Options for Managing
Treatment-Resistant Depression
RELEASE DATE:
November 1, 2019
EXPIRATION DATE:
November 30, 2021
FACULTY:
Kimberly E. Ng, PharmD, BCPS
Assistant Professor
St. John’s University
College of Pharmacy and Health Sciences
Department of Clinical Health Professions
Queens, New York
Farah Khorassani, PharmD, BCPS, BCPP
Assistant Clinical Professor
St. John’s University
College of Pharmacy and Health Sciences
Department of Clinical Health Professions
Queens, New York
FACULTY DISCLOSURE STATEMENTS
Drs. Ng and Khorassani have no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT:
Pharmacy
 Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-19-122-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE:
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER:
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients’ conditions and possible contraindications or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
GOAL:
To educate pharmacists on the management of treatment-resistant depression (TRD) and newly approved options for therapy.
OBJECTIVES:
After completing this activity, the participant should be able to:
- Summarize current therapy options for TRD.
- Describe new therapy options for TRD.
- Describe nonpharmacologic options for TRD.
- Identify the role of the pharmacist in TRD.
ABSTRACT: Depression affects 16 million Americans each year, with half of patients experiencing inadequate response to monotherapy with antidepressants. Up to 20% of people may suffer from chronic depression despite treatment. Treatment-resistant depression (TRD) can negatively impact an individual’s activities of daily living. Pharmacologic approaches for the management of TRD include optimizing current treatment with correct dosage and agent selection, switching therapy, and augmentation.
According to the CDC, depression affects one out of every six adults at some point in their life.1,2 About 16 million American adults are impacted by depression each year, mainly as a component of major depressive disorder (MDD). Women are almost twice as likely as men to have depression, and 80% of adults with depression experience difficulty in their daily activities.2 Due to the stigma surrounding mental-health disorders, many patients do not seek help or their care is substandard. Only 30% of patients who receive adequate treatment will achieve full recovery or remission.2
The Diagnostic and Statistical Manual of Mental Disorders, 5th Edition, characterizes MDD as the occurrence for at least 2 weeks of depressed mood and loss of interest in activities that were rather pleasurable in the past.3 These symptoms also must be accompanied by at least four of the following: changes in appetite or weight; sleep patterns; altered psychomotor activity; feelings of worthlessness or guilt; difficulty concentrating or making decisions; and recurrent thoughts of death or suicidal ideation. These signs and symptoms of depression result in significant stress and impaired social and occupational performance.4
According to the Agency for Healthcare Research and Quality (AHRQ), there is no consensus definition for treatment-resistant depression (TRD).2 The AHRQ conducted a literature review to identify a definition and found four basic definitions. The most common TRD definition for MDD required a minimum of two prior treatment failures and confirmation of prior adequate dose and duration. The TRD definition for bipolar disorder required one prior treatment failure. For all definitions, no consensus defined the adequacy of either dose or duration.2 Treatment nonresponse is a response that is poor enough with significant residual symptoms that a change in treatment plan is called for (failure to have at least a 50% reduction in symptoms measured by a standard rating scale such as the Hamilton Depression Rating Scale [HAM-D] or the Montgomery-Åsberg Depression Rating Scale score). Treatment response is defined as a >50% reduction in symptoms.5,6 The goal in the management of TRD is to obtain remission, defined as a >75% improvement in baseline symptoms. Patients with a 25% to 50% response are referred to as partial responders. Despite adequate dose and adequate length of treatment, 12% to 20% of patients with depression are said to fulfill criteria for TRD.5,6
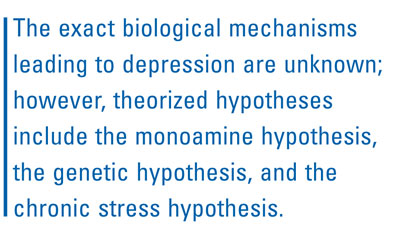 The exact biological mechanisms leading to depression are unknown; however, theorized hypotheses include the monoamine hypothesis, the genetic hypothesis, and the chronic stress hypothesis.7 The monoamine hypothesis suggests that depressive symptoms are mainly related to a deficit in the availability of norepinephrine, dopamine, or serotonin neurotransmitters, thereby explaining the mechanism of action of most antidepressant medications.
