Osteoarthritis 2019 Guideline Updates and Best Practices
RELEASE DATE
April 1, 2021
EXPIRATION DATE
April 30, 2023
FACULTY
Cortney Mospan, PharmD, BCACP, BCGP
Associate Professor of Pharmacy
Wingate University Levine College of Health Sciences
Wingate, North Carolina
FACULTY DISCLOSURE STATEMENTS
Dr. Mospan has no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT
 Pharmacy
Pharmacy
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-21-043-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER:
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients’ conditions and possible contraindications or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
GOAL
To provide pharmacists with comprehensive knowledge of osteoarthritis (OA) incidence; presentation versus other arthritic conditions; risk factors; and nonpharmacologic, pharmacologic, and dietary-supplement interventions.
OBJECTIVES
After completing this activity, the participant should be able to:
- Review the pathophysiology, etiology, and symptoms of OA.
- Recall the 2019 American College of Rheumatology/Arthritis Foundation (ACR/AF) guideline recommendations for nonpharmacologic, pharmacologic, and dietary-supplement interventions for OA.
- Discuss common dietary supplements used in OA treatment, along with associated safety and efficacy data.
- Identify an appropriate treatment plan for OA based on patient presentation.
ABSTRACT: Osteoarthritis (OA), the most common form of arthritis, typically results from an injury or obesity. The guideline for OA management and treatment released in 2019 by the American College of Rheumatology and the Arthritis Foundation provides several key updates to the 2012 guideline. These updates include broadening the use of duloxetine and further limiting situations in which tramadol and opioid therapies are considered. OA has a significant effect on existing comorbidities and the risk of new comorbidities. As the most accessible healthcare provider, the pharmacist can play a critical role in ensuring patient awareness of holistic treatment interventions that can be used to improve pain, functioning, and quality-of-life issues associated with OA.
Osteoarthritis (OA) is the most common form of arthritis, affecting more than 32.5 million adults in the United States.1,2 Informally referred to as a disease of “wear and tear,” OA typically results from an injury or obesity.1,2 OA is more likely to develop in women, and although it can occur at any age, it frequently presents in a person’s 50s.2 OA is the most common cause of disability in U.S. adults, and it can produce a level of physical disability equal to that for congestive heart failure, heart disease, and chronic obstructive pulmonary disease.3,4 Consequently, OA is the leading indication for joint replacement surgery.3 Additionally, OA has a significant impact on health-related quality of life (QOL), similar to that occurring in depression or advanced cancer.5
OA has a significant effect on existing comorbidities and the risk of new comorbidities. OA related difficulty with exercise and other physical activities can negatively impact patients with diabetes, heart disease, dyslipidemia, and hypertension, as physical activity can be considerably important for improving these disease states. OA is particularly concerning in geriatric patients given that they are 30% more likely to experience a fall and have a 20% greater risk of fracture. Weakened muscles, decreased joint functioning, and side effects from pain medications make falls more likely.2
This article will review the updated 2019 treatment guideline for OA from the American College of Rheumatology and the Arthritis Foundation (ACR/AF), provide key distinctions from the 2012 guideline, and highlight evidence for pharmacologic interventions.
