Novel Therapies Expand Hypogonadism Treatment Options
RELEASE DATE
June 1, 2022
EXPIRATION DATE
June 30, 2024
FACULTY
Justin J. Sherman, PharmD, MCS
Associate Professor of Pharmacy Practice
University of Mississippi School of Pharmacy
Jackson, Mississippi
FACULTY DISCLOSURE STATEMENTS
Dr. Sherman has no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT
 Pharmacy
Pharmacy
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-22-055-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients’ conditions and possible contraindications or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
GOAL
The purpose of this activity is to review the medications available for testosterone replacement therapy and to discuss the newer novel therapies more in-depth. All currently available therapies will be evaluated as to possible approaches for treatment and monitoring of hypogonadism.
OBJECTIVES
After completing this activity, the participant should be able to:
- Discuss an overall approach to the treatment of hypogonadism, including monitoring parameters.
- Compare and contrast the benefits and barriers of each testosterone replacement therapy, including new therapy modalities.
- Evaluate the possible role each medication may have in the individualized treatment of patients.
- Discuss follow-up monitoring parameters for evaluating efficacy of testosterone replacement therapy and adverse effects.
ABSTRACT: Management of hypogonadism with testosterone replacement therapy is complex, with a variety of disease state, medication, and monitoring considerations. Testosterone replacement traditionally has been available as transdermal and injection therapies and as subdermal pellets. New modalities of therapy recently approved include a testosterone nasal gel, an autoinjectable, and an oral capsule. Pharmacists should have a thorough knowledge of all testosterone replacement therapies to counsel patients and make recommendations regarding efficacy and potential adverse effects to optimize and individualize therapy for hypogonadism.
The various modalities of medications available has complicated the management of hypogonadism, determined by a deficiency or absence of endogenous testosterone (i.e., testosterone deficiency [TD]). Over the past few years, the FDA has approved several novel therapies of testosterone delivery. The purpose of this article is to review the current testosterone replacement therapy (TRT) options, to discuss new modalities of therapy, and to explore possible options for therapeutic management of this complex disease state.
GENERAL CONSIDERATIONS PRIOR TO MEDICATION TREATMENT
According to the Endocrine Society Clinical Practice Guidelines, hypogonadism is a clinical syndrome that results from failure of the testes to produce physiologic levels of the normal number of spermatozoa due to the disruption of one or more levels of the hypothalamic-pituitary-gonadal axis.1 Prior to the patient receiving TRT, a definitive diagnosis of TD should be made. While altered testosterone concentrations could be caused by other factors, TRT is intended for treating hypogonadism. Hypogonadism is characterized by symptoms coupled with reduced testosterone concentrations. Patients can present with a combination of physical, psychological, and sexual symptoms. The Androgen Deficiency in the Aging Male (ADAM) is one questionnaire that assesses symptoms.2 However, serum testosterone concentrations represent the most important diagnostic measure. A normal range for testosterone concentration should be 300 ng/dL to 1,000 ng/dL, and a reduction on two separate visits in the morning leads to an accurate diagnosis of hypogonadism.3
Primary hypogonadism is failure of testosterone to be produced at the level of the testes, leading to elevated serum gonadotropins.3 Some genetic diseases, such as Klinefelter syndrome and Noonan syndrome, as well as testicular injury, present as primary hypogonadism. Secondary hypogonadism is failure of gonadotropin production in the CNS. Genetic disorders, such as Kallmann syndrome, pituitary tumors, and hemochromatosis, can result in secondary hypogonadism. Of note, age-related (i.e., late onset) hypogonadism (ARH) results from decreasing testosterone production yearly in men after age 40 years.
Optimal medication management for TD treats associated signs and symptoms and maintains eugonadal serum testosterone concentrations.3 Purported benefits of TRT, historically, have included an increase in libido, sexual function, mood, bone density, erythropoiesis, and body composition in general. However, some evidence exists that patients may not experience all of these benefits.4 Also, certain patients should not take TRT. Symptoms of benign prostatic hyperplasia (BPH) and sleep apnea may worsen with TRT. In many cases, clinicians will not initiate TRT if the baseline prostate-specific antigen (PSA) concentration is greater than 3 ng/dL, and definitely not if a nodule is palpable on digital rectal examination (DRE).3 Increased hematocrit (HCT) and polycythemia may occur; if HCT is greater than 55%, the clinician should not initiate TRT. Gynecomastia, testicular atrophy, and infertility have also resulted from TRT. As well, a general increase in acne, oily skin, and hair loss can occur.
On March 3, 2015, the FDA issued a safety communication regarding TRT.5 Labeling for TRT medications was subsequently modified to include a possible increased risk for stroke and heart attack for patients with preexisting heart disease. Thus, physicians should only prescribe testosterone for patients with a diagnosis of hypogonadism. While several studies show TRT can increase the risk of stroke and heart attack for patients with preexisting conditions, other evidence is less clear.5 This ambiguity has led to a reduction overall in prescribing TRT; in fact, prescriptions decreased by roughly 33% between 2011 and 2018, regardless of whether men had pre-existing heart conditions.6
Regarding contraindications, men with known or suspected prostate cancer should not take TRT. An acceleration of current prostate cancer can occur with TRT. Since breast cancer can result in men subsequent to an undetected or inadequately treated prostate cancer, men with breast cancer should not take TRT. A contraindication no longer exists for men with a history of prostate cancer whose cancer has been eradicated; however, the patient’s provider should weigh risks versus benefits prior to starting TRT.3 Since testosterone may cause fetal harm, women who are pregnant should not use TRT—nor is it indicated for women in general.
