Oral Semaglutide: A New GLP-1 Receptor Agonist Product for the Treatment of Type 2 Diabetes
RELEASE DATE:
October 1, 2019
EXPIRATION DATE:
October 31, 2021
FACULTY:
Connor K. Smith, BS, PharmD Candidate 2021
College of Pharmacy and Pharmaceutical Sciences
Washington State University
Joshua J. Neumiller, PharmD, CDE, FAADE
Associate Professor
Department of Pharmacotherapy
College of Pharmacy and Pharmaceutical Sciences
Washington State University
Spokane, Washington
FACULTY DISCLOSURE STATEMENTS
Mr. Smith and Dr. Neumiller report no conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT:
Pharmacy
 Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-19-106-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE:
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER:
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients' conditions and possible contraindications or dangers in use, review of any applicable manufacturer's product information, and comparison with recommendations of other authorities.
GOAL:
To educate pharmacists about currently available data on the efficacy and tolerability of the oral GLP-1 receptor agonist semaglutide in patients with type 2 diabetes.
OBJECTIVES:
After completing this activity, the participant should be able to:
- State current recommendations for GLP-1 receptor agonist use in the management of type 2 diabetes.
- Review the pharmacology and characteristics of GLP-1 receptor agonists.
- Describe the mechanism of oral semaglutide that allows for oral GLP-1 receptor agonist drug delivery.
- Discuss key efficacy and tolerability data from clinical trials published to date with oral semaglutide.
ABSTRACT: Glucagon-like peptide-1 (GLP-1) receptor agonists are an increasingly popular class of glucose-lowering agents for the treatment of type 2 diabetes (T2DM), owing to their robust glucose-lowering effects, low risk of hypoglycemia, and potential to induce weight loss. More recently, agents within the GLP-1 receptor agonist class have also demonstrated cardiovascular and kidney benefits in completed cardiovascular outcome trials. Despite their popularity, GLP-1 receptor agonist use to date has been limited to patients willing and able to administer the medication by SC injection. A novel oral formulation of the GLP-1 receptor agonist semaglutide (Rybelsus) was recently approved for the treatment of T2DM. The formulation that allows for oral administration of semaglutide is described. Recently published efficacy and safety data are reviewed and the potential role of this novel GLP-1 receptor agonist in patient care is discussed.
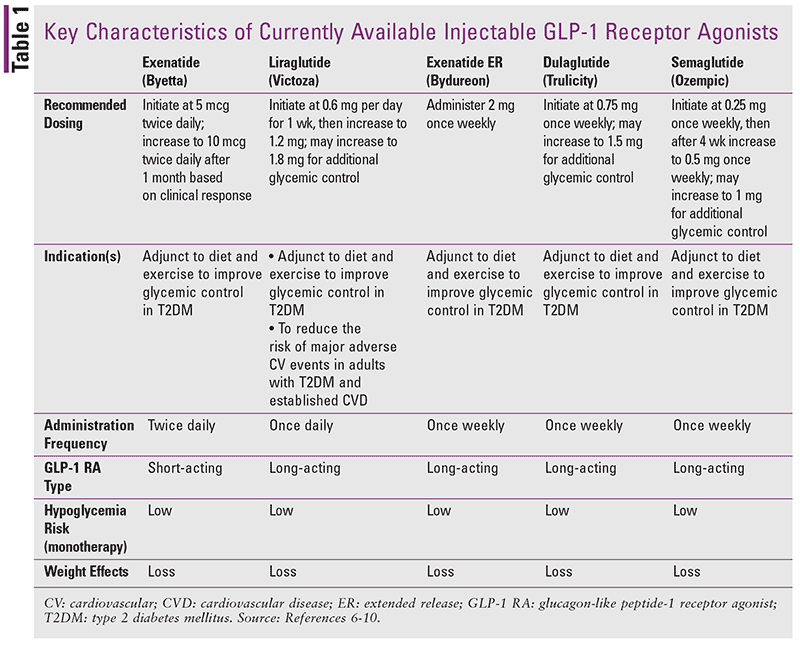
Over 30 million people in the United States live with diabetes, with another estimated 84 million people living with prediabetes.1 Type 2 diabetes mellitus (T2DM) accounts for roughly 90% to 95% of all diagnosed cases of diabetes in the U.S.2 Although the number of glucose-lowering agents available to treat T2DM has increased dramatically over the past 10 years, gaps continue to exist in care and outcomes. For example, data indicate that slightly more than half of patients with T2DM meet the general hemoglobin A1C goal of less than 7%, and suboptimal glycemic control continues to contribute to the development of complications like cardiovascular disease and microvascular complications.3 We now have 12 classes of glucose-lowering medications approved for the treatment of T2DM in the U.S. (SIDEBAR 1). Of the currently available classes of glucose-lowering therapies, the glucagonlike peptide-1 (GLP-1) receptor agonist class of medications has gained popularity in recent years. This increase in clinical use can be attributed in part to a number of favorable qualities including: 1) robust glucose-lowering efficacy; 2) a low risk of contributing to hypoglycemic events; 3) and promotion of weight loss.4,5 TABLE 1 provides a summary of key characteristics of currently available injectable GLP-1 receptor agonists.6-10
GLP-1 receptor agonists work to lower glycemia via several mechanisms. First, GLP-1 receptor agonists stimulate glucose-dependent insulin secretion from pancreatic β-cells. Second, they suppress inappropriately elevated glucagon secretion from pancreatic α-cells, thereby suppressing hepatic glucose overproduction. Third, GLP-1 receptor agonists (particularly the shorter-acting agents) delay gastric emptying, thus delaying carbohydrate absorption and promoting satiety.11 In addition, GLP-1 receptor agonists have been shown to influence satiety signaling within the central nervous system, promoting feelings of fullness that can result in less caloric intake and eventual weight loss.5
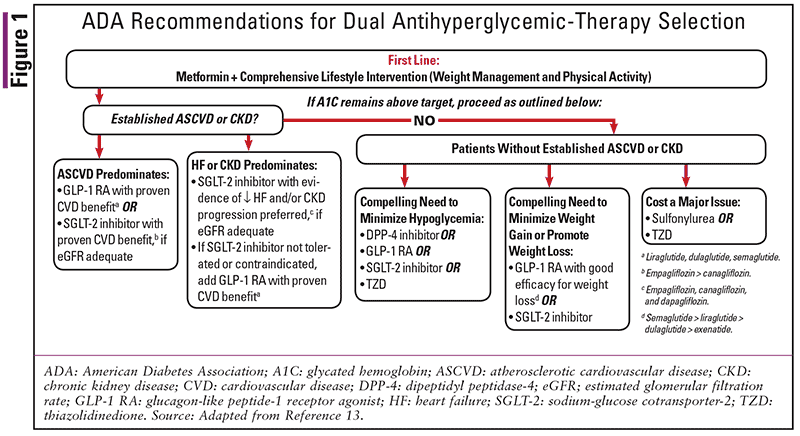