Risk Factors
Obesity is the best-established and strongest risk factor for OA development, particularly when the BMI exceeds 30 kg/m2, and the risk for knee OA is greater than for hip OA.6 Additional weight results in increased mechanical stress on joints, particularly weight-bearing joints such as the knees.1,7 Weight loss of just 2 kg is thought to decrease the risk of OA by 20% to 30%.8 It is theorized that metabolic disease and associated inflammation may also play a role.1 Female sex is a risk factor for OA, and incidence of the disease increases considerably at menopause. Estrogen has been theorized to play a protective role; however, a systematic review of 16 studies identified no clear association between sex hormones and OA in women.9 Other common risk factors for OA include age, genetics, and joint injury or overuse.1
Pathophysiology
The exact cause of OA is unknown, but it is thought that repeated and cumulative mechanical stress causes intrinsic alterations of articular tissue, resulting in degradation of collagen and proteoglycans in cartilage and leading to fibrillation, erosion, and cracking in the superficial cartilage layer.10 This spreads to deeper layers of the cartilage, and erosions form. The joints most commonly affected include (from most to least frequent) those of the hands, knee, and hip, although OA in hand joints is commonly asymptomatic.10 Beyond damage to and loss of articular cartilage, remodeling of subarticular bone occurs, along with osteophyte formations, weaking of periarticular muscles, and synovial inflammation. This often results from a disruption in the breakdown and repair of joint tissue.6
Presenting Symptoms
The most commonly experienced signs and symptoms of OA include stiffness, reduced range of motion, swelling, and pain.1,2 Pain typically occurs during use of the joint, after long periods of activity, or at the end of the day. Joint stiffness is most common upon waking or after periods of rest. Range of motion often improves after movement is initiated. Additional symptoms include joint instability, muscle weakness around the joint, and clicking or cracking sounds when the joint is bent.2
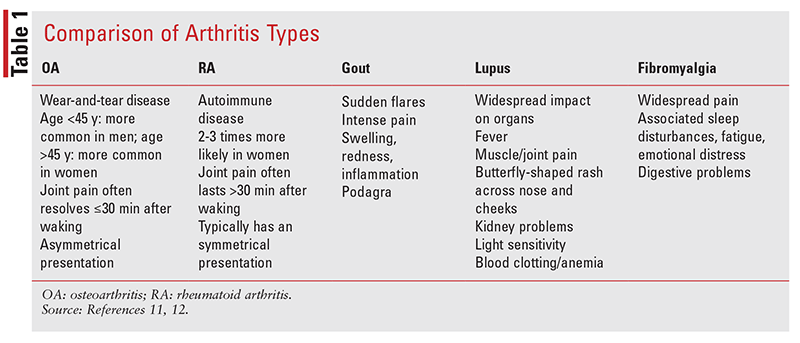
Upon questioning or during a physical examination, some patients may describe hip OA as pain in the buttocks or groin area, and sometimes the inside of the thigh. Knee OA often causes a “scraping” or “grating” feeling when the knee is in motion. In hand OA, bony spur growths lead to swollen, red, tender joints. Pain may also be present at the base of the thumb.2 TABLE 1 provides a comparison of OA and other common arthritic conditions.11,12
Holistic Management
In addition to pharmacologic interventions, comprehensive OA management requires behavioral, educational, physical, and psychosocial interventions. Although the pharmacist’s role generally focuses on pharmacologic interventions, involvement can include necessary behavioral and educational interventions and appropriate referrals to clinicians for physical and psychosocial interventions. Patient preferences and personal beliefs must be at the forefront of clinical decision making for OA, particularly for pharmacologic interventions that may be associated with substantial costs and use-related risks. Comorbidities, which are common, often include anxiety; altered sleep; depression; chronic, widespread pain; and impaired coping skills secondary to pain and functional limitations arising from OA. Monitoring patients for comorbidities and intervening promptly are crucial, and when appropriate, multimodal therapies to address comorbidities should be selected.13
Nonpharmacologic Interventions
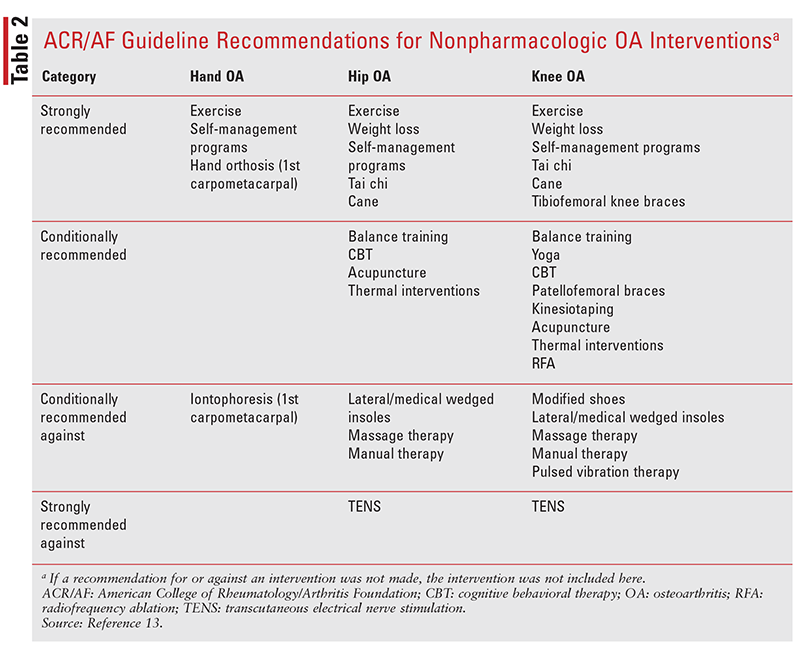
TABLE 2 provides a summary of the updated 2019 ACR/AF guideline recommendations for nonpharmacologic OA interventions based on disease location. The following text sections will discuss key information pharmacists should be aware of.
