Optimizing Polycystic Ovary Syndrome Outcomes
RELEASE DATE
September 1, 2021
EXPIRATION DATE
September 30, 2023
FACULTY
Ashley Barlow, PharmD
PGY2 Oncology Pharmacy Resident
The University of Texas MD Anderson Cancer Center
Houston, Texas
Brooke Barlow, PharmD
Neuromedicine ICU Pharmacy Specialist
UF Health Shands Hospital
Gainesville, Florida
FACULTY DISCLOSURE STATEMENTS
Drs. Barlow have no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT
 Pharmacy
Pharmacy
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-21-100-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients’ conditions and possible contraindications or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
GOAL
To review the pathophysiology, diagnosis, and treatment options for women with polycystic ovary syndrome (PCOS).
OBJECTIVES
After completing this activity, the participant should be able to:
- Discuss the pathophysiology of PCOS.
- Describe the signs, symptoms, and complications of PCOS.
- Review the supporting literature for pharmacologic treatment approaches for PCOS with a focus on metformin, contraceptives, and ovulation-inducing agents.
- Review the adverse effects of pharmacologic agents used for PCOS and patient-management considerations.
ABSTRACT: Polycystic ovary syndrome (PCOS) is a heterogeneous endocrine disorder that primarily affects women of reproductive age. PCOS is also a very complex disorder, and although it is common, many women remain undiagnosed. The syndrome is characterized by a defect in the hypothalamic-pituitary-ovarian axis that results in a triad of hyperandrogenism, anovulation, and ovarian cysts that carries a myriad of metabolic and reproductive consequences. Symptoms of PCOS manifest as obesity, insulin resistance resulting in acanthosis nigricans, acne, hirsutism, and infertility; PCOS can also result in significant patient morbidity, such as cardiovascular diseases and type 2 diabetes mellitus. The goals of pharmacologic and nonpharmacologic management are aimed at restoring ovulation, alleviating symptoms, and improving quality of life. Pharmacologic treatments are targeted at improving insulin resistance with agents such as metformin, regulating hormonal disturbances with oral contraceptives, or managing infertility with ovulation agents. Pharmacists play a critical role in the management of patients with PCOS, and this article aims at providing a comprehensive review of the syndrome, its presentation, diagnosis, and key management strategies to optimize patient outcomes.
Polycystic ovary syndrome (PCOS) is one of the most common causes of infertility that affects women of reproductive age.1,2 PCOS is a heterogeneous endocrine disorder characterized by the triad of hyperandrogenism, anovulation, and varying sizes of ovarian cysts. Disturbances in the hypothalamic-pituitary-ovarian (HPO) axis result in alterations of reproductive hormones, including gonadotropin-releasing hormone (GnRH), luteinizing hormone (LH), follicle-stimulating hormone (FSH), estrogen, and testosterone, causing disruptions in the normal menstrual cycle and resulting in oligomenorrhea (abnormal menstrual cycle) or amenorrhea (loss of menstrual cycle).1,3 According to the CDC, as many as 5 million women are affected by PCOS in the United States and 3.4% of women worldwide.4 While often diagnosed in women younger than age 30 years, PCOS is a lifelong condition that can bear significant consequences far beyond childbearing years.5 Additionally, while management tends to focus on treatment of infertility, PCOS can adversely affect metabolic and cardiovascular health, such as atherosclerotic disease.6
The development of obesity and metabolic syndrome among women with PCOS further heightens the risk of secondary sequelae, including a 2.26-fold increased risk of obstructive sleep apnea.7 Women with PCOS also experience higher rates of miscarriage; they are three times as likely to miscarry in the early months of pregnancy than are women without PCOS.8 Thus, the comprehensive care of these women involving pharmacologic and nonpharmacologic interventions that address the endocrine, metabolic, and cardiovascular manifestation of PCOS is critical to improve long-term health outcomes.9
PATHOPHYSIOLOGY
The best understanding of the pathophysiology of PCOS is knowledge that the disease is multifactorial, with a careful interplay between uncontrolled ovarian steroidogenesis, insulin resistance, and oxidative stress. Hyperandrogenism, evidenced by a raised level of serum-free testosterone, is the key hormonal abnormality that contributes to the development of polycystic ovaries.3,10,11 Excessive androgen secretion can result from excessive release of LH by the anterior pituitary, hyperinsulinemia, or reduced levels of sex hormone–binding globulin (SHBG), resulting in increased free-serum androgens. Decreased insulin sensitivity occurs independent of obesity or preexisting diabetes and is secondary to defective receptor binding through alterations in gene expression and oxidative damage.11 Elevated insulin can either be the cause of or contribute to the HPO axis dysregulation, as hyperinsulinemia potentiates GnRH release, which stimulates excess LH over FSH secretion.12 The elevated LH/FSH ratio causes ovarian androgen synthesis to dominate, resulting in decreased follicular maturation and reduced SHBG binding.12,13 Hyperinsulinemia and androgen excess can contribute to obesity, wherein excess adipose tissue that contains aromatase increases production of androgens.11 The complex positive feedback loop potentiated by hyperinsulinemia, hyperandrogenism, and oxidative damage is the key pathophysiologic mechanism underlying PCOS.