In addition to the potential benefits of GLP-1 receptor agonists already mentioned, several dedicated cardiovascular outcome trials have reported positive cardiovascular and kidney outcomes with agents from the GLP-1 receptor agonist class. One such investigation, known as the SUSTAIN-6 trial, was a moderate-sized trial that examined use of the once-weekly injectable agent semaglutide on cardiovascular outcomes when compared with placebo.12 The trial was powered to test noninferiority of semaglutide versus placebo on cardiovascular outcomes for the purpose of regulatory approval. The primary outcome was a composite of the first occurrence of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke. At trial completion, the primary outcome occurred in 108 patients (6.6%) in the semaglutide group versus 146 patients (8.9%) in the placebo group (hazard ratio [HR]: 0.74; 95% CI, 0.58-0.95).12 Based on current cardiovascular outcome data such as that provided in SUSTAIN-6, the American Diabetes Association (ADA) now recommends GLP-1 receptor agonists with proven cardiovascular benefit as a preferred option for second-line add-on therapy in patients with T2DM and established atherosclerotic cardiovascular disease (ASCVD) following metformin and lifestyle-change intensification.13 See FIGURE 1 for a summary of current recommendations from the ADA for second-line therapy in patients with T2DM.13 In addition to being recommended in patients with ASCVD, GLP-1 receptor agonists are recommended for consideration as an add-on to metformin in patients without established ASCVD in whom there is a compelling need to minimize hypoglycemia, minimize weight gain, or promote weight loss.13
Although GLP-1 receptor agonists provide clear benefits to appropriate patients with T2DM, there remain limitations and barriers to use. One notable barrier is that GLP-1 receptor agonists have historically been administered via SC injection only. This potentially leads to limitations in GLP-1 receptor agonist use in patients who are unwilling or unable to administer these medications subcutaneously. Because of their peptide structure, GLP-1 receptor agonists are susceptible to poor gastrointestinal permeability, cytosolic metabolism, and hepatic clearance mechanisms that result in poor oral bioavailability.14 Often labeled as the "Holy Grail" of drug delivery,15 oral delivery of peptide and protein-based medications has made remarkable progress in recent years. Advances in oral delivery of peptide-based medications have resulted in the development of an oral formulation of semaglutide (Rybelsus) which was recently approved by the FDA for the treatment of T2DM. This article will review the formulation of oral semaglutide that allows for oral administration, discuss recently published efficacy and safety data, and consider the potential role of this novel GLP-1 receptor agonist in patient care.
MECHANISM OF ORAL SEMAGLUTIDE DELIVERY
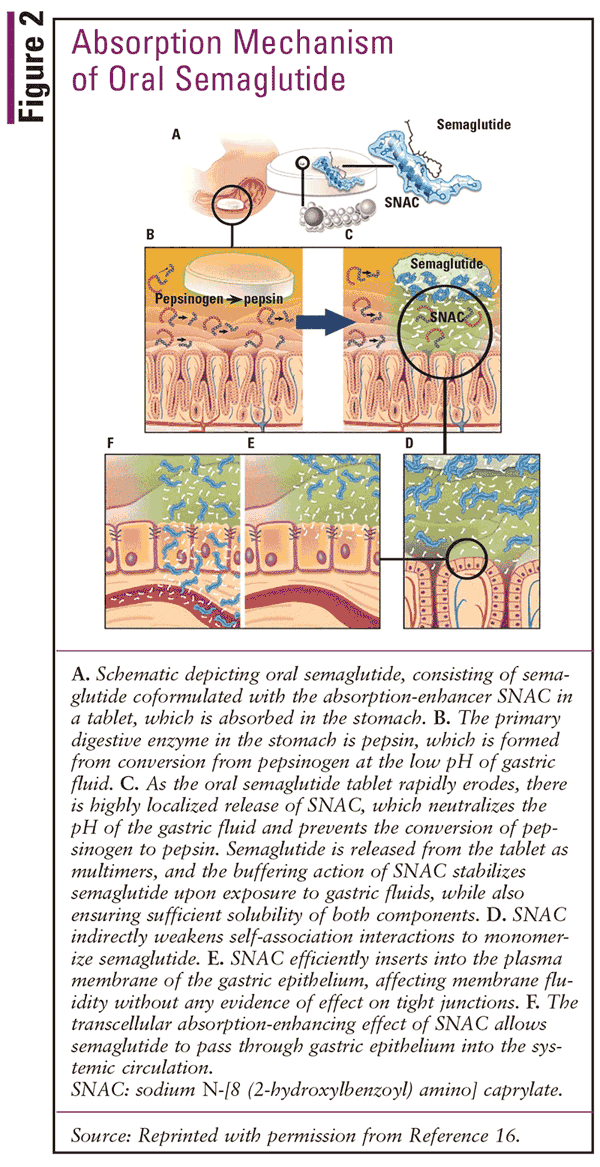
Oral delivery of peptide-derived drugs is often ineffective due to proteolytic degradation within the gastrointestinal tract leading to subtherapeutic drug exposure.16 Due to advancements in the use of absorption enhancers, oral semaglutide is coformulated in a manner that allows for a novel mechanism of oral administration and ultimate delivery to the systemic circulation (see FIGURE 2). Although a majority of oral drugs are absorbed in the small intestine, studies indicate that oral semaglutide is likely absorbed in the stomach.16 Furthermore, disintegration and absorption of the oral semaglutide dosage form in the stomach seem to ensue via a localized process where the tablet initially makes contact with the stomach wall. This is supported by the finding that the concentration of semaglutide is reduced dramatically in the stomach cells bordering the tablet's physical location.16,17 Hence, mechanistic studies indicate that oral semaglutide parks in the stomach, where it is then absorbed in a localized fashion with the help of the absorption enhancer sodium N-[8 (2-hydroxylbenzoyl) amino] caprylate (SNAC).16,18
SNAC was labeled as "generally recognized as safe" by the FDA in 2009 when used in combination with dietary supplements.19 In addition, the coformulation of SNAC and semaglutide was shown to have no extended effect on stomach mucosa; all changes were transient and fully reversible.16 SNAC works via a number of mechanisms to improve the absorption of semaglutide within the stomach. Due to its lipophilic properties, SNAC is able to embed into and modify the composition of plasma membranes. By incorporating into the lipid membrane of local gastric cells, SNAC disturbs the fluidity of the membrane and increases intracellular accumulation of semaglutide via a transcellular process.16,20 GLP-1 analogues, especially those that are fatty acid–acylated (liraglutide and semaglutide), have a tendency to form oligomers that can influence absorption.21 SNAC indirectly weakens these noncovalent interactions, which promotes the monomerization of semaglutide.16