Exercise is strongly recommended for all OA locations, but there is more evidence for hip and knee OA than for hand OA. Evidence is sparse in support of one type of exercise over another, but a wide range of appropriate options exist. Selection of an exercise regimen should consider patient preference, access, and safety for participation. Exercise programs are more effective if they are supervised and if specific plans are recommended; therefore, the pharmacist should help the patient locate a personal trainer, exercise physiologist, physical therapist, or OA-focused program in the community.
Balance exercises are only conditionally recommended, as they have not been found to reduce the risk of falls in OA.13
Weight loss has a dose-response relationship with symptoms and functional impairment. A 5% loss of body weight is associated with improved outcomes, and the benefit continues to increase up to 20% loss. Pharmacists can provide education on diet and pharmacologic interventions to support weight loss. Cane use is strongly recommended for patients with knee or hip OA when the disease is appreciably affecting joint stability or ambulation or is causing pain. Transcutaneous electrical stimulation (TENS) units have entered the OTC market, and patients may be interested in using this therapy to reduce medication burden. Studies of TENS units are of poor quality, and no benefit for knee OA has been demonstrated; therefore, the use of TENS therapy is strongly recommended against.13
Pharmacologic Interventions
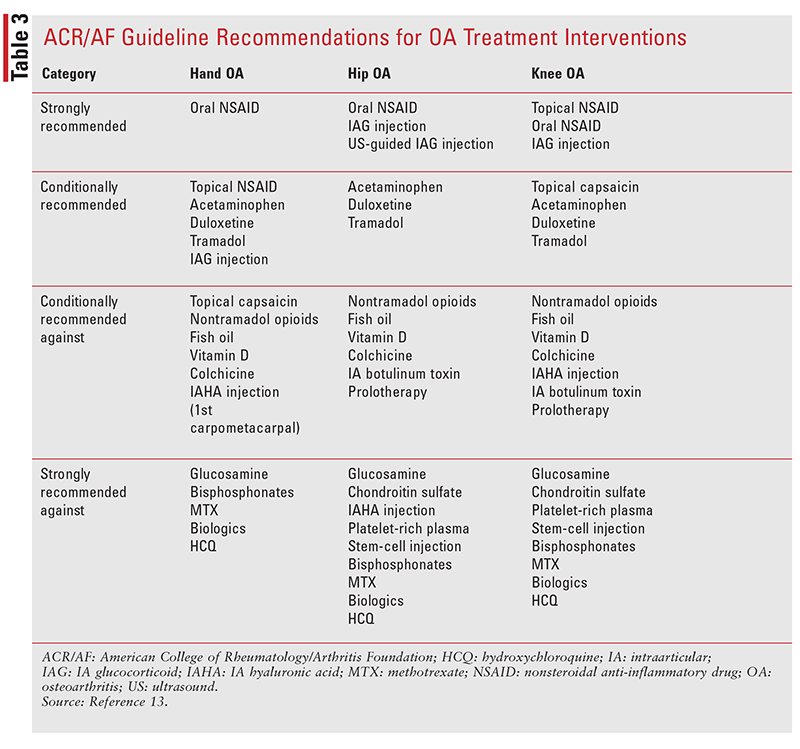
TABLE 3 provides a summary of the 2019 ACR/AF guideline recommendations for treatment interventions, including injectable therapies.13 The following text sections will discuss practical knowledge for pharmacists involved in dispensing, initiation, dose adjustment, or cessation of oral and topical pharmacologic interventions.