The exact biological mechanisms leading to depression are unknown; however, theorized hypotheses include the monoamine hypothesis, the genetic hypothesis, and the chronic stress hypothesis.7 The monoamine hypothesis suggests that depressive symptoms are mainly related to a deficit in the availability of norepinephrine, dopamine, or serotonin neurotransmitters, thereby explaining the mechanism of action of most antidepressant medications.
The pathophysiology of TRD is poorly understood, but it is thought to be multifactorial. Demographic factors such as female gender, older age, lower education level and economic status, and presence of life stressors have been associated with poor treatment response. Comorbid medical conditions such as endocrinopathies, immune-mediated disorders, neurologic disorders, autoimmune disorders, and psychiatric conditions increase the likelihood of poor response to treatment.6,8
MANAGEMENT STRATEGIES FOR TRD
Optimizing Existing Treatment
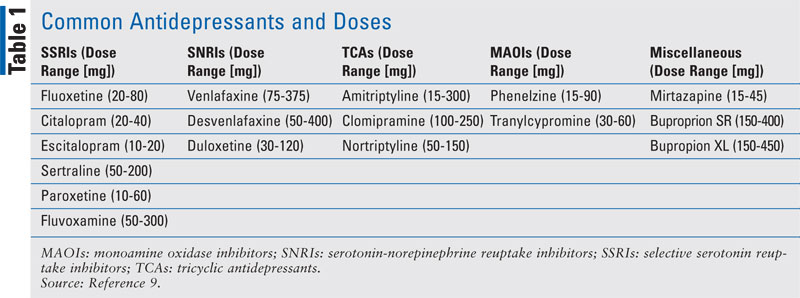
Antidepressants including selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), and monoamine oxidase inhibitors (MAOIs) are all options for TRD. Patients with TRD should first be assessed to guarantee they have received an adequate dose of medication for an adequate length of treatment.8 The definition of an adequate length of treatment may vary depending on the resource. In general, patients who fail to remit after 6 to 8 weeks of treatment at an optimized dose are considered a treatment failure. TABLE 1 lists common antidepressants classes, agents, and doses.9 If patients have prematurely discontinued past therapy or used inadequate doses, it may be appropriate to retrial medications prescribed in the past at higher doses that may be effective. If a patient is refractory to initial conventional doses, the dose should be titrated to maximum tolerable levels within the recommended dosing range and then reassessed. Those who respond but do not remit may be good candidates for augmentation of the initial antidepressant. Those who fail to respond may benefit from a switch to another agent.
Switching Antidepressants
If no response occurs after an adequate trial and duration of antidepressant, it is reasonable to consider switching. Switching may be effective in patients with mild illness. It is less costly, has fewer side effects and drug interactions, and has better patient adherence than augmentation. No guidelines exist for switching to another agent in the same class versus another antidepressant class. If there is initial SSRI failure, it is reasonable to consider another SSRI. After multiple SSRI failures, recommendations support switching to a different antidepressant class. When switching, it is recommended to cross taper to avoid antidepressant-withdrawal syndrome. The exception to this is when cross tapering with an MAOI to another antidepressant to avoid precipitating serotonin syndrome.6
Augmentation
When monotherapy with antidepressants fail, augmentation may be considered. Though additional benefit may be gained with augmentation, there is a greater potential for adverse effects with the addition of a second medication. Additionally, a second medication increases pill burden and cost. However, augmentation may be preferred when patients have a partial response to monotherapy with an antidepressant. The addition of a second agent may help with residual depression symptoms by targeting depression through a different mechanism of action.
The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trials were a group of landmark depression studies evaluating antidepressant effectiveness for MDD.10-13 Randomized, controlled trials were performed in sequence and evaluated different antidepressant classes as monotherapy or when combined. Patients who failed to achieve remission on an intervention were advanced to the next level and initiated on an alternative treatment. All patients were initiated on citalopram in level 1 of the trial. Patients who failed to achieve remission moved to level 2 and received either monotherapy with bupropion, sertraline, venlafaxine, citalopram + bupropion, citalopram + buspirone, or other strategies using cognitive behavioral therapy. Patients continued to advance to level 4 until they achieved remission.