SNAC also acts as a buffer to neutralize the acidic conditions of the stomach directly surrounding the tablet, which enhances the absorption of semaglutide in two ways.16 First, because of the peptide structure of semaglutide, the probability that it is degraded by pepsin in the stomach is significant. Pepsin activity is highest at a pH around 2 and is almost completely inactive at a neutral pH.22 Because pepsin is the major digestive enzyme in the stomach, SNAC's ability to locally neutralize the pH within the area immediately surrounding the disintegrating tablet stabilizes semaglutide against enzymatic degradation. Thus, this extension of the half-life of semaglutide allows for more drug to be absorbed into the stomach cells and reach systemic circulation.11 Second, the buffering activity of SNAC promotes enhanced solubility of semaglutide, providing higher concentrations of the drug to the gastric membrane.16 The interplay between both SNAC and semaglutide is also essential for absorption. Formulations with liraglutide and SNAC and semaglutide in combination with a close analogue of SNAC greatly reduced the bioavailability of the active medication in question.16,17 Adequate semaglutide absorption is also dependent on the quantity of SNAC in the tablet formulation. When 150 mg of SNAC was compared with 300 mg of SNAC, considerable reductions in systemic semaglutide concentrations were observed.16
Owing to the unique mechanism by which SNAC facilitates absorption of semaglutide within the stomach, it is important to consider the impact of meals on drug absorption. Buckley and colleagues conducted a study where participants received a dose of oral semaglutide in either a fasting or fed state.16 The results showed that almost no plasma semaglutide was measurable in the fed state, demonstrating that postprandial administration of oral semaglutide leads to a remarkable reduction in absorption. Therefore, oral semaglutide should be administered in the fasting state to facilitate absorption.16 While food appears to decrease absorption, multiple studies have suggested that the volume of the water ingested with the tablet does not affect the location or effectivess of the tablet erosion.23,24
CLINICAL STUDIES
Many clinical trials have been completed with oral semaglutide in support of the manufacturer's submission to the FDA seeking approval for the treatment of T2DM. The following is a brief overview of clinical trial data available to date.
Phase II, Dose-Ranging Trial
A phase II, randomized, dose-finding trial of oral semaglutide was published in late 2017.18 The objective of the trial was to compare the effects of oral semaglutide with placebo and open-label SC semaglutide on glycemic control in patients with T2DM.18 The trial was 26 weeks in duration with a 5-week follow-up period. There were 632 participants with T2DM and insufficient glycemic control—defined as an A1C between 7.0% and 9.5%—despite lifestyle interventions alone or treatment with a stable dose of metformin. Enrolled subjects were randomized to receive one of five possible doses of oral semaglutide once daily (2.5, 5, 10, 20, or 40 mg), placebo, or 1 mg SC semaglutide injection once weekly. For participants receiving 40 mg daily of oral semaglutide, three different dose-titration protocols were used where patients were titrated up to the full 40 mg dose in 6 weeks (fast dose escalation), 12 weeks (standard dose escalation), or 24 weeks (slow dose escalation). Significant reductions in A1C were observed in all oral semaglutide treatment groups when compared with placebo (dose-dependent range, -0.7% to -1.9%).18
When pooled, participants randomized to the 40-mg daily fast and standard dose-escalation groups exhibited an A1C reduction from baseline of 1.8% versus a reduction of 0.3% seen in the placebo group (P <.001). Injectable semaglutide resulted in a 1.9% mean reduction in A1C from baseline, which was also significant when compared with the placebo group (P <.001) but did not differ statistically from the 20 mg and 40 mg oral-semaglutide (standard dose escalation) groups. Oral semaglutide likewise resulted in reductions in weight during the trial. The 40 mg (standard-dose escalation) and SC semaglutide groups achieved the greatest mean reduction in body weight from baseline at -6.9 kg and -6.4 kg, respectively (P <.001 for both comparisons vs. placebo). Like the A1C data presented above, weight loss was similar with 20 mg and 40 mg oral semaglutide when compared with the injectable semaglutide group. In summary, in this 26-week dose-finding study, oral semaglutide demonstrated robust efficacy for A1C reduction and weight loss, with the 20 mg and 40 mg once-daily doses of oral semaglutide producing similar glycemic and weight benefits when compared with injectable semaglutide.