Acetaminophen: Previously, acetaminophen was conditionally recommended only for hip and knee OA, but the 2019 ACR/AF guideline conditionally recommends it for all affected locations. Although acetaminophen may be used, it is typically ineffective for OA, and most clinical trials have demonstrated small effect sizes (i.e., the benefit to patients is likely minimal to unnoticeable).13,14 For most patients, long-term treatment is no more effective than placebo. Acetaminophen is generally well tolerated; however, it is not without risks (e.g., hepatoxicity), and the risk increases if the patient overtreats with acetaminophen because of poor efficacy.15 Therefore, acetaminophen should be limited to short-term and episodic use, primarily in patients with intolerance or contraindications to nonsteroidal antiinflammatory drugs (NSAIDs).13
Duloxetine: The 2012 ACR/AF guideline makes no recommendation regarding the use of duloxetine, a serotonin norepinephrine reuptake inhibitor (SNRI). Duloxetine was only conditionally recommended in patients in whom total joint arthroplasty was not an option.14 In the 2019 guideline, duloxetine is conditionally recommended for all OA locations, although most evidence is from studies of knee OA.13,14 Studies have suggested that duloxetine alone or in combination with NSAIDs is efficacious for OA management, but some patients have reported tolerability problems.13
In a systematic review and meta-analysis evaluating the efficacy and safety of duloxetine in OA patients, the drug demonstrated moderate pain relief, functional improvement, and enhanced QOL over a period of 12 to 14 weeks.16 However, depressive symptoms did not improve significantly, which is an important detail given that approximately 21% of patients with OA have concomitant depression. Duloxetine patients were twice as likely as placebo patients to discontinue therapy, although serious side effects were not more frequent. Gastrointestinal (GI) side effects (e.g., constipation, dry mouth, nausea) were most common, being 4.5 times more likely to occur. Most studies used a dosage of 60 mg duloxetine daily, although a few investigated 60 mg to 120 mg daily.16 To minimize the risk of GI side effects, the lowest effective dose should be recommended. It should be noted that the efficacy of duloxetine is not a classwide effect; therefore, other SNRIs should not be recommended for OA.
Opioids: Opioids are conditionally recommended against by the 2019 ACR/AF guideline.13 The 2012 guideline strongly recommended opioids in patients who were unwilling to undergo or had contraindications to total joint arthroplasty after failing medical therapy.14 Although the 2019 guideline acknowledges that opioids may be appropriate in certain situations (e.g., no available surgical options, failure of other therapies, contraindications to first-line therapies [NSAIDs]), it no longer strongly recommends opioid use in these situations.13 The partial opioid agonist tramadol is preferred over nontramadol opioids, but no opioids have had use established beyond 1 year. Most trials showing a modest benefit of opioids for treatment of noncancer pain were limited to 3 to 12 months’ duration.13
Tramadol should be used cautiously and after all other conditional recommendations have been given a sufficient trial. Recent studies comparing tramadol with other OA treatments demonstrated that tramadol has significant morbidity and mortality risks versus NSAIDs, which themselves are not without risk.17,18 Tramadol carries a high rate of all-cause mortality, hip fracture, cardiovascular (CV) disease, and venous thromboembolism compared with selective cyclooxygenase-2 (COX-2) NSAIDs (celecoxib) and nonselective COX-2 NSAIDs (naproxen, diclofenac). However, compared with a weak opioid (codeine), tramadol had no difference in associated risks.17,18