In addition to strategies using monotherapy with TCAs (nortriptyline), mirtazapine, and MAOIs (tranylcypromine), augmentation with lithium, thyroid hormone (T3), and the combination of venlafaxine + mirtazapine was evaluated. Though patients in this trial were not classified as treatment-resistant, those who required three or more medication trials to achieve remission would meet criteria for treatment resistance. Therefore, the systematic and stepwise approach to antidepressant prescribing upon treatment failure modeled in the STAR*D trial is often followed in clinical practice.
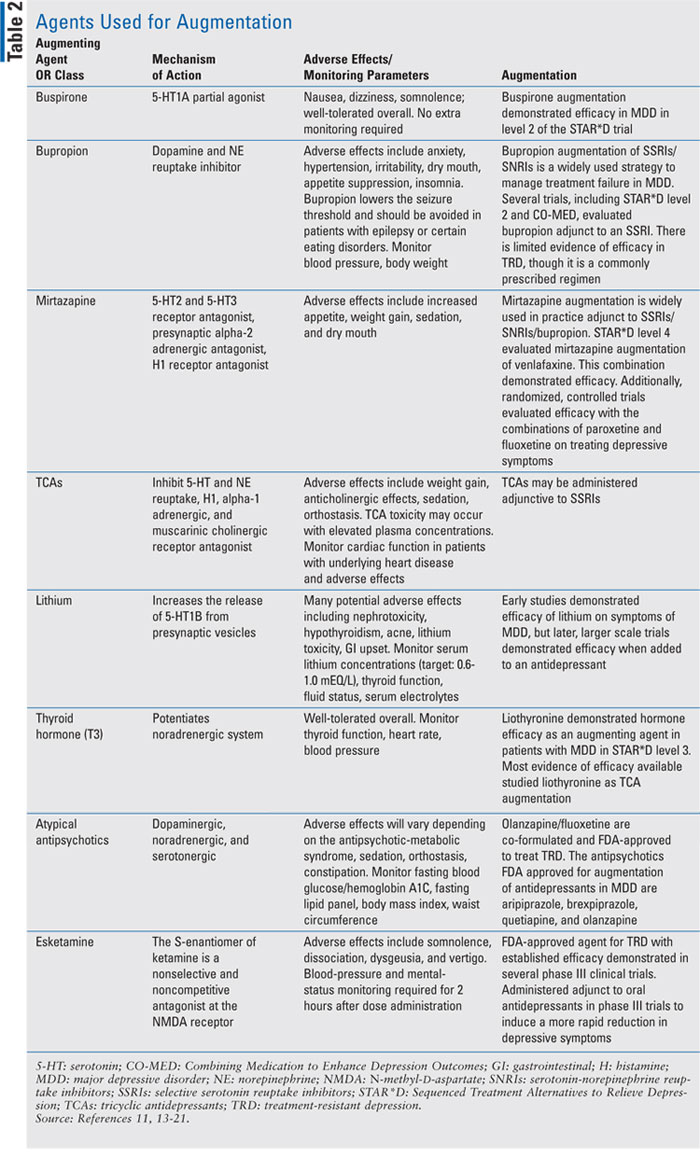
TABLE 2 lists agents that have been used to augment other antidepressants.11,13-21 Of these medications, mirtazapine, bupropion, and TCAs may also be used as monotherapy or adjunct to SSRIs/SNRIs. When augmenting, an agent within a different class with a different mechanism of action should be selected. For example, bupropion is a dopamine and norepinephrine reuptake inhibitor that is frequently used to augment SSRIs or SNRIs. Mirtazapine also has a different mechanism of action, making it a desirable and frequently prescribed antidepressant adjunct to SSRIs and SNRIs. Though MAOIs have a different mechanism of action than SSRIs and SNRIs, they may only be considered as monotherapy in TRD. MAOI augmentation is not recommended due to risk of serotonin syndrome when coadministered with other serotonergic agents.
Several agents are only indicated for augmentation. Lithium, T3, and atypical antipsychotics are frequently combined with SSRIs/SNRIs and less frequently with TCAs. Atypical antipsychotic augmentation of antidepressants were not evaluated in the STAR*D trial; however, aripiprazole, brexpiprazole, olanzapine, and quetiapine have gained FDA approval for the augmentation of antidepressants in MDD. These antipsychotics are prescribed at lower doses than those used when treating bipolar disorder and schizophrenia. Antipsychotic augmentation is typically reserved for patients who have failed several medication trials due to the adverse effect burden of antipsychotics. Weight gain, dyslipidemia, and type 2 diabetes are seen with antipsychotics at varying rates and are of greater burden than that of other agents used for augmentation. Of the approved agents, olanzapine and quetiapine have the greatest potential for inducing metabolic syndrome compared with aripiprazole and brexpiprazole.