Phase III, PIONEER Program
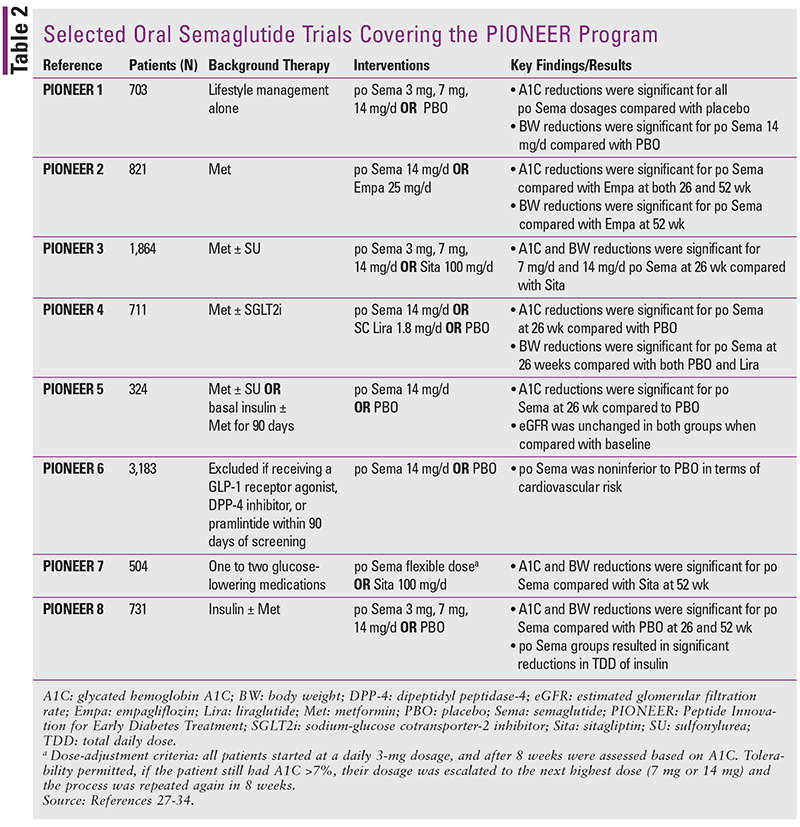
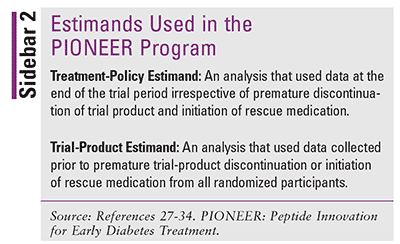
Multiple phase III efficacy trials have been fully published and/or presented in abstract form. The core phase III trial program for oral semaglutide is known as the Peptide Innovation for Early Diabetes Treatment (PIONEER) program. PIONEER 9 and PIONEER 10 are trials that enrolled Japanese participants with T2DM to meet regulatory requirements in Japan.25,26 Both trials are completed but are not yet published. Recruitment has also begun for PIONEER 12, which is projected to be completed in August of 2021. TABLE 2 provides a summary of key information from PIONEER 1-8.27-34 Of note, several trials in the PIONEER program used two estimands to further distinguish their analyses: 1) a treatment-policy estimand, which used data collected at the end of the study period (e.g., week 26 or 52) in the analysis regardless of treatment discontinuation or use of rescue medication; and 2) a trial-product estimand which includes data collected from all randomized participants prior to premature trial-product discontinuation or initiation of rescue medication (SIDEBAR 2). The following is a brief summary of available data from the PIONEER 1-8 studies.27-34
PIONEER 1
PIONEER 1 compared the efficacy and safety of oral semaglutide as monotherapy with placebo in patients with T2DM managed by diet and exercise alone.27 A total of 703 participants with uncontrolled T2DM (A1C of 7.0–9.5%) were randomized into four treatment groups that included a daily dose of either 3 mg, 7 mg, or 14 mg of oral semaglutide or placebo for 26 weeks. Placebo-adjusted reductions in A1C for oral semaglutide ranged from -0.6% to -1.1% for the treatment-policy estimand and -0.7% to -1.4% for the trial-product estimand (P <.001 for all doses vs. placebo). Placebo-adjusted reductions in body weight ranged from -0.1 kg to -2.3 kg for the treatment-policy estimand and -0.2 kg to -2.6 kg for the trial-product estimand (P <.001 for the 14-mg oral semaglutide dose for both estimands). The most common adverse events reported with oral semaglutide were mild-to-moderate transient gastrointestinal events—a finding consistent with the known tolerability of other available GLP-1 receptor agonists. A more detailed discussion of safety and tolerability are provided later.