Oral NSAIDs: This medication class is a mainstay of pharmacologic OA management and is strongly recommended despite the risks associated with prolonged use. Short-term efficacy for all OA locations has been well established. The lowest effective dose for the shortest time possible should be used.13 In patients with CV risk, naproxen appears to be the safest option. Ibuprofen carries an increased risk of recurrent myocardial infarction (MI) and mortality; however, the risks of vascular events, CV events, MI, and mortality are considerably lower than with diclofenac.19
It is essential to consider which risks of NSAID use are more critical in the specific patient in order to determine the appropriate NSAID based on COX enzyme selectivity. COX-1–selective agents (naproxen, ibuprofen) have greater GI risks (bleeding, ulcer), whereas COX-2–selective agents (meloxicam, diclofenac) have greater CV risks (blood-pressure elevation, thrombosis, MI). CV risks are greatest with COX-2–selective agents that were developed to lessen the GI risks associated with longterm NSAID use (celecoxib).19
Topical NSAIDs: In February 2020, the FDA approved diclofenac sodium topical gel, 1% (Voltaren Arthritis Pain Gel), for OTC transition.20 Topical NSAIDs are strongly recommended for knee OA and conditionally recommended for hand OA because most clinical studies evaluated topical NSAID use for knee OA. The 2019 ACR/AF guideline makes no recommendation regarding topical NSAIDs for hip OA, but it is thought that pharmacologic therapies applied topically are unable to penetrate the skin deeply enough to reach the hip joint.13
Topical diclofenac has demonstrated clinical success in 59% of patients over 6 to 12 weeks, but this may be due to the placebo effect of application route and perceived pain relief. Three studies comparing topical diclofenac with oral NSAIDs (diclofenac, ibuprofen) found at least equivalent pain relief and improvement in stiffness; however, it should be noted that the dosages of oral NSAIDs used were not maximally tolerated prescription dosages. Topical diclofenac provides relatively quick onset of action and pain relief, with signs of analgesia within 3 hours of application and symptom benefit by 1 week of therapy.21 Although long-term topical NSAID use is appropriate, OTC topical diclofenac gel without clinician oversight should be limited to 21 days.20 Owing to limited systemic absorption, topical NSAIDs are an excellent option for hand and knee OA in patients who have a history of GI bleed, are aged 75 years or older, or have any other reason to avoid the risks of oral NSAID use.14 Side effects, which are generally limited to localized reactions, include dry skin, redness, and pruritus.21
Topical Capsaicin: A key update in the 2019 ACR/AF guideline involves the use of topical capsaicin. Although it is still conditionally recommended for knee OA, topical capsaicin is now conditionally recommended against for hand OA based on a lack of direct evidence to support use, limited effect sizes or evidence of benefit in knee OA, and risk of eye contamination when applied to the hands.13,14 There is no recommendation on topical capsaicin for hip OA, and the depth of the joint suggests that there would be no meaningful effect.13
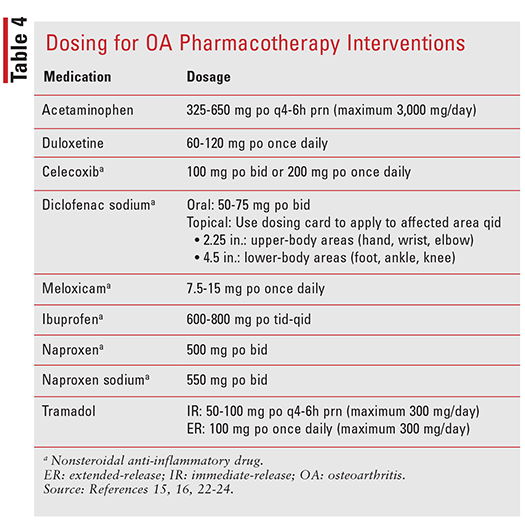
TABLE 4 provides dosing recommendations for pharmacologic interventions that are strongly or conditionally recommended by the 2019 ACR/AF guideline.15,16,22-24
Dietary Supplements
Patients are often interested in the use of dietary supplements to help manage symptoms of OA.
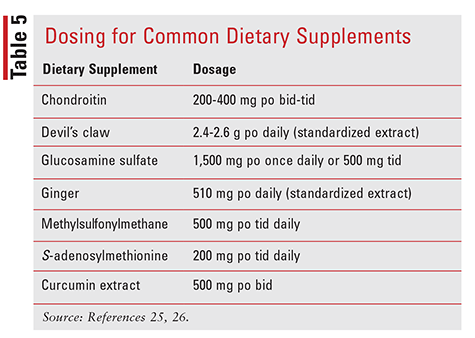
TABLE 5 provides dosing for commonly used dietary supplements.25,26 The following text sections will review the evidence for selected dietary supplements.