TESTOSTERONE
Testosterone is available in many formulations and administrative methods. Grouped in a class of TRT, it represents the only FDA-approved treatment for TD resulting from hypogonadism.7 TABLE 1 compares transdermal therapy options, injection therapies, and pellets, including dosing, dose adjustments, advantages, and disadvantages. More recent and novel medications will be discussed in detail, including nasal testosterone, an autoinjector, and oral therapies.
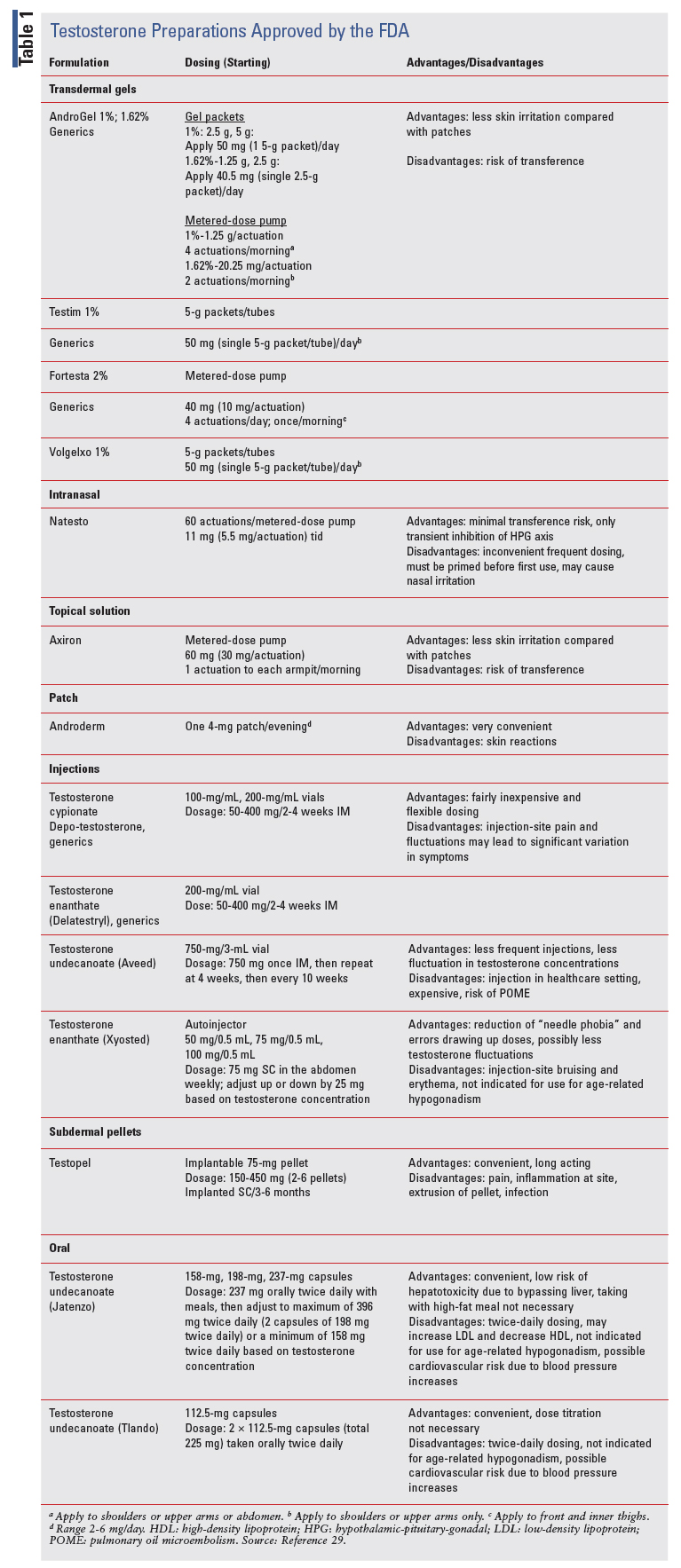
TRANSDERMAL THERAPIES
Transdermal testosterone is available as either patches, gels, or a topical solution.7 These formulations result in consistent serum testosterone concentrations, which fall quickly upon stopping therapy. Regarding patches, the starting dose is 4 mg per day, applied nightly to either the back, upper arms, abdomen, or thighs. Application should be to nonhairy areas, with sites rotated each day and no site reused for 7 days. Clinicians should obtain serum testosterone concentrations 2 weeks after initiation and increase doses to 6 mg daily or decrease to 2 mg daily depending on results. Skin irritation is the main adverse effect.