PIONEER 2
PIONEER 2 was a 52-week, randomized, open-label trial enrolling 821 patients with T2DM.28 The objective of the trial was to compare the addition of oral semaglutide (14 mg once daily) or the sodium-glucose cotransporter-2 (SGLT-2) inhibitor empagliflozin (25 mg once daily) to metformin monotherapy in patients with uncontrolled T2DM. Enrolled participants had a mean baseline A1C of 8.1% in both treatment groups. Treatment with oral semaglutide resulted in a mean A1C reduction from baseline of -1.3% at week 52 for both estimand calculations. In comparison, treatment with empagliflozin resulted in a mean A1C reduction from baseline of -0.9% and -0.8% for the treatment-policy estimand and trial-product estimand analyses, respectively (P <.0001 for oral semaglutide vs. empagliflozin for both analyses). Both treatment approaches resulted in a mean weight loss at 52 weeks, with oral semaglutide treatment resulting in a mean weight loss from baseline of 3.8 kg and 4.7 kg in the treatment-policy estimand and trial-product estimand analyses, respectively. The empagliflozin group achieved a mean weight loss of 3.6 kg and 3.8 kg in the two analyses, respectively. The weight loss achieved with oral semaglutide was greater than that achieved with empagliflozin treatment at week 52 in the trial-product estimand analysis (P <.05). When considering current recommendations from the ADA for add-on therapy to metformin in patients not achieving individualized glycemic goals, PIONEER 2 provides valuable data comparing the addition of either an oral GLP-1 receptor agonist or an SGLT-2 inhibitor.
PIONEER 3
PIONEER 3 compared the efficacy and safety of the addition of oral semaglutide or the DPP-4 inhibitor sitagliptin as add-on therapy in patients with T2DM receiving metformin with or without a sulfonylurea.29 The randomized, double-blind study enrolled 1,864 participants with a mean baseline A1C of 8.3% and BMI of 32.5. Participants were randomized to receive 3 mg, 7 mg, or 14 mg of oral semaglutide or 100 mg of sitagliptin once daily. The primary outcome of the trial was the change in A1C from baseline at week 26. Participants were also assessed at weeks 52 and 78 to assess durability of glucose lowering with the two treatment approaches. In terms of the primary outcome of reduction in A1C from baseline, although the 3-mg oral semaglutide group did not demonstrate superiority to sitagliptin 100 mg at 26 weeks, the 7-mg and 14-mg dosage groups did demonstrate statistically significant reductions in A1C from baseline when compared with sitagliptin. In the treatment-policy estimand analysis, estimated mean A1C reductions from baseline were -1.0% and -1.3% for oral semaglutide 7 mg and 14 mg daily, respectively, and -0.8% for sitagliptin (P <.001). A1C reductions were largely maintained over time; in the treatment-policy estimand analysis the 14 mg oral-semaglutide group had mean A1C reductions from baseline of -1.3%, -1.2%, and -1.1% at 26, 52, and 78 weeks, respectively. In terms of key secondary outcomes, mean changes from baseline in body weight at week 26 were -1.2 kg, -2.2 kg, and -3.1 kg for semaglutide 3 mg, 7 mg, and 14 mg, respectively. The sitagliptin treatment group, in contrast, achieved a mean weight loss from baseline of -0.6 kg (P <.001). Overall, the addition of oral semaglutide 7 mg or 14 mg compared favorably to the addition of sitagliptin 100 mg in patients with T2DM inadequately controlled on metformin ± sulfonylurea background therapy.
PIONEER 4
PIONEER 4 was an important trial comparing the addition of oral semaglutide or liraglutide to background metformin with or without an SGLT-2 inhibitor in patients with T2DM.30 A total of 711 patients with T2DM (mean baseline A1C of approximately 8% in all treatment groups) were enrolled and randomized to receive one of three interventions: 1) 14 mg daily of oral semaglutide; 2) 1.8 mg liraglutide; or 3) placebo for 52 weeks. In the treatment-policy estimand analysis, at week 26 oral semaglutide (1.2% mean A1C reduction from baseline) met criteria for noninferiority when compared with liraglutide (1.1% mean A1C reduction from baseline) and met criteria for superiority when compared with placebo (0.2% mean A1C reduction from baseline; P <.0001). At week 52, using the treatment-policy estimand analysis, however, the oral semaglutide group experienced significantly greater decreases in A1C compared with both the liraglutide and placebo groups. Oral semaglutide also resulted in a greater mean reduction in weight from baseline when compared with liraglutide and placebo (estimated mean change from baseline of -1.6 kg, -1.1 kg, and -0.3 kg, respectively [P = .0006 for oral semaglutide vs. liraglutide; P <.0001 for oral semaglutide vs. placebo]). As previously noted, PIONEER 4 is an important trial that compared the novel oral GLP-1 receptor agonist product against the market-leader injectable GLP-1 receptor agonist product, liraglutide. Overall, oral semaglutide compared favorably in terms of the efficacy endpoints examined.
PIONEER 5
The PIONEER 5 study enrolled patients with T2DM and moderate renal impairment (estimated glomerular filtration rate [eGFR] of 30–59 mL/ min/1.73 m2) to examine the efficacy and safety of oral semaglutide in patients with compromised kidney function.31 A total of 324 patients receiving either 1) a stable dose of a sulfonylurea, metformin, or both; or 2) basal insulin ± metformin for at least 90 days were enrolled in this randomized, doubleblind study. For 26 weeks, participants received either 14 mg of oral semaglutide or placebo once daily. In terms of efficacy, the treatment group receiving oral semaglutide achieved statistically significant reductions from baseline in A1C and body weight when compared with placebo. A daily dose of 14 mg of oral semaglutide for 26 weeks resulted in a mean decline in A1C from baseline of -0.8% (treatment-policy estimand) and -1.0% (trial-product estimand). In terms of body weight, oral-semaglutide treatment led to mean reductions of -2.5 kg (treatment-policy estimand) and -2.7 kg (trial-product estimand). The reported reductions in A1C and body weight from baseline were statistically significant when compared with those randomized to receive placebo (P <.001 for all comparisons). Additionally, follow-up at 31 weeks showed no difference in eGFR between the placebo and oral-semaglutide groups compared with baseline eGFR measurements, suggesting that treatment had no adverse effects on kidney function. Overall, PIONEER 5 provides some evidence that oral semaglutide may be an effective treatment option in patients with moderate renal impairment.
