Osteoporosis in Women’s Health
RELEASE DATE:
July 1, 2017
EXPIRATION DATE:
July 31, 2019
FACULTY:
Christina Andros, PharmD/MBA Candidate 2017
Chelsea Thompson, PharmD Candidate 2017
Farbod Khaleghi, PharmD Candidate 2017
Jared Ostroff, PharmD, BCACP
Clinical Assistant Professor–Ambulatory Care
Marissa Wolff, PharmD, BCPS
Clinical Assistant Professor
Department of Pharmacy Practice
Western New England University
College of Pharmacy
Springfield, Massachusetts
FACULTY DISCLOSURE STATEMENTS:
The authors have no actual or potential conflicts of interest in relation to this activity. Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT:
Pharmacy
 Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-17-048-H01-P
Credits: 2.0 hours (0. 20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE:
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@jobson.com
DISCLAIMER:
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients’ conditions and possible contraindications or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
GOAL:
To educate pharmacists about the management of osteoporosis, including both nonpharmacologic and pharmacologic therapies.
OBJECTIVES:
After completing this activity, the participant should be able to:
- Define osteoporosis and describe its pathophysiology and impact on women’s health.
- Summarize treatment guidelines for the pharmacologic and nonpharmacologic treatment options for osteoporosis.
- Identify the role of the pharmacist in the diagnosis and treatment of osteoporosis in accordance with current practice guidelines.
ABSTRACT: Osteoporosis is a progressive, multifactorial disease that is marked by an increase in the risk of bone fractures due to bone loss. Comorbidities, diet, social history, and medication use all play a role in a woman’s risk of osteoporosis. T scores are used to measure bone mineral density and to determine if a patient is a candidate for pharmacologic therapy. Calcium and vitamin D supplementation may be used in osteoporosis management, as well as prescription medications, including bisphosphonates and hormone therapies. As the population continues to age, the incidence of osteoporosis also increases. As accessible healthcare professionals, pharmacists have the opportunity to make simple yet effective interventions in patients with osteoporosis, such as OTC recommendations and selection of supplementation.
Currently in the United States, osteoporosis affects more than 10 million Americans, and about 80% of them are women. Roughly one in two Caucasian women experiences an osteoporosis-related fracture in her lifetime. It is estimated that by 2020, onehalf of Americans over the age of 50 years will have osteopenia or osteoporosis.1
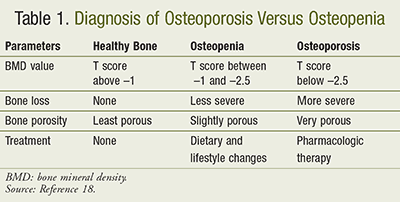
Bone mineral density (BMD) testing is the gold standard for assessing for osteoporosis or osteopenia. It is calculated by comparing one’s BMD to that of a healthy, 30-yearold adult. T scores are assigned after completion of BMD testing. A normal T score is greater than or equal to –1.0. Osteopenia is defined as a low bone mass with a T score between –1.0 and –2.5, and a T score of less than or equal to –2.5 is defined as osteoporosis. Severe or established osteoporosis is a T score of less than or equal to –2.5 with one or more fractures having occurred in the patient’s lifetime (TABLE 1).1
THE PATHOPHYSIOLOGY OF OSTEOPOROSIS
Bone is constantly remodeled by osteoclasts and osteoblasts working in coordination to ensure homeostasis of calcium levels in the blood. Osteoblasts create new bone by absorbing serum calcium and building new bone. Working against this force are osteoclasts, which break down bone to ensure that calcium levels in the blood remain between 8.5 and 10.2 mg/dL.1 The damage resulting from osteoporosis starts with bone resorption and consistent bone turnover. During bone resorption, osteoclasts—cells that break down bone—increase the concentration of calcium in the blood and release minerals into the bloodstream. In osteoporosis, bone removal is greater than bone replacement in the body because of increased osteoclast activity, leading to a decrease in bone mass and an increased risk of fractures.1