Gels and a topical solution quickly became popular due to ease of use. These formulations are applied from either packets or a pump (i.e., Axiron) to similar body sites as the patch, with the exception of the pump that is delivered in a cupful to each axilla daily. Gels are available in concentrations of 1%, 1.62%, and 2%.8 Patients should be counseled to allow for drying time and to wash their hands thoroughly to prevent transference. Testosterone delivered to a patient’s spouse or kids could be harmful. These formulations cause less skin irritation than patches.
INJECTION THERAPIES
Esterified formulations of testosterone, given intramuscularly, include cypionate, enanthate, and undecanoate. Hallmarks of this TRT modality include relatively low cost and extended dosing intervals.
However, injection-site pain and wide fluctuations of testosterone concentrations (especially with cypionate and enanthate) may be experienced. Formulations differ in their pharmacologic properties. Cypionate and enanthate versions are usually injected every 2 to 4 weeks but could be injected weekly depending upon serum testosterone concentration and mood fluctuations.8 Patients may use a 22- or 23-gauge needle on a syringe to pull the dose from a vial and inject themselves. Serum testosterone concentrations may be obtained a week after therapy initiation, with subsequent dosage adjustment.
The undecanoate formulation (i.e., Aveed) has the longest carbon side chain, which considerably increases the duration of action.9 A healthcare professional gives it at baseline, at 4 weeks, and then every 10 weeks thereafter. While this formulation exhibits less fluctuations in testosterone concentration and mood, several considerations limit its use. It must be administered in a physician’s office or clinic because a rare pulmonary oil microembolism (POME) may occur. Possible respiratory symptoms, including cough and shortness of breath after injection, requires a healthcare professional to monitor the patient in the office for at least 30 minutes. Also, it requires the physician to be trained in a Risk Evaluation Mitigation Strategy.
SUBCUTANEOUS PELLETS
Testosterone-eluting pellets may be implanted subcutaneously in a prescriber’s office under local anesthesia. Pellets that provide 150 mg to 450 mg of testosterone are inserted into the fat of the thigh, abdomen, or buttock. While this modality is not commonly used, once inserted it provides a reliable serum testosterone concentration for 3 to 6 months. Adverse effects include extrusion of the pellet, infection, and skin irritation.8
RECENTLY APPROVED THERAPIES
Adverse events with therapies previously discussed include hepatotoxicity from first-pass metabolism, skin irritation, transference, injection-site reactions, and symptom variances based on administration. Recent development of novel therapies maintain eugonadal serum testosterone concentrations without such adverse events. These include testosterone nasal gel, an autoinjectable, and an oral capsule.
As with previously discussed TRT, clinicians should consider several issues prior to starting therapy. The clinician should ensure that the patient has hypogonadism due to either primary hypogonadism or hypogonadotropic hypogonadism. Two limitations of use for the new modalities are ARH and males younger than age 18 years. Safety and efficacy have not yet been established for patients with ARH.7,8
Also, clinicians should ensure efficacy of therapy for continued treatment. Testosterone concentrations should be taken after the start of therapy, at different intervals depending upon the TRT modality. If total testosterone concentrations consistently exceed 1,050 mg/dL or are below 300 mg/dL, discontinue treatment in favor of a different treatment or no therapy.
Testosterone Nasal Gel
Endo International produced the first and only testosterone nasal gel (Natesto); the FDA approved it for hypogonadism on May 29, 2014. This modality takes advantage of the high permeability and superior bioavailability that the nasal mucosa provides. Absorption leads to maximum serum concentrations within 60 minutes with a half-life of 10 to 100 minutes.8 First-pass metabolism is also not an issue with this modality.
A metered-dose pump delivers 5.5 mg of testosterone with each pump. The recommended dose regimen is one pump in each nostril, delivering a total of 11 mg, three times daily.10 The need for multiple daily dosing stems from the half-life, which is relatively short compared with other TRT modalities. Administration, delivered with a full pump, should be at least 6 to 8 hours apart. Patients should prime the pump on first use by inversion and then pumping 10 times directly into a sink and then washing the gel away with warm water. The steps for administration can be found in the package insert.10 Patients should not concomitantly administer other nasal medications with testosterone nasal gel except for sympathomimetic decongestants (e.g., oxymetazoline). Unknown interactions may occur between such medications.10
Recent novel therapies maintain serum testosterone concentrations without adverse events such as hepatotoxicity, skin irritation, transference, injection-site reactions, and symptom variances based on administration.
Also, patients should discontinue use of the nasal gel if severe rhinitis develops. Of note, seasonal allergies do not impact absorption of the nasal gel significantly; thus, discontinuation in such circumstances is not needed.11 No specific recommendations exist for patients with chronic nasal conditions or nasal anatomy alterations.
With regards to contraindications and most warnings and precautions, testosterone nasal gel shares those aforementioned concerns. However, due to its unique modality of nasal administration, pharmacists should be aware of specific adverse reactions. Nasopharyngitis, rhinorrhea, epistaxis, nasal discomfort, and nasal scabbing were all experienced in clinical trials.10 All were considered mild or moderate in severity, with the exception of a single case of upper respiratory infection. If nasal symptoms occur during treatment, patients should be further evaluated as to referral to a specialist or discontinuation of the nasal gel. Other adverse reactions commonly experienced (>3%) include an increase in PSA concentration, headache, bronchitis, and sinusitis.