PIONEER 6
PIONEER 6 was a randomized, double-blind, placebo-controlled trial comparing oral semaglutide to placebo designed to rule out an excess in cardiovascular risk with oral semaglutide among patients with T2DM.32 The primary outcome for the trial was the first occurrence of a major adverse cardiovascular event (death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke). A total of 3,183 participants were enrolled. Patients were eligible to enroll within the study if they were aged 50 years or older and had established cardiovascular disease or chronic kidney disease. Alternatively, participants could also be enrolled if they were aged 60 years or older and had cardiovascular risk factors. Participants were randomly assigned to a target dose of 14 mg of oral semaglutide once daily or placebo. Participants in the oral semaglutide arm underwent a dose-escalation schedule (3 mg daily for 4 weeks, followed by 7 mg daily for 4 weeks, then 14 mg daily for the remainder of the trial) to minimize gastrointestinal side effects. The median treatment duration for participants was 15.9 months. The primary endpoint occurred in 61 of 1,591 participants (3.8%) in the oral semaglutide group compared with 76 of 1,592 participants in the placebo group (4.8%), validating noninferiority (P <.001). Based on these findings, PIONEER 6 supports preliminary cardiovascular safety for the treatment of T2DM. Furthermore, cardiovascular results for oral semaglutide were similar to the results observed in the SUSTAIN-6 trial with injectable semaglutide, suggesting that the oral and SC formulations share similar cardiovascular safety profiles.7
PIONEER 7
The PIONEER 7 trial, like PIONEER 3, compared oral semaglutide against 100-mg sitagliptin, although this trial differs in that it allowed for flexible dose adjustments of oral semaglutide. The 52-week randomized, open-label trial enrolled a total of 504 patients with a mean baseline A1C of 8.3%.33 Participants enrolled were receiving stable daily doses of one or two glucose-lowering drugs (metformin, sulfonylureas, SGLT-2 inhibitors, or thiazolidinediones). The primary endpoint was the percentage of patients achieving an A1C of less than 7%. All participants in the oral-semaglutide group initiated oral semaglutide at 3 mg once daily; at 8 weeks and every 8 weeks thereafter, participants were assessed based on A1C response to determine if a dose adjustment was warranted. If permitted based on individual tolerability, the dose of oral semaglutide was increased if the patient had an A1C remaining above 7.0% at each visit, respectively. After 52 weeks of treatment, the proportion of participants in the oral semaglutide group achieving an A1C of less than 7.0% was 58.3% (treatment-policy estimand) and 62.8% (trial-product estimand), compared with 25.2% (treatment-policy estimand) and 28.3% (trial-product estimand) for the group receiving 100 mg of sitagliptin daily. PIONEER 7 provides data supporting oral semaglutide use as add-on to background oral glucose-lowering agents to improve glycemic control. Of note, this trial employed an individualized titration schedule on a variety of background regimens, which is likely more applicable to typical clinical practice.
PIONEER 8
PIONEER 8 examined the addition of oral semaglutide in patients with T2DM receiving insulin with or without metformin.34 The trial had a total of 731 patients with a baseline mean A1C of 8.2% and a baseline total daily dose of insulin ranging from 56 to 62 units, depending on the treatment group. Enrolled participants were randomized to receive 3 mg, 7 mg, or 14 mg of oral semaglutide or placebo once daily for 52 weeks. In both the trial-product and treatment-policy estimand analyses, all oral semaglutide groups demonstrated a significantly greater reduction in A1C from baseline when compared with placebo at 26 and 52 weeks. Furthermore, total insulin use was reduced significantly in the 7-mg and 14-mg oral semaglutide groups when compared with placebo, suggesting an insulin-sparing effect with treatment.
USE IN PATIENTS WITH HEPATIC IMPAIRMENT
One clinical trial has been published assessing the safety, tolerability, and pharmacokinetics of oral semaglutide in patients with hepatic impairment.35 A total of 56 patients was assessed using the ChildPugh criteria and grouped into either normal, mild, moderate, or severe hepatic impairment. All patients received 5 mg of oral semaglutide once daily for the first 5 days, then received a dose of 10 mg once daily for the remaining 5 days of the study. Because GLP-1 receptor agonists are believed to be metabolized in various areas throughout the body and not specifically in the liver,35 no significant variations were expected within any of the groups. This expectation was verified based on findings of this study. The half-life, Cmax, and tmax were similar when comparing all hepatic-impairment groups. Safety and tolerability issues were also similar across groups, suggesting that dose adjustment is not required in patients with hepatic impairment.
USE IN PATIENTS WITH RENAL IMPAIRMENT
Two trials are available examining the impact of renal impairment on oral semaglutide safety, tolerability, and pharmacokinetics. The first study, PIONEER 5, is reviewed above.31 The second study enrolled 60 patients with varying degrees of renal impairment and administered 5 mg of oral semaglutide daily for the first 5 days, followed by a 10-mg dose once daily for the remaining 5 days of the study.36 Similar to the findings previously noted for the PIONEER 5 study, no differences were seen in any pharmacokinetic or safety parameters based on baseline kidney function, including participants receiving hemodialysis. No dose adjustments are recommended for patients with renal impairment.37
DRUG INTERACTIONS
Previous trials investigating potential drug interactions with SC semaglutide have yielded few notable interactions, most likely due to the nonspecific mechanisms of metabolism seen with semaglutide.38 Because of the unique absorption characteristics of oral semaglutide, it is important to consider how other commonly used drugs may be affected when given concomitantly with the oral GLP-1 receptor agonist. In one study, the pharmacokinetics of oral semaglutide were assessed when taken simultaneously with the proton pump inhibitor omeprazole.39 Study of this potential interaction is particularly important due, in part, to the effect of omeprazole on stomach pH and the complicated interplay between SNAC and gastric pH to allow for oral semaglutide absorption.