RISK FACTORS
PCOS most commonly develops during adolescence in women with high risk for comorbidities of metabolic syndrome, such as obesity, type 2 diabetes, hypertension, dyslipidemia, and cardiovascular disease. Insulin resistance affects 50% to 70% of women with PCOS and is only exacerbated in those with preexisting, uncontrolled diabetes.1,3 Mental health disorders, such as depression, anxiety, bipolar disorder, or binge-eating disorders, also occur more frequently in women with PCOS.13,14 Stress, unhealthy lifestyle, and poor nutrition have been identified as modifiable risk factors. Family history of PCOS heightens a woman’s risk, with evolving research suggesting genetic factors such as polymorphisms in single-nucleotide polymorphisms that result in defective gene transcription of hormonal receptors may play a role in genetic predisposition.14
CLINICAL PRESENTATION AND DIAGNOSIS
PCOS acquired its name from the ultrasound examination of multiple ovarian cysts, which represent immature follicles. The clinical metabolic and reproductive manifestations of PCOS vary widely depending on an individual’s degree of hormonal abnormalities; however, common features include menstrual dysfunction, infertility, hirsutism, acne, and obesity.10 High levels of androgen result in masculinizing features such as acne, hirsutism, male-pattern baldness, and weight gain. Menstrual irregularities can include oligomenorrhea, amenorrhea, abnormal uterine bleeding, and infertility. Features of insulin resistance include hyperglycemia, acanthosis nigricans, hypercholesterolemia, and obesity.
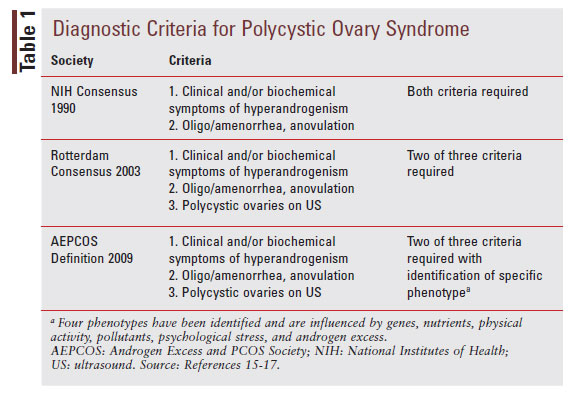
The diagnosis of PCOS may be made based on patient history, physical exam, and laboratory findings suggestive of the syndrome. Some diagnostic criteria may indicate the further need for radiographic features to confirm the diagnosis. Three diagnostic criteria exist for the identification of PCOS (TABLE 1).15-17 Each of these diagnostic tools incorporate criteria for hyperandrogenism and features of ovarian dysfunction (menstrual irregularities or radiographic findings of polycystic ovaries), but they vary in the need for radiographic imaging versus basing the diagnosis on clinical features alone. Regardless of the criteria used, it is essential to exclude other potential causes of these symptoms, including thyroid dysfunction, hyperprolactinemia, premature ovarian failure, or adrenal hyperplasia.18