Chondroitin: The 2012 ACR/AF guideline conditionally recommended against the use of chondroitin for knee and hip OA, but the 2019 guideline strongly recommends against it. A notable change is that hand OA now has a conditional recommendation for use based on one trial that demonstrated analgesic efficacy.13,14 A systematic review and meta-analysis reported that chondroitin failed to achieve clinically important structural improvement in joints.27 In animal studies, chondroitin was found to maintain joint viscosity, stimulate repair of cartilage, and inhibit enzymes responsible for cartilage breakdown.28 The treatment effect of chondroitin has been shown to be delayed compared with NSAIDs.28 Adverse effects for chondroitin have been shown to be no greater than for placebo.27 Chondroitin is often derived from animal sources (e.g., bovine cartilage), which has raised concerns about disease transmission. Although there are no reports of disease transmission, it is crucial to select a chondroitin product from a company with verifiable good manufacturing practices.26
Glucosamine: Many patients use glucosamine with the goal of relieving OA symptoms and slowing or reversing disease progression.26 The ACR/AF long conditionally recommended against glucosamine use; however, it is now strongly recommended against in the 2019 guideline. Hand OA, for which there was previously no recommendation, is included in the strong recommendation against glucosamine. The change in recommendation is due to discrepancies in efficacy between publicly funded and industry-sponsored clinical trials that raised concerns about publication bias.13,14 There is also evidence of sizable placebo effects. Despite the lack of data supporting its use, glucosamine remains one of the most popular dietary supplements in the U.S. For most patients this is likely without consequence, but it is worth noting that glucosamine can cause minor elevations in glucose.13 The systematic review and meta-analysis mentioned in the previous section found glucosamine to be less effective than chondroitin, but neither agent had clinically significant efficacy.27 Interestingly, long-term glucosamine use was less effective than shortterm use. Adverse effects of glucosamine were no greater than those for placebo.27
Glucosamine has been shown in vitro to stimulate the synthesis of glycosaminoglycans and proteoglycans, supporting cartilage development. This agent has modest anti-inflammatory benefits that are significantly less potent than those of indomethacin. Some studies have shown it to be better tolerated than ibuprofen.28 Because it is derived from shellfish chitin, glucosamine should not be recommended to patients with a shellfish allergy.
S-adenosylmethionine (SAM-e): Some patients may be interested in using this product based on proposed analgesic effects, and trials have consistently shown reductions in pain; however, concerns about cost and product quality have been prevalent. It is thought that SAM-e increases chondrocytes and cartilage thickness while also decreasing cytokineinduced chondrocyte damage.26 In a meta-analysis, the Agency for Healthcare Research and Quality determined that SAM-e was more effective than placebo and comparable to NSAIDs for reducing OA pain.29
Methylsulfonylmethane (MSM): This nutrient, commonly found in combination with glucosamine- and chondroitin-containing products, has been proposed to have anti-inflammatory and analgesic properties. Although MSM has been well tolerated in clinical trials and causes no more side effects than placebo, trials have had a maximum duration of 12 weeks. Based on insufficient evidence, MSM is not recommended. It has been shown to modestly reduce pain and swelling but has no impact on joint stiffness.26
Curcumin: This nutrient has been growing in popularity for treatment of OA and other arthritic conditions. Some studies showing a benefit have been industry-sponsored; still, curcumin extract does have a significant effect on OA symptoms, albeit only over the short term (4-6 weeks).27,30 Curcumin is theorized to reduce pain, inflammation, and associated stiffness by blocking inflammatory cytokines and COX enzymes. Patients choosing to use this product should be instructed to get curcumin extract because whole turmeric can act as a blood thinner and is often contaminated with lead.30
The Pharmacist’s Role