Drug interactions that may be common with nasal gel testosterone include a decrease in blood glucose, thus increasing insulin requirements in patients with concomitant diabetes. More frequent monitoring for patients with warfarin may be required since international normalized ratio may fluctuate more when taking this medication. Finally, in patients with cardiac, renal, or hepatic disease, testosterone may increase fluid retention when given with corticosteroids. However, no recommendations of dose adjustment exist for patients with renal or hepatic disease.10
Advantages of using this modality include a simple, noninvasive administration, lower dosing needed due to efficient absorption, no transference versus other gel formulations, and avoidance of first-pass metabolism. Of note, twice-daily or three-times-daily dosing has been suggested to achieve therapeutic serum testosterone concentrations without reducing luteinizing hormone and follicular-stimulating hormone below normal ranges.12 Thus, the body may potentially restore the hypothalamic-pituitary-gonadal axis due to multiple dosing.9 Clinically, spermatogenesis for fertility may be preserved and has been shown to be maintained at 6 months after the start of therapy.13 Also, clinicians should monitor testosterone concentration 4 weeks after the start of therapy. Disadvantages include the inconvenience of frequent dosing, the need for priming, specific nasal-related adverse events, and a higher cost. Each product delivers 60 actuations, so cost is generally around $825 for a month’s supply.9
Testosterone Enanthate
On October 2, 2018, the FDA approved the first autoinjectable testosterone enanthate (Xyosted) developed by Antares Pharma. This subcutaneous, weekly, abdominal self-injection with three dosage strengths and a narrow peak-to-trough ratio produces a normal testosterone concentration. In October 2017, the FDA had declined to approve it over concerns of clinically significant increase in blood pressure. Upon reevaluation, the FDA approved it with a boxed warning regarding a possible increase in major adverse cardiovascular events (MACE).14
Testosterone enanthate, as an autoinjectable, is available as 0.5-mL solution in a single-dose syringe with a fine-gauge needle. It is available in three dosage strengths: 50 mg/0.5 mL, 75 mg/0.5 mL, and 100 mg/0.5 mL. The suggested starting dose is 75 mg. The steps for administration can be found in the package insert.15 The clinician should measure total testosterone concentrations at the following intervals: 7 days after the most recent dose, following 6 weeks of dosing, following 6 weeks of a dose adjustment, and then periodically while on treatment. Achievement of a trough testosterone concentration between 350 ng/ dL and 650 ng/dL is the goal for using this particular medication.7 Once initiating the starting dose regimen, the patient should receive a dose increase of 25 mg (100 mg/0.5 mL dose regimen) if the concentration is less than 350 ng/dL. Conversely, if the concentration is greater than 650 ng/dL, the patient should begin taking 25 mg less per week (50 mg/0.5 mL–dose regimen).7
In 2017 when the FDA originally did not approve this medication, they identified two reasons. A phase III trial showed clinical efficacy for the autoinjection maintaining steady blood testosterone concentrations. However, the FDA questioned a clinically meaningful increase in blood pressure, as well as occurrences of depression and suicidality.16 Systolic blood pressure increased by an average of 4 mmHg from baseline in the first 3 months of treatment. Ten percent of patients taking the trial medication (autoinjectable testosterone enanthate) were either started on antihypertensives or required a change in their antihypertension medication regimen during the first year.16
Subsequent to the blood pressure increase, the FDA required a black box warning.16 The clinician should consider the patient’s baseline cardiovascular risk prior to initiating this medication. In addition to possible increase in blood pressure, MACE includes the potential of nonfatal myocardial infarction, nonfatal stroke, and cardiovascular death. The concomitant disease state of hypertension is not a contraindication. However, blood pressure should be adequately controlled prior to initiation of this autoinjectable. Six weeks after the start of therapy, the clinician should evaluate for new-onset hypertension or exacerbations of preexisting hypertension. Benefits versus risks should further be evaluated periodically after the first 6 weeks of therapy. Of note, the black box warning includes that this medication should only be used to treat men with hypogonadal conditions associated with structural or genetic etiologies. Thus, it is not the intent of the autoinjectable testosterone enanthate to be used for men with ARH.
Unless otherwise noted in the black box warnings, the contraindications, warnings, and precautions of this medication are similar to the other medications discussed. Adverse reactions experienced by >5% of patients in clinical trials include an increase in hematocrit and PSA concentration, hypertension, injectionsite bruising, and headache.15 Drug interactions are similar to those previously discussed for the testosterone nasal gel, with the addition of the following: concomitant medications that can increase blood pressure can lead to additional increases with autoinjectable testosterone enanthate. With regard to the geriatric population, long-term data are insufficient to determine safety concerning cardiovascular risks and development of prostate cancer.