TREATMENT
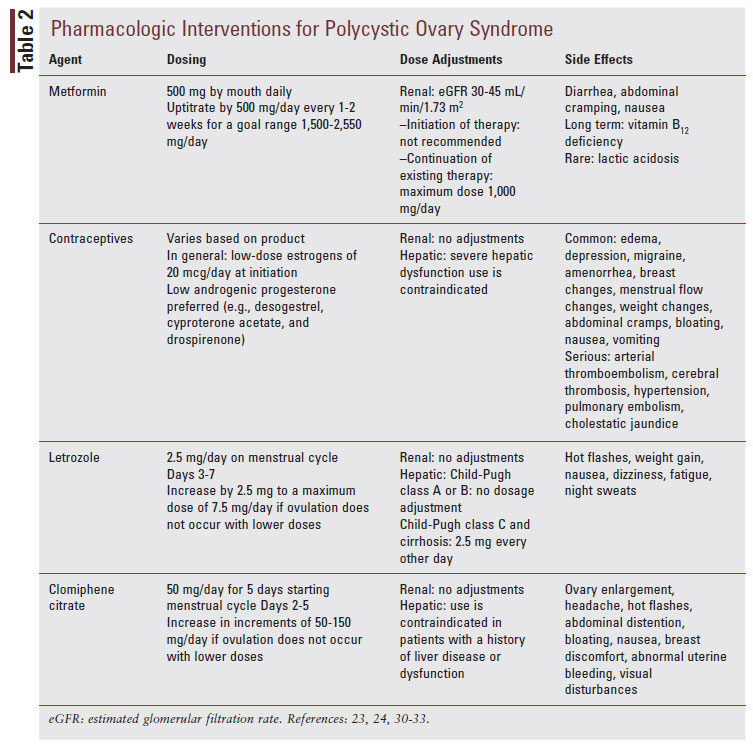
The three pillars of PCOS management include lifestyle modification, surgery, and pharmacologic interventions. The goal of this multimodal treatment approach is to alleviate discomforting manifestations associated with PCOS, increase quality of life and well-being, improve the chances of restoring fertility, and prevent long-term diseaserelated complications, such as type 2 diabetes, cardiovascular disease, and endometrial hyperplasia.18 When discussing these variable treatment options available for PCOS, important key points to determine from the patient that will guide therapeutic decision making are the desire for pregnancy, maintenance of cosmetic appearance, and the presence of concomitant metabolic abnormalities.19 See TABLE 2 for a summary of the pharmacologic interventions for PCOS.
Nonpharmacologic Interventions
All patients with PCOS benefit from lifestyle interventions and should receive counseling on weight loss, dietary modifications, exercise, and behavioral interventions.18 Increased physical activity and corresponding weight reduction can significantly improve insulin resistance, decrease androgen levels, and reduce the risk of developing type 2 diabetes. In order to empower patients to achieve these goals, pharmacists and other providers can set SMART (Specific Measurable, Achievable, Realistic, and Timely) goals.19 For example, according to current international guidelines, a 5% to 10% weight loss is considered successful weight loss within 6 months of starting to actively lose weight. For exercise planning for adults aged 18 to 64 years, a minimum of 150 min/week of moderate intensity physical activity or 75 min/ week of vigorous intensity should be recommended to improve metabolic performance and overall strength.19 The guidelines provide recommendations for dietary modifications; however, no single diet plan is currently recommended for patients with PCOS. In addition to lifestyle modifications, behavioral counseling is recommended to appropriately address the psychologic stressors associated with PCOS manifestations.19 Pharmacists can reference these guidelines and develop SMART goals for all pillars of the lifestyle interventions that benefit PCOS tailored to each patient, her goals, access, and personal preferences.