Corticosteroids are the most common iatrogenic cause of osteoporosis. They work through two mechanisms, one involving RANK ligand (RANKL) and one involving peroxisome proliferator–activated receptor gamma (PPARy2). Glucocorticoids increase RANKL, a membrane protein involved in regulating apoptosis, and in doing so they stimulate osteoclasts, which increases bone resorption.2 This is an early-stage side effect that is usually transient. PPARy2 is a nuclear receptor that regulates fatty acid storage and glucose metabolism, and it is upregulated in response to glucocorticoids, which leads to a downstream effect of decreasing osteoblasts and long-term effects of decreasing bone formation. In addition to steroids, there are many other drug classes that have been shown to increase patients’ risk of osteoporosis. These include anticonvulsants, proton pump inhibitors (PPIs) (long-term use), heparin, progestins, selective serotonin receptor antagonists, thiazolidinediones, aromatase inhibitors, and immunosuppressants. In patients maintained on regimens containing these drug therapies, it is important to weigh the risks versus benefits for potential osteoporosis development.2
Osteoporosis can also be impacted by one’s genetics. For example, women are at much higher risk for developing osteoporosis compared with men. Genetic polymorphisms are also being studied to define a possible link between genetic makeup and an increased risk of osteoporosis. Other factors, such as estrogen deficiency, the aging process, calcium or vitamin D deficiency, untreated hyperthyroidism, smoking, alcohol intake, and history of a previous fracture can contribute to a person’s risk of developing osteoporosis.4
Estrogen deficiency is common in women who are postmenopausal. As this hormone decreases in the body, bone loss rapidly follows. This same physiological change also occurs in young women who cease menstruation because of low body weight or BMI secondary to anorexia nervosa, for example. In the absence of estrogen, T cells promote osteoclast recruitment, differentiation, prolonged survival via interleukin (IL)-1, IL-6, and tumor necrosis factor, all of which affect osteoclast regulation. In contrast to postmenopausal bone loss, which is associated with an increase in osteoclast activity, bone loss that is due to aging is associated with a decline in osteoblasts.3 Women begin to lose bone after the age of 30 years, when bone resorption exceeds bone formation.
Osteoporosis may also be caused by a deficiency in calcium and/or vitamin D. Without enough calcium being absorbed, hyperparathyroidism can result. Parathyroid hormone is released in response to low calcium levels and increases calcium resorption from bone, leading to increased serum concentrations of calcium but decreased bone formation. A deficiency in vitamin D, on the other hand, can lead to impaired calcium absorption in the body and can also cause secondary hyperparathyroidism. Additionally, a high level of thyroxine in the body has been linked to an increase in bone loss.5
Fracture history also plays a large role in determining the cause and risk of developing osteoporosis. History of a previous osteoporotic fracture or the fracture of a parent’s hip can increase one’s risk. These fractures represent the imbalance in bone formation and destruction and can occur from low-energy falls as well, such as from a standing or sitting position. Fractures due to low trauma are strongly associated with osteoporosis, as they are likely caused by low BMD.3
CLINICAL PRESENTATION AND DIAGNOSIS