The rate of adherence to chronic medications for OA is approximately 50%. Adherence to OA medications is greater in patients who are female, are older, have a better perceived state of health, and have been clearly advised by their healthcare provider as to the purpose of their arthritis medication. In a qualitative study, patients with OA reported differing adherence to analgesics compared with other medications for chronic disease, with reasons for this including fears about addictive properties; pill burden; noninclusion in pill boxes; lack of efficacy; and strict interpretation of directions such as “as needed.”31 Patients frequently decreased the dose or frequency of their analgesics, sometimes in order to avoid undesirable side effects such as constipation. Interestingly, most patients took NSAIDs as prescribed, not realizing that they had analgesic properties, and the use of dietary supplements was common. The study noted that many patients pushed themselves to have a high pain tolerance and viewed pain and lessened mobility as a part of aging.31
Pharmacists can play a key role in addressing barriers to OA adherence and QOL in patients such as those identified by the qualitative study discussed above. Being frontline healthcare providers, pharmacists often have considerably more points of contact with patients compared with primary care providers. Pharmacists can intervene to screen for medication adherence, efficacy, and presence of side effects. Although NSAIDs are first-line treatment for OA, they carry risks and are included in the 2019 American Geriatrics Society Beers Criteria because of the risk of GI bleeding, peptic ulcer disease, and CV and renal effects.32 Nearly one-third of patients taking NSAIDs report being unaware of contraindications for use, such as peptic ulcer disease and chronic kidney disease.32 In a survey, although 62% of patients knew potential side effects of NSAID use, only 27% were aware of the FDA’s recommendation to use NSAIDs at the lowest effective dose for the shortest possible duration in order to reduce the risk of developing these side effects.33 Knowledge is also suboptimal regarding awareness of NSAID side effects and strategies to mitigate these effects. Whereas 73% of surveyed patients taking NSAIDs were aware of side effects, fewer than one-half knew that NSAIDs should be taken with meals in order to prevent GI adverse effects. Additionally, 54% of patients were taking OTC NSAIDs without any discussion of their use with their provider.33
To help patients better understand OA and their OA medications (including risks and benefits) and to optimize the treatment approach, pharmacists should engage in shared decision making with patients. Shared decision making is a communication process in which patients and clinicians collaborate to determine optimal decisions for the patient’s health based on what is most important to the patient.34 The foundational steps of shared decision making include 1) clear, accurate, unbiased evidence about all reasonable treatment options, including risks, benefits, and burdens of each option as well as no treatment; 2) tailoring how information is communicated and presented to individual patients; and 3) patient goals, informed preferences, and concerns, including burdens of disease and treatment.34 Pharmacists should use these steps in advising patients with OA on the potential benefits and risks of pharmacologic interventions of OA as well as the potential impact of untreated or undertreated OA.
Conclusion
Most pharmacologic interventions for OA are associated with considerable risks. Interventions with fewer safety concerns often have limited clinical benefit, and in OA, several health complications and comorbidities are associated with progressing disease. Pharmacists should be well informed regarding safety and efficacy data associated with pharmacologic and complementary/alternative medicine interventions in order to appropriately advise patients on a holistic approach to OA management.
REFERENCES
1. CDC. Osteoarthritis (OA). www.cdc.gov/arthritis/basics/osteoarthritis. htm. Accessed February 1, 2021.
2. Arthritis Foundation. Osteoarthritis. www.arthritis.org/diseases/ osteoarthritis. Accessed February 1, 2021.
3. Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population-health perspective. Am J Nurs. 2012;112(3 suppl 1):S13-S19.
4. Guccione AA, Felson DT, Anderson JJ, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health. 1994;84:351-358.
5. Groessl EJ, Kaplan RM, Cronan TA. Quality of well-being in older people with osteoarthritis. Arthritis Rheum. 2003;49:23-28.
6. Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105:185-199.
7. Breedveld SC. Osteoarthritis—the impact of a serious disease. Rheumatology (Oxford). 2004;43(suppl 1):i4-i8.
8. Felson DT. Does excess weight cause osteoarthritis and, if so, why? Ann Rheum Dis. 1996;55:668-670.
9. de Klerk BM, Schiphof D, Groeneveld FP, et al. No clear association between female hormonal aspects and osteoarthritis of the hand, hip and knee: a systematic review. Rheumatology. 2009;48:1160-1165.
10. Buckwalter JA, Anderson DD, Brown TD, et al. The roles of mechanical stresses in the pathogenesis of osteoarthritis: implications for treatment of joint injuries. Cartilage. 2013;4:286-294.
