering, fever, and gastrointestinal symptoms.22
 With formal publication in the MMWR in January 2018, Shingrix became the preferred vaccination over Zostavax for preventing herpes zoster (Shingles).21,31 Both vaccines are included in the vaccination schedule and may be used; however, Shingrix should be used unless there is a contraindication, cost barrier, and so on. If such a barrier exists, Zostavax remains indicated by the ACIP for use in adults aged 60 years and older.21 As with Zostavax, the ACIP has recommended the use of Shingrix in immunocompetent adults only.21 Zostavax should not be used in immunocompromised patients owing to a risk of causing disease, but Shingrix has not been studied in immunocompromised patients, and it is unknown if efficacy will be reduced in patients on immunosuppressive therapies.21,22,32 Shingrix has been approved for use in all adult aged patients >50 years, but patients taking moderate-high doses of immunosuppressant medications were excluded from clinical trials. Patients who are taking <20 mg of prednisone, topical corticosteroids, or inhaled corticosteroids are not considered immunocompromised and should be vaccinated.21,22
With formal publication in the MMWR in January 2018, Shingrix became the preferred vaccination over Zostavax for preventing herpes zoster (Shingles).21,31 Both vaccines are included in the vaccination schedule and may be used; however, Shingrix should be used unless there is a contraindication, cost barrier, and so on. If such a barrier exists, Zostavax remains indicated by the ACIP for use in adults aged 60 years and older.21 As with Zostavax, the ACIP has recommended the use of Shingrix in immunocompetent adults only.21 Zostavax should not be used in immunocompromised patients owing to a risk of causing disease, but Shingrix has not been studied in immunocompromised patients, and it is unknown if efficacy will be reduced in patients on immunosuppressive therapies.21,22,32 Shingrix has been approved for use in all adult aged patients >50 years, but patients taking moderate-high doses of immunosuppressant medications were excluded from clinical trials. Patients who are taking <20 mg of prednisone, topical corticosteroids, or inhaled corticosteroids are not considered immunocompromised and should be vaccinated.21,22
Shingrix is indicated for vaccination in all immunocompetent adults aged 50 years and older and is preferred over Zostavax.21 Unlike Zostavax, the ACIP has recommended Shingrix for patients aged 50 and older.21 Zostavax is FDA-approved for persons aged 50 years and older, but is recommended by the ACIP only for ages 60 and older, due to many concerns. One of these is its cost-effectiveness under a quality-adjusted lifeyear saved model.33
It is unclear whether there is any transferability of lack of cost effectiveness from Zostavax to Shingrix. If patients have previously received Zostavax, they still should receive the Shingrix series.21,29,30 Patients who have previously had herpes zoster but have not been vaccinated should also receive the Shingrix series, as disease can recur. If patients have herpes zoster or postherpetic neuralgia and have not been vaccinated with Shingrix, they should be vaccinated after the acute stage of illness is over and symptoms have resolved.21
Shingrix may be coadministered with other indicated vaccines as long as they are administered in different anatomical locations.18 Unlike Zostavax, there are no concerns for change in efficacy of Pneumovax (PPSV23 [pneumococcal polysaccharide vaccine]) when administered together.21,22,32 Shingrix has been studied only for coadministration with Fluarix (IIV4) at this time, with no evidence of interference with immune response to Fluarix, and studies are ongoing with Pneumovax (PPSV23) and Tdap (Tetanus, Dipitheria, Pertussis, Boostrix).21
Pharmacists should be mindful of the Vaccine Adverse Events Reporting System (VAERS) with all vaccines, particularly concerning Shingrix, because of its limited clinical use thus far. Adverse events that occur following vaccination of a patient may be reported to VAERS, and reporting is encouraged for any clinically significant adverse event, even if the association with the vaccine is uncertain. Instructions for how to submit a report to VAERS is available at http://vaers.hhs.gov/index.html or by telephone at 1-800-822-7967.21
Mumps
Since the introduction of the first ACIP mumps vaccination recommendation in 1977 and the subsequent recommendation for a second booster dose in 1989, the incidence of mumps in the U.S. has declined drastically.34 In the past decade, outbreaks have been occurring with greater frequency with a larger number of patients affected; recently, there were 150 mumps outbreaks over 18 months, with a total of 9,200 cases. Analysis of mumps cases that occurred in 2016-2017 found that the median patient age was 21 years and that 75% of patients received two measles, mumps, and rubella (MMR) doses.34 One-half of outbreaks occurred in university settings, and complications were low in vaccinated invidividuals.34