 Aside from the aforementioned agents, several other medications have been evaluated for TRD. These agents include methylphenidate, pindolol, lamotrigine, omega-3 fatty acids (FAs), gabapentin, modafanil, and pramipexole. Limited evidence exists to support their treatment efficacy as augmenting agents for MDD, and they are infrequently prescribed.22
Aside from the aforementioned agents, several other medications have been evaluated for TRD. These agents include methylphenidate, pindolol, lamotrigine, omega-3 fatty acids (FAs), gabapentin, modafanil, and pramipexole. Limited evidence exists to support their treatment efficacy as augmenting agents for MDD, and they are infrequently prescribed.22
Esketamine
Intranasal esketamine recently earned FDA approval for the treatment of TRD. With the exception of olanzapine/fluoxetine, it is the only agent with an FDA approval for this indication. It works as a nonselective and noncompetitive antagonist at the N-methyl-D-aspartate receptor. Patients self-administer esketamine in a clinicbased setting under the supervision of healthcare staff for blood-pressure and mental-status monitoring due to transient blood-pressure increases and dissociation reactions noted in phase III clinical trials.21
 Esketamine is administered as an adjunct to oral antidepressants and has demonstrated efficacy in reducing depressive symptoms to a greater extent than oral antidepressants alone.19,20 Overall, esketamine has a favorable risk-to-benefit profile with adverse effects reported as mild and mostly occurring within the 2-hour mandatory postdose monitoring period. Dissociation is reported with the highest incidence (41%) but dissipated within 2 hours of administration in most clinical trials. Esketamine is contraindicated in patients with a history of aneurysmal vascular disease or intracerebral hemorrhage. Additionally, it has a black box warning for abuse and misuse and should be avoided in patients with substanceuse disorder or at a higher risk of abuse.
Esketamine is administered as an adjunct to oral antidepressants and has demonstrated efficacy in reducing depressive symptoms to a greater extent than oral antidepressants alone.19,20 Overall, esketamine has a favorable risk-to-benefit profile with adverse effects reported as mild and mostly occurring within the 2-hour mandatory postdose monitoring period. Dissociation is reported with the highest incidence (41%) but dissipated within 2 hours of administration in most clinical trials. Esketamine is contraindicated in patients with a history of aneurysmal vascular disease or intracerebral hemorrhage. Additionally, it has a black box warning for abuse and misuse and should be avoided in patients with substanceuse disorder or at a higher risk of abuse.
Patients with moderate-to-severe depression who have failed traditional strategies for managing TRD may be candidates for esketamine therapy. Additionally, literature is emerging suggesting esketamine’s efficacy in rapidly reducing suicidal thoughts in patients with MDD.23 Patients with chronic suicidality may also benefit from esketamine; however, further studies must be conducted to validate its efficacy in this population.
NONPHARMACOLOGIC STRATEGIES
Several nonpharmacologic strategies for TRD exist, including psychotherapy, transcranial magnetic stimulation, vagus nerve stimulation, and electroconvulsive therapy (ECT). Adding cognitive behavior therapy or interpersonal therapy to pharmacotherapy has been shown to increase remission rates in partial responders and in patients with TRD.24-26 Of the aforementioned interventions, ECT has demonstrated the greatest efficacy in reducing depressive symptoms, with remission rates ranging from 50% to 89% in patients unresponsive to a prior antidepressant trial.27 Though ECT demonstrates superior efficacy to oral antidepressants, its use is limited by the need for patients to undergo anesthesia and neuromuscular blockade, the small number of psychiatrists able to perform the procedure, and cost.