Metformin
In order to achieve metabolic control and prevent long-term metabolic complications, pharmacologic intervention with metformin, an agent approved by the FDA for type 2 diabetes, is most commonly used as an off-label therapy.19 The guidelines recommend initiating metformin in addition to lifestyle modifications for women with PCOS to aid in lowering BMI, optimize hormonal manifestations, and improve metabolic complications.19 Metformin acts as an insulin-sensitizing agent for women with PCOS by decreasing hepatic glucose production through inhibition of gluconeogenesis and increased insulinmediated glucose uptake in the liver, visceral fat, and muscles. In turn, this can influence the androgen excess observed in PCOS, as metformin has been shown to reduce circulating androgens and increase SHBG synthesis, which overall decreases the freeandrogen fraction.20 Not all women obtain a clinically meaningful benefit from metformin; its greatest effect is observed in women with high-risk metabolic features such as risk factors for diabetes and impaired glucose tolerance.19 Consistent with its pharmacologic mechanisms, several large metaanalyses have demonstrated that metformin initiation results in improved menstrual cycles and ovulation rates in most women with PCOS.21,22
These data resulted in widespread use implementation of metformin in women with PCOS regardless of current glucose parameters in an effort to improve menses and ovulatory performance. Unfortunately, there is no standardized dosing regimen of metformin in PCOS, and a variety of regimens have been used in the currently available literature, with variable target dose ranging from 1,500 to 2,550 mg/ day.20 It is known that metformin at a higher dose of 1,700 to 2,000 mg/day is associated with a greater improvement of BMI.19,23
The side-effect profile of metformin in women with PCOS is in line with the side effects seen in patients treated for type 2 diabetes, namely gastrointestinal distress resulting in diarrhea and nausea.23,24 Gastrointestinal side effects are self-limiting and dose dependent. These side effects can be minimized by starting metformin at a lower dose of 500 mg with uptitration by 500 mg weekly or every other week, based on tolerance and/or the use of extended-release preparations.24 Long-term use of metformin is associated with a deficiency in vitamin B12, and therefore patients should be monitored accordingly depending on the duration of therapy.24
More severe side effects include lactic acidosis in those with renal dysfunction, heart failure, or liver failure, and use should be avoided in patients with compromised end-organ function or creatinine clearance <45 mL/min for initiation of therapy or <30 mL/min for continuation of therapy.24 Use is generally off-label, and health professionals need to inform women and discuss the evidence, possible concerns, and side effects.
Contraceptives
For women with significant manifestations of hyperandrogenism and/or irregular menstrual cycles, combined oral contraceptive pills (COCPs) are an effective treatment option. COCPs improve menstrual irregularity and are effective when the treatment of hirsutism or acne is needed. The mechanism by which COCPs elicit their pharmacologic effects is related to halting the overproduction of endogenous androgens. It is important to emphasize that both the estrogen and progesterone components of COCPs play an important role in the pharmacologic effect. The estrogen component promotes hepatic synthesis of SHBG, which subsequently reduces circulating concentrations of free testosterone available to bind to the androgen receptor.25 The progesterone component acts as a negative regulator of LH, thereby reducing the LH surge and production of androgens from the ovaries.25 In addition, it is suggested that some formulation of progestogens have a direct antagonistic effect on the androgen receptor and blunt the activity of 5-alpha reductase, an enzyme that converts testosterone to the highly potent androgen dihydrotestosterone.26
Many formulations, generations, and combinations of COCPs are available to treat PCOS. Unfortunately, there is no specific recommendation provided in current guidelines for which product or combination is most appropriate for patients with certain PCOS manifestations.19 In terms of estrogen dosing, there is a significant amount of controversy and variety among international guidelines for PCOS due to the risks and relative benefits of estrogen therapy. Estrogens are well known to result in thromboembolic and adverse cardiovascular side effects. It is prudent to take this risk into consideration in women with PCOS due to their baseline cardiovascular risk and the common comorbid conditions, such as obesity, that increase the risk of thromboembolic events. In addition, women with PCOS experience high rates of hypertension associated with the cardiovascular sequelae, with the risk and severity increased with estrogen-containing contraceptives.25
The international PCOS guidelines state that the lowest effective dose of ethinyl estradiol should be used that is able to control symptoms and that dosages of ethynyl estradiol of >35 mcg should not be considered first line due to the undue risk of serious adverse venous thromboembolic events.19 If these high doses are needed for women to achieve optimal suppression of ovarian androgens and management of hyperandrogenic features, additional monitoring for thromboembolic events or progression of cardiovascular disorders to optimize safety is recommended. Unfortunately, nonoral estrogens, such as the transdermal or vaginal ring preparations, have not been well studied for the management of hirsutism and cannot be recommended at this time.19
Another consideration for deciding on the appropriate COCP is the progesterone component. All progesterones have varying androgenic properties, and given that PCOS is driven by excessive androgens, a progesterone with the least amount of androgenic activity is preferred. Progestins with lower androgenic activity include desogestrel, cyproterone acetate, and drospirenone; however, these preparations have also been associated with a potential for a higher risk of venous thromboembolism.19,25 In terms of duration of COCP therapy, most studies investigating the potential efficacy of these therapies for hirsutism lasted 6 to 12 months.19 Although long-term studies show the safety of COCP for contraceptive purposes, for PCOS it is unclear how long COCPs should be continued.