The study enrolled 54 healthy patients who received 5 mg of oral semaglutide daily for the first 5 days, followed by 10 mg daily for the remaining 5 days with or without 40 mg of omeprazole taken daily for the 10-day duration of the study. Although there was a slight increase in oral semaglutide exposure when taken in combination with omeprazole (possibly due to the increase in gastric pH), the increase observed was not statistically significant and was considered by the investigators to likely not be clinically relevant. This conclusion was reached not only because of the subtle increase in exposure but also due to the wide therapeutic index of semaglutide. In another study, the pharmacokinetics of lisinopril, warfarin, digoxin, and metformin were observed when coadministered with oral semaglutide and SNAC.40 Oral semaglutide or SNAC alone did not appear to alter the exposure of lisinopril, warfarin, or digoxin. While an increase in metformin exposure was observed, it was not considered clinically relevant by the investigators.
TOLERABILITY AND ADVERSE EVENTS
The most common adverse events associated with GLP-1 receptor agonist use in general are gastrointestinal in nature, with mild-to-moderate diarrhea, nausea, and vomiting being the most commonly reported events.41 Gastrointestinal symptoms, however, tend to be transient and less severe with proper dose titration.41 This finding was consistent with oral semaglutide, with mild-to-moderate gastrointestinal adverse events being the most commonly reported events in clinical trials. In the phase II dose-ranging study discussed previously, patients receiving larger doses of semaglutide (i.e., 40 mg vs. 5 mg) experienced more gastrointestinal events, although continued treatment generally resulted in lessening of symptoms.18 The prescribing information for oral semaglutide notes that the most common side effects with use include nausea, abdominal pain, diarrhea, decreased appetite, vomiting, and constipation. Like other GLP-1 receptor agonists, oral semaglutide also carries a black box warning for potential risk of thyroid C-cell tumors and a contraindication for use in patients with a personal or family history of medullary thyroid cancer or in patients with multiple endocrine neoplasia syndrome type 2.37
DOSAGE AND ADMINISTRATION
The recommended starting dose of oral semaglutide is 3 mg once daily for 30 days. After 30 days on the 3-mg dose, the dose should be increased to 7 mg once daily. The dose may be increased again to 14 mg once daily if additional glycemic control is needed after 30 days of treatment with the 7-mg dose. Patients should be counseled to take the medication with no more than 4 ounces of plain water on an empty stomach; it is recommended to be taken 30 minutes before consuming any food, nonwater beverages, or other oral medications. Waiting less than 30 minutes or taking with food or beverages other than plain water will lessen the effect of the medication. Additionally, waiting more than 30 minutes to eat after taking the medication may increase the absorption of oral semaglutide.37
CONCLUSION
In consideration of clinical trials published thus far, oral semaglutide has demonstrated efficacy in terms of A1C and weight reduction when used as an addon to a variety of background glucose-lowering regimens in patients with T2DM and inadequate glycemic control. Notably, oral semaglutide has compared favorably when studied head-to-head against a variety of agents, including an SGLT-2 inhibitor and injectable liraglutide. Because of the ever-expanding use of GLP-1 receptor agonists, the availability of an oral agent within this therapeutic class has the potential to further increase GLP-1 receptor agonist use and provide an alternative route of administration for patients unable or unwilling to take SC injections.
REFERENCES
- CDC. Diabetes and prediabetes. National Center for Chronic Disease Prevention And Health Promotion. www.cdc.gov/chronicdisease/ resources/publications/factsheets/diabetes-prediabetes.htm. Accessed August 4, 2019.
- CDC. Estimates of diabetes and its burden in the United States. National diabetes statistics report, 2017. www.cdc.gov/diabetes/pdfs/ data/statistics/national-diabetes-statistics-report.pdf. Accessed August 4, 2019.
- Lipska KJ, Yao X, Herrin J, et al. Trends in drug utilization, glycemic control, and rates of severe hypoglycemia, 2006-2013. Diabetes Care. 2017;40(4):468-475.
- Lund A, Knop FK, Vilsbøll, T. Glucagon-like peptide-1 receptor agonists for the treatment of type 2 diabetes: differences and similarities. Eur J Intern Med. 2014;25(5):407-414.
- Neumiller JJ. Incretin pharmacology: a review of the incretin effect and current incretin-based therapies. Cardiovasc Hematol Agents Med Chem. 2012;10(4):276-288.
- Ozempic (semaglutide) prescribing information. Plainsboro, NJ: Novo Nordisk, Inc; April 2019.
- Byetta (exenatide) prescribing information. Wilmington, DE: AstraZeneca Pharmaceuticals LP; December 2018.
- Victoza (liraglutide) prescribing information. Plainsboro, NJ: Novo Nordisk, Inc; June 2019.
- Trulicity (dulaglutide) prescribing information. Indianapolis, IN: Eli Lilly & Co; January 2019.
- Bydureon (exenatide extended-release) prescribing information. Wilmington, DE: AstraZeneca Pharmaceuticals LP; July 2019.
- Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27(4):740-756.
- Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
- American Diabetes Association. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl. 1):S90-S102.
- Muheem A, Shakeel F, Jahangir MA, et al. A review on the strategies for oral delivery of proteins and peptides and their clinical perspectives. Saudi Pharm J. 2016;24(4):413-428.