Complementary and Alternative Medicine
Complementary and alternative medicine (CAM) is defined as a group of diverse medical and healthcare systems, practices, and products that are not presently considered to be part of conventional medicine. Complementary refers to things that are not considered mainstream or conventional but are Western biomedical concepts. Alternative approaches are considered to be outside traditional Western medical concepts. Many who suffer from psychiatric illnesses, including depression, have used CAM. Patients with MDD are more likely to use CAM, with one study revealing 79% of patients reported using CAM but did not disclose its use to their psychiatrist.28
Omega-3 Fatty Acids
Omega-3 FAs found in fish oil, seafood, and some vegetables are important in brain development and function. Deficiencies in omega-3 FA compounds such as eicosapentaenoic acid (EPA) and docosahexaenoic acid are proposed to be risk factors for MDD. Studies of cultures that include diets with fish oil have lower rates of depressive disorders. Omega-3 FAs potentially improve serotonergic and dopaminergic neurotransmission through cell-membrane microstructure and signal transduction. Adjunct EPA has been evaluated in persistent depression. One study reported improvements in depressive symptoms compared with baseline and controls. Another study with adjunct omega-3 FA supplements for 8 weeks showed improvements in HAM-D scores. A minimum treatment of 4 weeks with omega-3 FA, with EPA content more relevant than DHA, may help patients manage TRD.6
SAMe
S-adenosylmethionine (SAMe) is a natural compound found in the body and is a source of methyl groups used in the synthesis of catecholemines and neuronal membranes. Since catecholemines play a role in neuropathology of depression, SAMe may have a therapeutic role in regulating mood disorders by increasing monoamine synthesis and serotonin turnover, inhibition of norepinephrine reuptake, and augmentation of dopaminergic activity. Clinical trials with SAMe for the treatment of depression have reported response rates comparable with TCAs when used as monotherapy. SAMe is also recommended as adjunctive therapy in mild-to-moderate MDD. Evidence for use in TRD has come from 6-week-long treatments of SAMe at doses of 800 mg/day to 1,600 mg/day for benefits.6
St. John’s Wort
Many placebo-controlled and active comparator trials have been conducted on St. John’s wort. However, there is a lack of consensus on efficacy for the treatment of MDD. A number of doubleblind studies have demonstrated superiority over placebo while others have not. Randomized studies have failed to show differences between approved antidepressant medications. There are data to support that St. John’s wort has better tolerability than TCAs and SSRIs. The Hypericum Depression Trial Study Group conducted a trial of patients with MDD in which patients were randomly assigned St. John’s wort, sertraline, or placebo. St. John’s wort and sertraline had similar results to placebo on the primary outcome of change in HAM-D score and Clinical Global Impressions rating scales. A limitation of use for St. John’s wort is its significant drug interactions. As an inducer of cytochrome P450 3A4, it can potentially decrease the efficacy of medications such as antiretrovirals, immunosuppressants, antineoplastics, and anticoagulants.28
ROLE OF THE PHARMACIST
Pharmacists are accessible healthcare professionals who can play a vital role in helping patients with TRD. Pharmacists can assist with medication selection, provide dosage recommendations to optimize therapy, and reduce polypharmacy. Pharmacists can also help to monitor patient response until remission. Identification of drug interactions and adverse reactions with recommendations for alternative therapies may be necessary. Medication adherence is a challenge in the management of depression as nonadherence can be related to forgetfulness, psychosocial stress, anxiety about possible adverse effects, low motivation, or inadequate knowledge and skills to manage disease symptoms and treatments.
Patients may lack a self-perceived need for treatment or a perceived effect to treatment and have negative beliefs about the efficacy of treatment. Approximately one-third of patients will discontinue antidepressants within the first month of treatment, and 44% of patients are reported to discontinue therapy by the third month of treatment.29 More than 50% of depressed patients will withdraw treatment prematurely. Early termination of antidepressants is associated with a 77% increased risk for relapse.29 Pharmacists can help to ensure medication adherence by providing medication counseling so patients understand how to use medication, the importance of taking medication consistently, and side effects that may occur. When patients are informed, they are more likely to continue treatment despite side effects.
REFERENCES
- Center for Disease Control (CDC). Mental health conditions: depression and anxiety. www.cdc.gov/tobacco/campaign/tips/diseases/depression-anxiety.html. 2018. Accessed October 18, 2019.
- Gaynes BN, Gary Asher M, Gerald Gartlehner M, et al. Technology assessment program—technical assessment definition of treatment-resistant depression in the Medicare population. www.ahrq.gov. Accessed August 30, 2019.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington, VA: American Psychiatric Association; 2013.