Other important side effects to consider with COCPs include migraines, weight gain, breast tenderness, irritability, mood changes, fluid retention, and changes in appetite. Certain side effects such as weight gain can be very distressing in women with preexisting weight challenges, and therefore choosing a preparation with a progesterone component with the least amount of androgen activity is prudent. Patients should be screened for relative and absolute contraindications to COCPs set forth by the World Health Organization (WHO), such as smoking history, venous thromboembolism risk, stroke history, uncontrolled hypertension, lipid abnormalities, and gallbladder issues.27 Most side effects can be influenced by the progesterone or estrogen component, so modifying a patient’s doses or formulation will help improve compliance and tolerability of treatment.
When contraception is not necessary (i.e., if a patient desires to become pregnant in the near future or is not sexually active), menstrual irregularity alone can be managed with cyclical medroxyprogesterone acetate. Medroxyprogesterone acetate is dosed at 5 to 10 mg/day for 10 to 14 days every 1 to 2 months.28 This can be offered for protection against endometrial cancer or when there is a desire to have fewer menstrual cycles, preference for a nonoral therapy, or contraindication to estrogens due to the side effects mentioned above.28
Ovulation Agents
The use of induction ovulation agents improves the chances of fertility in women with PCOS. The most common agents include letrozole and clomiphene citrate, with or without metformin.19,29 Clomiphene citrate has gained FDA approval for ovulation induction, whereas letrozole and metformin use are off-label.24,30,31 Letrozole is a nonsteroidal aromatase inhibitor, which is the enzyme responsible for catalyzing the conversion of androgens to estrogens.30 By inhibiting the formation of excessive plasma estrogen, there is a break on the negative feedback loop and subsequent increase in FSH release.32 Clomiphene citrate is a selective estrogen receptor modulator that acts as an antiestrogen. More specifically, clomiphene citrate competitively inhibits the binding of estradiol to its receptors in the hypothalamus and pituitary, which blocks the negative feedback effect of endogenous estrogens, such as estradiol. The inhibition of negative feedback results in an increased secretion of GnRH, as well as FSH and LH. The increase in FSH ultimately stimulates follicular growth and mid-cycle LH surge, which results in successful ovulation.33
Although clomiphene citrate was long considered the recommended therapy for ovulation induction for women with PCOS, contemporary data suggest that letrozole is superior to clomiphene citrate in improving the rates of live births in oligoovulatory women with PCOS.34 In addition, letrozole reduces the frequency of multiple pregnancies and has an improved tolerability profile.19 Therefore, according to the international guidelines and the American College of Obstetrics and Gynecology, letrozole is considered a first-line agent for ovulation induction for women with PCOS and anovulatory infertility with no other infertility factors to improve ovulation, pregnancy, and live birth rates.19,29 Clomiphene citrate is an alternative in the setting of contraindications to letrozole or if letrozole is deemed to be ineffective at ovulation induction at maximal dose.19
For dosing these treatments, the schedule and timing is based on the phase of the menstrual cycle to align with a period of natural ovulatory cycle. Letrozole is initiated at a starting dose of 2.5 mg administered on menstrual cycle Days 3 to 7.19,32 The dose can be increased by 2.5-mg increments to a maximum dose of 7.5 mg/day if ovulation has not occurred with lower doses. Dosing for clomiphene citrate usually starts at 50 mg/day for 5 days starting menstrual cycle Days 2 to 5.31 The dosage can be increased to 150 mg/day. If ovulation cannot be achieved with these high doses, then a resistance to clomiphene citrate is suggested and metformin can be added. Studies suggest that pretreatment with metformin for 1 month may be able to resensitize the ovaries to clomiphene and that metformin was able to significantly improve the ovulatory response with clomiphene citrate.31 If after six ovulatory cycles pregnancy is not achieved, clomiphene failure is suggested, and alternative strategies are recommended.32