Since 2012, the CDC has had guidance in place for health departments to steer the use of a third MMR dose in outbreak settings, but data were insufficient to recommend for or against the use of MMR during outbreaks.35 The routine vaccination recommendation for prevention of mumps includes MMR vaccine at 12 to 15 months, with a second dose at 4 to 6 years, before school entry. Two doses are also recommended for adults at high risk for exposure (e.g., students attending college, international travelers).35
The effectiveness of two doses of MMR is 88%, but it is well validated that effectiveness decreases with time since vaccination.36 Epidemiologic data presented at the October 2017 ACIP meeting involved three outbreak locations with high coverage of two-dose MMR completion. A study at the University of Iowa found a lower mumps-attack rate in students who received three doses compared with two, and the threedose regimen was associated with a 78% lower risk of mumps.37
Other studies reviewed by the ACIP also found lower attack rates among patients vaccinated with a third dose.36 It was also found that students who received their two doses of MMR 13 years or more before the outbreak were nine or more times likely to contract mumps than those who received their second dose within 2 years of the outbreak.36 From a safety perspective, 14,368 vaccination records of patients who received a third dose were reviewed. Patient ages ranged from 9 to 28 years, and no serious adverse events were reported; all adverse events were mild and reported at low rates.36
As a result of the evidence reviewed, the ACIP made a permanent, and firmer, recommendation that all persons previously vaccinated with two doses of a mumps-containing vaccine who are identified by public-health personnel at increased risk due to an outbreak should receive a third vaccine dose to improve protection against mumps and its complications.36,38 The recommendation was made to clarify for local health departments facing a local outbreak when the vaccine may be beneficial and provide greater flexibility in utilizing resources as necessary.39 A routine third dose for all persons entering college was considered, but there was not enough evidence to support such a recommendation at this time.39
Conclusion
Although the 2018 ACIP Adult Immunization Schedule stayed relatively constant from 2017, two major changes occurred that have significant implications. For the first time, the ACIP made a preferential recommendation against use of a particular influenza immunization—FluMist—owing to established efficacy concerns. The ACIP preferentially recommends the new herpes zoster vaccine Shingrix for herpes zoster prevention over the established Zostavax vaccine. Pharmacists should implement these recommendations into practice systems for optimal patient-immuization outcomes.
REFERENCES
- Walton LR, Orenstein WA, Pickering LK. The history of the United States Advisory Committee on Immunization Practices (ACIP). Vaccine. 2015;33:405-414.
- Smith JC. The structure, role, and procedures of the U.S. Advisory Committee on Immunization Practices (ACIP). Vaccine. 2010;28S:A68-A75.
- Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2017–2018 influenza season. MMWR Recomm Rep.2017;66(RR-02):1-20.
- Grohskopf L. Influenza WG considerations and proposed recommendations. www.cdc.gov/vaccines/acip/meetings/downloads/ slides-2017-06/flu-06-grohskopf.pdf. Accessed January 5, 2018.
- Walker M. ACIP reinstates FluMist for the 2018 – 2019 season. www.medpagetoday.com/meetingcoverage/acip/71298. Accessed February 23, 2018.
- Centers for Disease Control and Prevention. The flu season. www.cdc.gov/flu/about/season/flu-season.htm. Accessed January 5, 2018.
- Centers for Disease Control and Prevention. Key facts about seasonal flu vaccine. www.cdc.gov/flu/protect/keyfacts.htm. Accessed February 23, 2018.
- Centers for Disease Control and Prevention. Health care personnel and flu vaccination, internet panel survey, United States, November 2017. www.cdc.gov/flu/fluvaxview/hcp-ips-nov2017. htm. Accessed February 2, 2018.
- Skowronski DM, Chambers C, De Serres G, et al. Early season co-circulation of influenza A(H3N2) and B(Yamagata): interim estimates of 2017–2018 vaccine effectiveness, Canada, January 2018. Euro Surveill. 2018;23(5):pii=18-00035.
- American Academy of Family Physicians. CDC: 2017-18 flu season in U.S. dominated by H3N2 virus. www.aafp.org/news/ health-of-the-public/20171213fluupdate.html. Accessed January 31, 2018.
- Drug Store News. CDC: 2017/2018 flu season strongest since 2009. www.drugstorenews.com/news/cdc-0101-flu-season-strongest-00//. Accessed February 2, 2018.
- CDC Health Alert Network. Seasonal influenza A(H3N2) activity and antiviral treatment of patients with influenza. https:// emergency.cdc.gov/han/han00409.asp. Accessed February 23, 2018.
- Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Recomm Rep. 2018;67(6):180-185.
- Centers for Disease Control and Prevention. Weekly U.S. influenza surveillance report. www.cdc.gov/flu/weekly/#S6. Accessed February 23, 2018.
- Casado I, Dominguez A, Toledo D, et al. Repeated influenza vaccination for preventing severe and fatal influenza infection in older adults: a multicentre case-control study. CMAJ. 2018;190(1):E3-E12.
- Flannery B, Reynolds SB, Blanton L, et al. Influenza vaccine effectiveness against pediatric deaths: 2010 – 2014. Pediatrics. 2017;139(5):e20164244.