- Fekadu N, Shibeshi W, Engidawork E. Major depressive disorder: pathophysiology and clinical management. J Depress Anxiety. 2017;6(1):1-3.
- Al-Harbi KS. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence. 2012;6:369.
- Shivakumar K, Amanullah S, eds. Complex Clinical Conundrums in Psychiatry: From Theory to Clinical Management. New York, NY: Springer International Publishing. 2018.
- Aan het Rot M, Mathew SJ, Charney DS. Neurobiological mechanisms in major depressive disorder. Can Med Assoc J. 2009;180(3):305-313.
- Johnson KK, White BM, Dreger D, et al. Psychiatry Board Review Manual endorsed by the Association for Hospital Medical Education; 2006. www.turner-white.com. Accessed August 30, 2019.
- Micromedex Healthcare Series. www.thomsonhc.com.ezproxy. samford.edu/. Accessed September 1, 2019.
- Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurementbased care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006:28-40.
- Fong P, Boss D, Yap T, et al. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depressions. N Engl J Med. 2006;354(12):1231-1242.
- Fava M, Rush AJ, Wisniewski SR, et al. A comparison of mirtazapine and nortriptyline following two consecutive failed medication treatments for depressed outpatients: A STAR*D report. Am J Psychiatry. 2006;163(7):1161-1172.
- McGrath PJ, Stewart JW, Fava M, et al. Tranylcypromine versus venlafaxine plus mirtazapine following three failed antidepressant medication trials for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1531-1541.
- Wisniewski SR, Fava M, Trivedi MH, et al. Acceptability of second-step treatments to depressed outpatients: a STAR*D report. Am J Psychiatry. 2007;164(5):753-760.
- Rush AJ, Trivedi MH, Stewart JW, et al. Combining Medications to Enhance Depression Outcomes (CO-MED): acute and long-term outcomes of a single-blind randomized study. Am J Psychiatry. 2011;168(7):689-701.
- Lam RW, Wan DDC, Cohen NL, Kennedy SH. Combining antidepressants for treatment-resistant depression: a review. J Clin Psychiatry. 2002;63(8):685-693.
- Thase ME. What role do atypical antipsychotic drugs have in treatment-resistant depression? J Clin Psychiatry. 2002;63(2): 95-103.
- Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T3 augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1519-1530.
- Daly EJ, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression. JAMA Psychiatry. 2018;75(2):139-148.
- Popova V, Daly EJ, Trivedi M, et al. Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: a randomized double-blind active-controlled study. Am J Psychiatry. 2019;176(6):428-438.
- Spravato (esketamine) Nasal Spray, CIII Highlights of Prescribing Information. www.fda.gov. Accessed July 31, 2019.
- Ng A. Toolbox: psychotropic medications for augmentation or combination in treatment-resistant depression. https://mhc.cpnp.org/doi/pdf/10.9740/mhc.n207188. Accessed July 25, 2019.
- Canuso CM, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2018;175(7):620-630.
- Keller MB, McCullough JP, Klein DN, et al. A comparison of nefazodone, the cognitive behavioral-analysis system of psychotherapy, and their combination for the treatment of chronic depression. N Engl J Med. 2000;342(20):1462-1470.
- Thase ME, Greenhouse JB, Frank E, et al. Treatment of major depression with psychotherapy or psychotherapy-pharmacotherapy combinations. Arch Gen Psychiatry. 1997;54(11): 1009.
- Fava GA, Savron G, Grandi S, Rafanelli C. Cognitive-behavioral management of drug-resistant major depressive disorder. J Clin Psychiatry. 1997;58(6):278-284.
- Thase ME, Rush AJ. Psychopharmacology: The Fourth Generation of Progress. ACNP. New York: Raven Press; 1995. https://acnp.org/digital-library/psychopharmacology-4th-generation-progress/. Accessed August 22, 2019.
- Freeman MP, Fava M, Lake J, et al. Complementary and alternative medicine in major depressive disorder. J Clin Psychiatry. 2010;71(6):669-681.
- Alekhya P, Sriharsha M, Priya Darsini T, et al. Treatment and disease related factors affecting non-adherence among patients on long term therapy of antidepressants. J Depress Anxiety. 2015;4(2):175.