In terms of adverse effects, it appears that letrozole has an improved tolerability profile compared with clomiphene citrate. In a pivotal trial comparing clomiphene and letrozole, 33% of women receiving clomiphene experienced hot flashes, while fatigue and dizziness occurred in 22% and 12% of women taking letrozole, respectively.34 Contrary to the side effects observed in women receiving long-term aromatase inhibitors for breast cancer, letrozole is associated with mild side effects such as nausea and abdominal discomfort when used short term for ovulation induction without the occurrence of musculoskeletal disturbances.34 One unique consideration with regards to side effects of ovulation induction agents includes the risk of teratogenicity. Both clomiphene and letrozole portend fetal harm and are category X during pregnancy.30,31 However, one major benefit to the use of letrozole is that it has a shorter half-life of only approximately 48 hours compared with the 2-week half-life of clomiphene, which reduces the exposure duration to a fetus.30,35
THE PHARMACIST’S ROLE
Pharmacists can take an active approach in the management of PCOS. Pharmacists are in a pivotal position to identify patients at risk and should encourage these patients to be screened for PCOS. Through patient education, expanding awareness, and continuing investigation into the complexities of this syndrome, the millions of women affected by PCOS can enhance their health and overall quality of life and possibly reduce or prevent the various associated comorbidities. Specifically, with their knowledge of the treatments, pharmacists can empower patients to choose a therapy that works best for them to maximize their quality of life while minimizing side effects. In addition, pharmacists can reference current guidelines and perform counseling strategies on how to create and target SMART goals. Since most therapies used for PCOS are offlabel treatments, it is important for both hospital and community-based clinicians to provide logic for use in PCOS for agents used off-label to help patients obtain treatments without an unmanageable cost.
Pharmacists are also one of the key providers in managing birth-control recommendations on an outpatient basis in those states that give pharmacists prescriptive authority. For women with PCOS, these patients should be referred to an appropriate specialist, as the prescription of COCPs is much more complex than regular contraceptive uses. Where pharmacists can be of assistance is in the screening of contraindications based on the WHO contraceptive guidelines and choosing a formulation with the appropriate estrogen and lowest androgenic progestin to optimize symptom control.
REFERENCES
- Azziz R, Woods KS, Reyna R, et al. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89(6):2745-2749.
- Gibson-Helm M, Teede H, Dunaif A, Dokras A. delayed diagnosis and a lack of information associated with dissatisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102(2):604-612.
- Goodarzi M. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7(4):219-231.
- CDC website. Polycystic Ovary Syndrome and Diabetes Facts. www.cdc.gov/diabetes/basics/pcos.html. Accessed July 3, 2021.
- Vidya Bharathi R, Swetha S, Neerajaa J, et al. An epidemiological survey: effect of predisposing factors for PCOS in Indian urban and rural population. Middle East Fertility Society Journal. 2017;22(4):313 316.
- Orio F, Palomba S, Cascella T, et al. Early impairment of endothelial structure and function in young normal-weight women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89(9):4588-4593.
- Kumarendran B, Sumilo D, O'Reilly MW, et al. Increased risk of obstructive sleep apnea in women with polycystic ovary syndrome: a population-based cohort study. Eur J Endocrinol. 2019;180(4):265 272.
- Boomsma CM, Fauser BC, Macklon NS. Pregnancy complications in women with polycystic ovary syndrome. Seminars in Reproductive Medicine. 2008;26(1):72-84.
- Hillman SC, Bryce C, Caleyachetty R, Dale J. Women's experiences of diagnosis and management of polycystic ovary syndrome: a mixed-methods study in general practice. Br J Gen Pract. 2020;30(694):e322-e329.
- El Hayek S, Bitar L, Hamdar LH, et al. Polycystic ovarian syndrome: an updated overview. Front Physiol. 2016;7:124.
- Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (pcos): the hypothesis of pcos as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37(5):467-520.
- Strauss JF. Some new thoughts on the pathophysiology and genetics of polycystic ovary syndrome. Ann N Y Acad. Sci. 2003;997:42- 48.
- Bulsara J, Patel P, Soni A, Acharya S. A review: brief insight into polycystic ovarian syndrome. Endocrine and Metabolic Science. 2021;3:100085.
- Ajmal N, Khan SZ, Shaikh R. Polycystic ovary syndrome (pcos) and genetic predisposition: a review article. Eur J Obstet Gynecol Reprod Biol X. 2019;3:100060.
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group, authors. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19-25.
- Azziz R, Carmina E, Dewailly D, et al. Task Force on the Phenotype of the Polycystic Ovary Syndrome of The Androgen Excess and PCOS Society. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456-488.
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GR, eds. Polycystic Ovary Syndrome. Boston, MA: Blackwell Scientific Publications. 1992:377-384.
- Lee TT, Rausch ME. Polycystic ovarian syndrome: role of imaging in diagnosis. RadioGraphics. 2012;32(6):1643-1657.
- Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertility and Sterility. 2018;110(3):364-379.
- Pasquali R, Gambineri A. Targeting insulin sensitivity in the treatment of polycystic ovary syndrome. Expert Opin Ther Targets. 2009;13(10):1205-1226.
- Palomba S, Falbo A, Zullo F, Orio F. Evidence-based and potential benefits of metformin in the polycystic ovary syndrome: a comprehensive review. Endocr Rev. 2009;30(1):1-50.
- Tang T, Lord JM, Norman RJ, Yasmin E, Balen AH. Insulin sensitising drugs (metformin, rosiglitazone, pioglitazone, d-chiro inositol) for women with polycystic ovary syndrome, oligo amenorrhea and subfertility. Cochrane Database Syst Rev. 2010(1):CD003053.
- Fulghesu AM, Romualdi D, Di Florio C, et al. Is there a dose response relationship of metformin treatment in patients with polycystic ovary syndrome? Results from a multicentric study. Hum Reprod. 2012;27(10):3057-3066.
- Glucophage (metformin) [prescribing information]. Princeton, NJ: Bristol-Myers Squibb; May 2018.
- de Melo AS, Dos Reis RM, Ferriani RA, Vieira CS. Hormonal contraception in women with polycystic ovary syndrome: choices, challenges, and noncontraceptive benefits. Open Access J Contracept. 2017;8:13-823.
- Nader S, Diamanti-Kandarakis E. Polycystic ovary syndrome, oral contraceptives and metabolic issues: new perspectives and a unifying hypothesis. Hum Reprod. 2007;22(2):317-322.
- World Health Organization. Medical Eligibility Criteria for Contraceptive Use. 4th ed. Geneva: WHO; 2008.
- Bagis T, Gokcel A, Zeyneloglu HB, Tarim E, Kilicdag EB, Haydardedeoglu B. The effects of short-term medroxyprogesterone acetate and micronized progesterone on glucose metabolism and lipid profiles in patients with polycystic ovary syndrome: a prospective randomized study. The Journal of Clinical Endocrinology & Metabolism. 2002;87(10):4536-4540.
- American College of Obstetrics and Gynecology practice bulletin no. 194: polycystic ovary syndrome: correction. Obstetrics & Gynecology. 2020;136(3):638-638.
- Femara (letrozole) [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; April 2018.
- Clomiphene [prescribing information]. Chestnut Ridge, NY: Par Pharmaceutical; June 2016.
- Guang H, Li F, Shi J. Letrozole for patients with polycystic ovary syndrome. Medicine (Baltimore). 2018;97(44):e13038.
- Adashi EY. Clomiphene citrate: mechanism(S) and site(S) of action—a hypothesis revisited. Fertil Steril. 1984;42(3):331-344.
- Legro RS, Brzyski RG, Diamond MP, et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371(2):119-129.
- Nestler JE, Jakubowicz DJ, Evans WS, et al. Effects of metformin on spontaneous and clomiphene-induced ovulation in the polycystic ovary syndrome. N Engl J Med. 1998;338:1876-1880.