Advantages of using autoinjectable testosterone enanthate include reduction of “needle phobia” and errors in drawing up doses. Also, an autoinjectable formulation may prevent drug contamination and accidental needle sticks, plus provide a standardized method of dosing that may be less subject to “patient errors.”17 Testosterone enanthate is available in vial formulation and is less expensive than the autoinjectable form. The cost of the autoinjectable is approximately $450 per month versus $30 to $60 per month for the vial formulation. The vial formulation may be dosed every 2 to 3 weeks in comparison with the autoinjectable formulation used weekly. However, dosing every 2 to 3 weeks may result in higher fluctuation of testosterone concentrations. In the clinical study leading to FDA approval, 90% of patients achieved testosterone concentrations in the normal range by Week 12 of therapy.7 Injections via the autoinjectable formulation also are less painful.7 Disadvantages include cost; due to this formulation being an injection, disadvantages also include injection-site bruising and erythema. A final disadvantage would be that this medication is not indicated for use for ARH.
Testosterone Undecanoate (Jatenzo)
Of the testosterone formulations, oral testosterone would have the potential for high patient satisfaction due to its convenience. In some disease states, such as multiple sclerosis and rheumatoid arthritis, research shows that patients prefer oral medications over other formulations.18,19 Historically, though, first-pass metabolism yields decreasing oral bioavailability. Supraphysiologic doses and dosing with meals high in fat have been needed to attain therapeutic serum concentrations.20 Achieving consistent testosterone concentrations has been a problem. Also, hepatotoxicity seen with oral formulations have posed limitations. New oral formulations are absorbed through the lymphatic system and bypass liver metabolism. The undecanoate ester of testosterone is more lipophilic in comparison with cypionate and enanthate, which are absorbed more through the hepatic portal circulation.8
Testosterone undecanoate can decrease high-density lipoprotein (HDL) concentrations while increasing low-density lipoprotein (LDL) concentrations. For example, in a small phase II pharmacokinetic study, the investigators reported a 19% decrease in HDL cholesterol in the steady-state arm of the study.8 The increase in LDL and decrease in HDL concentrations possibly suggests that this formulation may increase cardiovascular risk.8
Serious hepatotoxicity occurred with the first oral testosterone formulation, methyltestosterone, which was approved for use in the U.S. over 60 years ago.21
Advantages of autoinjectable testosterone enanthate include reduction of “needle phobia” and errors in drawing up doses. Also, an autoinjectable formulation may prevent drug contamination and accidental needle sticks.
No oral testosterone formulations existed on the market when the FDA approved the first oral capsule of testosterone undecanoate (Jatenzo, Clarus Therapeutics) on March 27, 2019.21 The path to FDA approval had been problematic. The FDA rejected initial filings of new drug applications (NDA) from two companies of separate oral testosterone formulations.22 Concerning safety data—increases in heart rate and blood pressure in studies of both potential medications—led to an advisory committee initially rejecting approval. However, reevalution has since led to FDA approval with the limitation of a black box warning and a requirement that further postmarketing trials will be done to more clearly delineate effect on cardiovascular parameters. Notably, a previous FDA Advisory Committee meeting in 2014 had discussed possible cardiovascular effects of all TRT products. Since then, the FDA has required all manufacturers of TRT products to evaluate ambulatory blood pressure during all clinical studies.21
The FDA required a black box warning—identical to the one for the injectable testosterone enanthate, as previously discussed—for oral testosterone undecanoate regarding a possible increase in MACE. Oral testosterone undecanoate is not contraindicated for patients with concomitant hypertension. However, blood pressure should be adequately controlled before starting this TRT formulation and reevaluated within 6 weeks. Of note, this formulation of oral testosterone undecanoate should only be used to treat men with hypogonadism associated with structural or genetic etiologies. It is not appropriate at this time for ARH.
Oral testosterone undecanoate has a unique self-emulsifying drug-delivery system (SEDDS).21 Combining hydrophilic and lipophilic components in SEDDS promotes absorption by the intestinal lymphatics to reduce first-pass hepatic metabolism. Also, absorption can occur with a typical meal rather than having to ingest a high-fat meal. With this unique delivery system, one study demonstrated adequate serum testosterone concentrations after dosing with oral testosterone undecanoate 200 mg twice daily.23
Capsules are available in the following dose strengths: 158 mg, 198 mg, and 237 mg.24 The recommended starting dose regimen is 237 mg by mouth in the morning and in the evening, with meals. After at least 7 days of initiation, serum testosterone should be drawn 6 hours after the morning dose. The clinician should adjust the dose regimen based on the serum testosterone concentration at that time, with the minimum and maximum dose regimen of 158 mg twice daily and 396 mg twice daily (198 mg strength times two capsules, taken twice daily).
Contraindications, warnings, and precautions with this medication are similar to those of the medications discussed previously. Adverse reactions, which occurred in at least 2% of patients during clinical trials, include polycythemia, dyspepsia, increased hematocrit, diarrhea, eructation, peripheral edema, nausea, headache, prostatomegaly, and hypertension.24 Drug interactions are similar to those discussed for autoinjectable testosterone enanthate. Regarding the potential of cardiovascular disease (CVD) and prostate cancer, long-term safety data are insufficient for assessing the risks.