- Centers for Disease Control and Prevention. Pregnant women and flu vaccination, internet panel survey, United States, November 2017. www.cdc.gov/flu/fluvaxview/pregnant-women-nov2017. htm. Accessed February 2, 2018.
- Centers for Disease Control and Prevention. Frequently asked flu questions 2017 – 2018 influenza season. www.cdc.gov/flu/ about/season/flu-season-2017-2018.htm. Accessed January 31, 2018.
- Centers for Disease Control and Prevention. National earlyseason flu vaccination coverage, United States, November 2017. www.cdc.gov/flu/fluvaxview/nifs-estimates-nov2017.htm. Accessed February 2, 2018.
- Immunization Action Coalition. Influenza vaccine products for the 2017 – 2018 influenza season. www.immunize.org/catg.d/ p4072.pdf. Accessed January 5, 2018.
- Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Recomm Rep. 2018;67(3):103-108.
- Shingrix (zoster vaccine recombinant, adjuvanted) package insert. Research Triangle Park, NC: GlaxoSmithKline Biologicals; 2017. www.gsksource.com/pharma/content/dam/GlaxoSmithKline/ US/en/Prescribing_Information/Shingrix/pdf/SHINGRIX.PDF. Accessed January 5, 2018.
- Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271-2284.
- Schmader KE, Oxman MN, Levin MJ, et al. Persistence of the efficacy of zoster vaccine in the Shingles prevention study and the short-term persistence substudy. Clin Infect Dis. 2012;55(10):1320-1328.
- Morrison VA, Johnson GR, Schmader KE, et al. Long-term persistence of zoster vaccine efficacy. Clin Infect Dis. 2015;60(6):900-909.
- Bharucha T, Ming D, Breuer J. A critical appraisal of 'Shingrix', a novel herpes zoster subunit vaccine (HZ/Su or GSK1437173A) for varicella zoster virus. Human Vaccin Immunother. 2017;13(8):1789-1797.
- Centers for Disease Control and Prevention. Shingles surveillance. www.cdc.gov/shingles/surveillance.html. Accessed February 2, 2018.
- Johnson BH, Palmer L, Gatewood J, et al. Annual incidence rates of herpes zoster among an immunocompetent population in the United States. BMC Infect Dis. 2015;15:502.
- Cunningham AL, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375(11):1019-1032.
- Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372(22):2087-2096.
- Centers for Disease Control and Prevention. Updates – 2018 Adult Immunization Schedule. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/adult-immunization-02-kim.pdf. Accessed January 31, 2018.
- Zostavax (zoster vaccine live) package insert. Whitehouse Station, NJ: Merck & Co., Inc.: 2017. www.merck.com/product/usa/ pi_circulars/z/zostavax/zostavax_pi2.pdf. Accessed January 5, 2018.
- Le P, Rothberg MB. Cost-effectiveness of herpes vaccine for persons aged 50 years. Ann Intern Med. 2015;163(7):489-497.
- Marin M. Update on mumps epidemiology, United States. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/mumps-02-marin-508.pdf. Accessed January 5, 2018.
- Marin M. Considerations for the use of the 3rd dose of MMR vaccine for persons at increased risk because of a mumps outbreak and proposed recommendations. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/mumps-04-marin-508.pdf. Accessed January 5, 2018.
- Marin M, Marlow M, Moore KL, Patel M. Recommendation of the Advisory Committee on Immunization Practices for use of a third dose of mumps virus-containing vaccine in persons at increased risk for mumps during an outbreak. MMWR Recomm Rep. 2018;67(1):33-38.
- Cardemil CV, Dahl RM, James L, et al. Effectiveness of a third dose of MMR vaccine for mumps outbreak control. N Engl J Med. 2017;377:947-956.
- McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS. Prevention of measles, rubella, congenital rubella syndrome and mumps, 2013 summary: recommendations from the ACIP. MMWR Recomm Rep. 2013;62(RR-04):1-34.
- Jenco M. ACIP: give 3rd mumps vaccine dose during outbreaks. AAP News. www.aappublications.org/news/2017/10/26/ Mumps102617. Accessed January 5, 2018.
- Shimabukuro T. Reports of shoulder dysfunction following inactivated influenza vaccine in the Vaccine Adverse Event Report System (VAERS), 2010-2016. www.cdc.gov/vaccines/acip/meetings/ downloads/slides-2017-10/vaccine-safety-03-shimabukuro-508.pdf. Accessed January 5, 2018.
- Atanasoff S, Ryan T, Lightfoot R, Johann-Liang R. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010;28:8049-8052.
- IOM (Institute of Medicine). Adverse effects of vaccines: evidence and causality. Washington, DC: The National Academies Press.
- Health Resources and Services Administration. Vaccine injury table. www.hrsa.gov/sites/default/files/vaccinecompensation/vaccineinjurytable.pdf. Accessed January 5, 2018.