11. CDC. Arthritis types. www.cdc.gov/arthritis/basics/types.html. Accessed February 1, 2021.
12. Arthritis.com. Rheumatoid arthritis vs. osteoarthritis. www.arthritis. com/about-arthritis/rheumatoid-arthritis-vs-osteoarthritis. Accessed February 1, 2021.
13. Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2020;72:149-162.
14. Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64:465-474.
15. Acetaminophen. Lexi-Drugs [online database]. Riverwoods, IL: Lexicomp, Inc; 2021. http://online.lexi.com. Accessed February 1, 2021.
16. Osani MC, Bannuru RR. Efficacy and safety of duloxetine in osteoarthritis: a systematic review and meta-analysis. Korean J Intern Med. 2019;34:966-973.
17. Zeng C, Dubreuil M, LaRochelle MR, et al. Association of tramadol with all-cause mortality among patients with osteoarthritis. JAMA. 2019;321:969-982.
18. Li L, Lu N, Xie H, et al. OP0191. Association of tramadol with all-cause mortality, cardiovascular disease, venous thromboembolism and hip fractures among patients with osteoarthritis. A population-based study [abstract]. Ann Rheum Dis. 2020;79(suppl 1):118-119.
19. Antman EM, Bennett JS, Daugherty A, et al. Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115:16341642.
20. FDA. FDA approves three drugs for nonprescription use through Rx-to-OTC switch process. www.fda.gov/news-events/pressannouncements/fda-approves-three-drugs-nonprescription-use-throughrx-otc-switch-process. Accessed February 1, 2021.
21. Bariguian Revel F, Fayet M, Hagen M. Topical diclofenac, an efficacious treatment for osteoarthritis: a narrative review. Rheumatol Ther. 2020;7:217-236.
22. Tramadol. Lexi-Drugs [online database]. Riverwoods, IL: Lexicomp, Inc; 2021. http://online.lexi.com. Accessed February 1, 2021.
23. Celebrex (celecoxib) package insert. New York, NY: Pfizer, Inc; May 2019.
24. Voltaren Arthritis Pain. What is Voltaren? www.voltarengel.com/ what-is-voltaren/. Accessed February 1, 2021.
25. Chou R, McDonagh MS, Nakamoto E, Griffin J. Analgesics for osteoarthritis: an update of the 2006 comparative effectiveness review. Comparative Effectiveness Reviews (No. 38). Rockville, MD: Agency for Healthcare Research and Quality; 2011.
26. Gregory PJ, Sperry M, Wilson AF. Dietary supplements for osteoarthritis. Am Fam Physician. 2008;77:177-184.
27. Liu X, Machado GC, Eyles JP, et al. Dietary supplements for treating osteoarthritis: a systematic review and meta-analysis. Br J Sports Med. 2018;52:167-175.
28. Wang Y, Prentice LF, Vitetta L, et al. The effect of nutritional supplements on osteoarthritis. Altern Med Rev. 2004;9:275-296.
29. Hardy ML, Coulter I, Morton SC, et al. S-adenosyl-L-methionine for treatment of depression, osteoarthritis, and liver disease: summary. AHRQ Evidence Report Summaries (No. 64). Rockville, MD: Agency for Healthcare Research and Quality (US); 2003.
30. Arthritis Foundation. Supplement and herb guide for arthritis symptoms. www.arthritis.org/health-wellness/treatment/complementarytherapies/supplements-and-vitamins/supplement-and-herb-guide-forarthritis-symptoms. Accessed February 1, 2021.
31. Sale JE, Gignac M, Hawker G. How “bad” does the pain have to be? A qualitative study examining adherence to pain medication in older adults with osteoarthritis. Arthritis Rheum. 2006;55:272-278.
32. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67:674-694.
33. Arain A, Rasheed M, Sallam N, et al. Patient’s knowledge and use of oral non-steroidal anti-inflammatory drugs in a rheumatology clinic. Kans J Med. 2019;12:132-135.
34. MGH Health Decision Sciences Center. What is shared decision making? https://mghdecisionsciences.org/about-us-home/shared-decisionmaking/#:~:text=Shared%20decision%20making%20(SDM)%20 is,what%20matters%20most%20to%20patients. Accessed February 1, 2021.