Advantages of oral testosterone undecanoate would foremost be the ease of dosing this formulation. Due to the SEDDS component of this oral medication, patients can take it with a regular meal and not have to eat a high-fat meal for optimal absorption. Also, bypassing the liver should allow this medication to not lead to hepatotoxicity. Disadvantages include twice-daily dosing, as opposed to gels and patches that are once daily and injections that range from weekly to every 10 weeks (i.e., testosterone undecanoate injection). Clinicians will need to monitor for increased LDL and decreased HDL concentrations. Also, pricing will limit consumption of this medication, at approximately $900 to $1,000 for a 30-day supply. Finally, the lack of indication for ARH limits use of oral testosterone undecanoate for a major group needing TRT.
Testosterone Undecanoate (Tlando)
Oral testosterone undecanoate is the newest in the pipeline. As stated previously, the FDA rejected the first NDA for the second potential oral formulation of testosterone undecanoate (Antares Pharma). Concerns for increased blood pressure findings during clinical studies caused the initial rejection. Also, the FDA determined that the phase III trial failed to meet all three secondary end points and used an impractical titration regimen.25 The company conducted two new phase III trials and submitted another NDA. The primary efficacy end -point was met with a 225mg, twice-daily regimen without the need for titration.26 An extended-release version for once daily is currently in trials. The FDA granted approval for men with a deficiency or absence of endogenous testosterone on March 29, 2022.
The FDA will require a black box warning for possible cardiovascular risk due to increases in blood pressure, similar to the first FDA-approved oral testosterone undecanoate. Due to this increased risk, this second FDA-approved oral testosterone undecanoate should not be used to treat patients with ARH. It is only approved for treatment of men with hypogonadism associated with structural or genetic etiologies. Contraindications, warnings, and precautions with this medication are similar to those of the medications discussed previously. Adverse reactions, which occurred in at least 2% of patients during clinical trials, include blood prolactin, hypertension, increased hematocrit, upper respiratory tract infection, increase weight, headache, and musculoskeletal pain.
The recommended dosage is two 112.5-mg capsules (total dosage of 225 mg) taken twice daily, once in the morning and once in the evening. It will be available as a 112.5-mg capsule and will not be substitutable with other oral testosterone undecanoate products.
Advantages include ease of dosing this formulation and that dose titration will not be necessary. Disadvantages include twice-daily dosing, increased blood pressure that may lead to cardiovascular risk, and that it is not indicated for use in men with ARH.
THERAPY MANAGEMENT/PLACE IN THERAPY
Clinicians have a variety of tools for treatment of hypogonadism caused by TD. Management presents as a complexity due to the plethora of medication options, but clinicians must also consider many concerns and gray areas of therapy prior to starting and continuing treatment with TRT. First, nonmedication concerns should be addressed.
Does the Patient Need Treatment?
While several guidelines now exist regarding treatment of hypogonadism, the provider for the patient must diagnose this disease state definitively.1,3,27 Primary and secondary hypogonadism should be treated; pros and cons should be weighed first for ARH. For men with erectile dysfunction, TRT is inappropriate. They should be prescribed phosphodiesterase inhibitors (i.e., sildenafil). True hypogonadism presents with both symptoms of sexual dysfunction and low serum testosterone concentrations. Medication therapy is helpful for patients with sexual dysfunction, but the evidence is either mixed or shows little benefit for symptoms such as decreased lean body mass, low energy, or cognitive impairment.27 Also, testosterone concentrations below 300 ng/dL may not need TRT if symptoms are not also evident. This is why diagnostic criteria are clear that a patient presenting with symptoms should have two separate morning serum testosterone concentrations below the therapeutic range.
Of interest, the American College of Physicians (ACP) guidelines on treating men with ARH suggest that clinicians should have a discussion with patients regarding potential benefits, harms, costs, and patient preference.27 Clinicians should not initiate TRT to improve energy, vitality, physical function, or cognition—only to improve sexual dysfunction in this subgroup of men. If TRT is initiated for a man with ARH, symptoms should be reevaluated within a year and periodically thereafter for efficacy. Clinicians should discontinue treatment if sexual function is not improved.
Ruling Out Contraindications and Considering Possible Increased Cardiovascular Risk
Contraindications have been discussed, such as current breast cancer and known or suspected prostate cancer. However, TRT may be avoided for those with ARH (no medications are approved for this indication, and the two newest FDA-approved medications have a contraindication for this diagnosis) with prior prostate cancer or significant CVD, unless the benefits clearly outweigh the risks. Data are still unclear 7 years after the FDA issued a safety warning regarding the possible increased risk of heart attack and stroke in men with CVD.28 Observational and randomized studies have produced conflicting cardiovascular data, and meta-analyses have been inconclusive.28 Randomized, controlled trials should be targeted with this objective before guidelines can clarify when to use TRT in patients with current or history of CVD. At this time, it may be prudent to avoid TRT if the patient has had a recent CVD event. Finally, pharmacists should counsel patients that TRT is linked to many other problems, such as BPH, infertility, clots, and high red blood cell counts.