- Shen WC. Oral peptide and protein delivery: unfulfilled promises? Drug Discov Today. 2003;8(14):607-608.
- Buckley ST, Bækdal TA, Vegge A, et al. Transcellular stomach absorption of a derivatized glucagon-like peptide-1 receptor agonist. Sci Transl Med. 2018;10(467).
- Knudsen LB, Lau J. The discovery and development of liraglutide and semaglutide. Front Endocrinol. 2019;10:155.
- Davies M, Pieber TR, Hartoft-Nielsen ML, et al. Effect of oral semaglutide compared with placebo and subcutaneous semaglutide on glycemic control in patients with type 2 diabetes: a randomized clinical trial. JAMA. 2017;318(15):1460-1470.
- Emisphere. Emipshere's SNAC carrier achieves provisional GRAS status for use with nutrients added to foods and dietary supplements. http://ir.emisphere.com/static-files/a5eee7ce-1065-4f74-a6c43647e64446b5. Accessed August 4, 2019.
- Brayden DJ, Gleeson J, Walsh EG. A head-to-head multi-parametric high content analysis of a series of medium chain fatty acid intestinal permeation enhancers in Caco-2 cells. Eur J Pharm Biopharm. 2014;88(3):830-839.
- Frederiksen TM, Sønderby P, Ryberg LA, et al. Oligomerization of a glucagon-like peptide-1 analog: bridging experiment and simulations. Biophys J. 2015;109(6):1202-1213.
- Piper DW, Fenton BH. pH stability and activity curves of pepsin with special reference to their clinical importance. Gut. 1965;6(5):506-508.
- Bækdal TA, Borregaard J, Donsmark M, et al. Evaluation of the effects of water volume with dosing and post-dose fasting period on pharmacokinetics of oral semaglutide. Diabetes. 2017;66(suppl 1):1179-P.
- Novo Nordisk AS. Tablet formulation comprising semaglutide and a delivery agent. US9993430B2. U.S. Patent. June 12, 2018. https:// patents.google.com/patent/US9993430B2/en18. Accessed August 4, 2019.
- U.S. National Library of Medicine. Dose-response, safety and efficacy of oral semaglutide versus placebo and versus liraglutide, all as monotherapy in Japanese subjects with type 2 diabetes (PIONEER 9). https://clinicaltrials.gov/ct2/show/NCT03018028?term=oral+semaglutide &phase=2&rank=6. Accessed August 4, 2019.
- U.S. National Library of Medicine. Safety and efficacy of oral semaglutide versus dulaglutide both in combination with one OAD (oral antidiabetic drug) in Japanese subjects with type 2 diabetes (PIONEER10). https://clinicaltrials.gov/ct2/show/NCT03015220?term=oral+semagl utide&phase=2&rank=5. Accessed August 4, 2019.
- Aroda VR, Rosenstock J, Terauchi Y, et al. PIONEER 1: Randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care. June 11, 2019. 2019;42(9)1724-1732.
- Montanya E, Rosenstock J, Canani LH, et al. Oral semaglutide vs. empagliflozin added on to metformin monotherapy in uncontrolled type 2 diabetes: PIONEER 2. Abstract 54-OR. American Diabetes Association 79th Scientific Sessions; June 7-11, 2019; San Francisco, CA.
- Rosenstock J, Allison D, Birkenfeld AL, et al. Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea. JAMA. 2019;321(15):1466-1480.
- Pratley R, Amod A, Hoff ST, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet. 2019;394(10192):39-50.
- Mosenzon O, Blicher TM, Rosenlund S, et al. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): a placebo-controlled, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7(7):515-527.
- Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381(9):841-851.
- Pieber TR, Bode B, Mertens A, et al. Efficacy and safety of oral semaglutide with flexible dose adjustment versus sitagliptin in type 2 diabetes (PIONEER 7): a multicentre, open-label, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7(7):528-539.
- Zinman B, Aroda VR, Buse JB, et al. Oral semaglutide as add-on to insulin in T2DM: PIONEER 8. Abstract 985-P. American Diabetes Association 79th Scientific Sessions; June 7-11, 2019; San Francisco, CA.
- Bækdal TA, Thomsen M, Kupčová V, et al. Pharmacokinetics, safety, and tolerability of oral semaglutide in subjects with hepatic impairment. J Clin Pharmacol. 2018;58(10):1314-1323.
- Granhall C, Søndergaard FL, Thomsen M, Anderson TW. Pharmacokinetics, safety and tolerability of oral semaglutide in subjects with renal impairment. Clin Pharmacokinet. 2018;57(12):1571-1580.
- Rybelsus (semaglutide tablets) prescribing information. Plainsboro, NJ: Novo Nodisk; September 2018.
- Hall S, Isaacs D, Clements JN. Pharmacokinetics and clinical implications of semaglutide: a new glucagon-like peptide (GLP)-1 receptor agonist. Clin Pharmacokinet. 2019;57(12):1529-1538.
- Bækdal TA, Breitschaft A, Navarria A, Hansen CW. A randomized study investigating the effect of omeprazole on the pharmacokinetics of oral semaglutide. Expert Opin Drug Metab Toxicol. 2018;14(8):869877.
- Bækdal TA, Borregaard J, Hansen CW, et al. Effect of oral semaglutide on the pharmacokinetics of lisinopril, warfarin, digoxin, and metformin in healthy subjects. Clin Pharmacokinet. 2019;58(9)1193-1203.
- Neumiller, JJ, Campbell RK. Liraglutide: a once-daily incretin mimetic for the treatment of type 2 diabetes mellitus. Ann Pharmacother. 2009;43(9):1433-1444.