How to Choose Medication Therapy
Providers and patients should have a shared decision-making approach that considers patient preference, functionality, treatment burden, cost, dosing interval, and side-effect profiles when choosing medications.7 Patient adherence represents a critical factor to successful implementation of TRT. Hepatotoxicity has historically caused oral testosterone (i.e., methyltestosterone) to be withdrawn from the market. The new oral testosterone undecanoate formulations (i.e., Jatenzo and soon-to-be fully FDA-approved Tlando) may offer a preferred choice by patients, but presently high cost and twice daily dosing serve as limitations. Also, the current FDA-approved oral medication on the market has a black box warning that it may only be prescribed for men with hypogonadal conditions associated with structural or genetic etiologies. This is due to the possible increased CVD, which may make this medication untenable for men with ARH. While not an oral formulation, a buccal medication was available (i.e., Striant, Endo Pharmaceuticals). This consisted of placement of a bioadhesive along the gum line but has been discontinued by the manufacturer.
Patients widely accept transdermal therapies, including patches and gels, due to the lipophilicity of testosterone. Cost is an issue, though, due to the need for daily dosing, as well as the risk of skin irritation (e.g., patches) and transference (e.g., gels). This latter condition results from nonabsorbed testosterone being transferred to children and spouses of men not using the gels appropriately. Historically, the transdermal therapies, especially gels, have not been covered well by insurance; however, this has been less of a problem over the past few years. While not a transdermal therapy, the nasal gel represents a unique TRT medication. It avoids transference problems and firstpass effects, but use may be limited due to three times daily dosing and cost. Of interest, the clinical guideline from the ACP suggests that transdermal therapies should not be considered as initial therapy for men with ARH.27 Rather, intramuscular TRT should be considered due to lower cost and equal efficacy and harms.
Esterified formulations, including cypionate, enanthate, and undecanoate, represent the injectable testosterone therapies. The span of time for injections ranges from once weekly to once every 10 to 14 weeks. While cost is generally less of an issue with injectable TRT, IM injection can lead to irritation at the site and mood lability. Treatment often leads to sub- and supraphysiologic testosterone concentrations. Vial formulations of cypionate and enanthate, given every 2 to 4 weeks, may yield such fluctuations with up to half the time below targeted concentrations.7 Mood swings, decreased libido, and fatigue may result. Also, supraphysiologic concentrations may lead to elevated hematocrit. Testosterone undecanoate injection (i.e., Aveed) has an advantage over the cypionate and enanthate vial formulations that injection is at baseline, 4 weeks later, and then every 10 weeks thereafter. However, injections must be administered in a clinic or office due to potential for POME, and cost is an issue, as it is for the novel injectable formulations. Testosterone enanthate autoinjector (i.e., Xyosted) has advantages inherent with autoinjection. A significant limitation, however, is the current black box warning against dispensing to patients with ARH due to possible increased cardiovascular risk.
Finally, subdermal pellets containing testosterone that elutes slowly can be implanted SC in the office under local anesthesia. While not often used, this formulation is long-acting, convenient, and produces a consistent serum testosterone concentration in the normal range.7 Dosing can be as long as every 3 to 6 months. However, skin irritation and infection can occur, with an expense not often covered by insurance. An ongoing trial is investigating whether a generic version has equal efficacy and safety as the current subdermal pellet FDA-approved (i.e., Testopel). If successful, this has the potential to offer a lower-cost alternative.
Follow-Up Considerations
In general for all medications, sexual desire should improve after 3 weeks and then reach a plateau within 6 weeks.9 Correction of erectile dysfunction associated with low testosterone concentrations may take up to 6 months. If, after 6 months of normal testosterone concentration restoration, men do not exhibit associated better sexual symptoms, a risk-versus benefit discussion should ensue. The goal of normalizing testosterone concentrations should be in the mid-normal range to prevent sub- and supratherapeutic dosing consequences. If not previously described for certain medications, clinicians should monitor serum testosterone concentrations after the initiation of TRT at 3, 6, and 12 months, and then yearly thereafter.
Monitoring for specific adverse events with each medication has been previously described. For all TRT, clinicians should monitor additionally for hematocrit, prostate safety, bone mineral density, and sleep apnea.9 After TRT initiation, hematocrit should be checked at the same intervals as serum testosterone concentrations, maintaining hematocrit at <54% at all times. Hematocrit above 54% is often associated with development of blood clots. Prostate monitoring, historically, has included obtaining a PSA concentration and digital rectal examination. Monitoring should occur within 3 to 12 months after TRT initiation.9 After a year of normal-range testosterone concentrations, the American Urological Association suggests monitoring to follow prostate-cancer screening guidelines.3 In men with abnormal bone mineral density at baseline, follow-up monitoring should occur after 1 to 2 years of TRT initiation. Pharmacists should counsel patients with sleep apnea that TRT may transiently worsen the severity.
CONCLUSION
While the treatment of hypogonadism still has several unknowns, a growing development of novel therapies gives the potential of having even more effective choices. In the future, successful novel treatments for this disease state will be of low cost, easily administered by the patient, and a low risk versus benefit. Pharmacists are at the forefront of counseling patients effectively with this disease state and must be attentive to individualized needs to manage this disease state well.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
- Bhasin S, Brito JP, Cunningham GR, et al. Testosterone therapy in men with hypogonadism: an Endocrine Society clinical practice guide line. J Clin Endocrinol Metab. 2018;103(5):1715-1744.
- Mohamed O, Freundlich RE, Dakik HK, et al. The quantitative ADAM questionnaire. Int J Impot Res. 2010;22(1):20-24.
- Mulhall JP, Trost LW, Brannigan RE, et al. Evaluation and manage ment of testosterone deficiency: AUA guideline. J Urol. 2018;200:423.
- Weigh risks and benefits of testosterone for “low T.” Pharm Lett. March 2020;360308.
- Drug Information Group. College of Pharmacy–Chicago. What is the controversy surrounding testosterone replacement therapy? https:// dig.pharmacy.uic.edu/faqs/2020-2/july-2020-faqs/what-is-the-contro versy-surrounding-testosterone-replacement-therapy/. Accessed March 21, 2022.
- Dunleavy BP. Fewer men receive testosterone replacement therapy after FDA warnings. UPI.com. September 29, 2021. www.upi.com/ Health_News/2021/09/29/testosterone-therapy-use-heart-disease-risk study/3351632925835/. Accessed March 22, 2022.
- Kresch E, Patel M, Lima TFN, Ramasamy R. An update on the available and emerging pharmacotherapy for adults with testosterone deficiency available in the USA. Expert Opin Pharmacother. 2021;22(13):1761-1771.
- Carrasquillo R, Chu K, Ramasamy R. Novel therapy for male hypo gonadism. Curr Urol Rep. 2018;19:63.
- Barbonetti A, D’Andrea S, Francavilla S. Testosterone replacement therapy. Andrology. 2020;8:1551-1566.
- Natesto [package insert]. Dublin, Ireland: Endo International; 2016.
- Rogol AD, Westfield G, Guidry M, et al. Seasonal allergies do not significantly impact the absorption of Natesto® (testosterone) nasal gel, in hypogonadal men. Endocrine Rev. 2017;38(3):8-10.
- Gronski MA, Greber ED, Gottesman IS, et al. Efficacy of nasal tes tosterone gel (Natesto®) stratified by baseline endogenous testosterone levels. J Endocr Soc. 2019;3(9):1652-1662.
- Masterson T, Molina M, Ibrahim E, Ramasamy R. Natesto effects on reproductive hormones and semen parameters. Eur Urol Focus. 2018;4(3):333-335.
- Brooks M. FDA clears first autoinjectable testosterone, Xyosted. October 2, 2018. www.medscape.com/viewarticle/902818_. Accessed March 2, 2022.
- Xyosted [package insert]. Ewing Township, NJ: Antares Pharma, Inc; 2019.
- Busko M. FDA rejects testosterone autoinjector Xyosted. October 23, 2017. www. medscape.com/viewarticle/887436_. Accessed March 14, 2022.
- Rothrock JF, Freitag FG, Farr SJ, Smith EF 3rd. A review of needle-free sumatriptan injection for rapid control of migraine. Headache. 2013;53(suppl 2):21-33.
- Edel Y, Sagy I, Pokroy-Shapira E, et al. A cross-sectional survey on the preference of patients with rheumatoid arthritis for route of administration of disease-modifying anti-rheumatic drugs. Isr Med Assoc J. 2020;22:154-159.
- Sanchez Martinez I, Cerdan Sanchez M, Lopez Roman J, et al. Possible influence on the route of treatment administration on treatment adherence in patients with multiple sclerosis. Clin Ther. 2020;42:e87-e99.
- Shoskes JJ, Wilson MK, Spinner ML. Pharmacology of testosterone replacement therapy preparations. Transl Androl Urol. 2016;5:834-843.
- Patel M, Muthigi A, Ramasamy R. Jatenzo: challenges in the development of oral testosterone. Int J Impot Res. August 5, 2021.
- Ault A. FDA panel votes down oral testosterone replacement prod ucts. January 11, 2018. https://www.medscape.com/viewarticle/891186. Accessed March 3, 2022.
- Yin AY, Htun M, Swerdloff RS, et al. Reexamination of pharmaco kinetics of oral testosterone undecanoate in hypogonadal men with a new self-emulsifying formulation. J Androl. 2021;33:190-201.
- Jatenzo [package insert]. Northbrook, IL: Clarus Therapeutics; 2019.
- Monaco K. FDA panel: two thumbs down for new oral testoster one drugs. MedPage Today. January 10, 2018. www.medpagetoday. com/endocrinology/generalendocrinology/70432. Accessed March 7, 2022.
- DelConte A, Papangkorn K, Kim K, et al. A new oral testosterone (TLANDO) treatment regimen without dose titration requirement for male hypogonadism. Andrology. 2022:10(4):669-676.
- Qaseem A, Horwitch CA, Vijan S, et al. Testosterone treatment in adult men with age-related low testosterone: a clinical guideline from the American College of Physicians. Ann Intern Med. 2020;172:126 133.
- Kaur H, Werstuck GH. The effect of testosterone on cardiovascu lar disease and cardiovascular risk factors in men. CJC Open. 2021;3(10):1238-1248.
- Comparison of testosterone products. Pharm Lett. March 2020.