Osteoporosis presents various challenges for patients and providers alike. Osteoporosis is described as a silent disease and is often asymptomatic prior to incidence of fracture. Therefore, it is important to understand the common clinical presentation of osteoporosis to promote prevention, prevent progression, and choose the most efficacious treatment option for the patient.1 Most importantly, with proper diagnosis and recognition of symptoms, progression of the disease and incidence of fracture can be further prevented. Risk factors for osteoporosis include female sex, advanced age, history of fracture in a first-degree relative, dementia, tobacco use, inadequate physical activity, recurrent falls, thyroid dysfunction, and excessive alcohol intake.4
Owing to the asymptomatic nature of the disease and the fact that osteoporosis is the most common bone disease in humans, appropriate screening should be initiated to detect risk. BMD testing should be performed in accordance with the 2010 American Association of Clinical Endocrinologists (AACE) Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis to properly detect the disease.4 The guidelines suggest BMD testing should be performed in all women aged 65 years and older and in postmenopausal women in general based on their specific risk-factor profile. Furthermore, BMD should be performed in postmenopausal women aged 50 years and older who have had a fracture in adulthood in order to diagnose and determine the degree of osteoporosis. Imaging should also be performed in all women aged 70 years and older if a BMD T score is less than or equal to –1.0 at the spine, total hip, or femoral neck. In women aged 65 to 69 years, if a BMD T score is less than or equal to –1.5 at the spine, total hip, or femoral neck, and in postmenopausal women aged 50 years and older with specific risk factors, vertebral imaging should also be performed.4 The AACE guidelines advise it is also important to check for secondary causes of osteoporosis and to look for biochemical markers of bone turnover, which can serve as monitoring tools following the initiation of treatment.4
When fractures occur, they are located most commonly in the vertebrae, proximal femur, and distal forearm. In patients presenting with osteoporosis, a low bone mass is a common contributing factor to fracture, even when the fracture results from a traumatic injury. Although the majority of vertebral fractures are clinically difficult to detect initially, pain, disability, and deformity are symptoms most often associated with this type of fracture. Furthermore, vertebral fractures are a strong predictive risk factor for future fracture risk.4 Alternatively, wrist fractures are less disabling, but they can interfere with daily living and make seemingly simple tasks quite difficult for the patient. The symptoms of thoracic fractures may lead to restrictive lung disease. Lumbar fractures can influence the anatomy of the abdomen and may cause the patient to present with constipation, abdominal pain, distention, reduced appetite, and premature satiety. Pelvic and humeral fractures are also common sites of osteoporosis-associated fracture and lead to increased risk of morbidity and mortality.1
While the clinical presentation varies based on the site, severity, and extent of the fracture, it is important to not underestimate the psychosocial impact that osteoporosis and osteoporosis-related fractures can have on the patient. Osteoporosis is often associated with a reduction in mobility and ability to carry out usual tasks of daily living. Therefore, it is not uncommon for patients presenting with osteoporosis and osteoporosisrelated fractures to have concomitant depression.1
TREATMENT AND MANAGEMENT
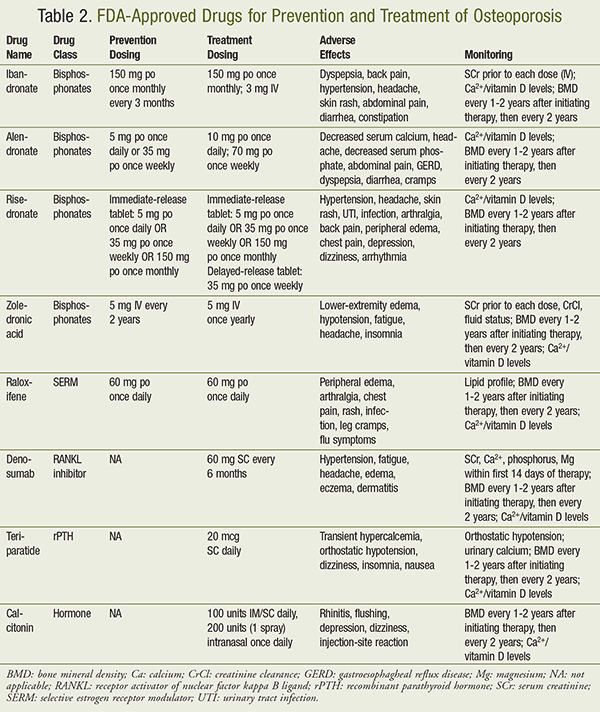
According to the 2014 National Osteoporosis Foundation (NOF) Clinician’s Guide to Prevention and Treatment of Osteoporosis, postmenopausal women aged 50 years and older should be considered for pharmacologic treatment if they meet the following parameters: history of hip/vertebral fracture; T score less than or equal to –2.5 at the femoral neck/total hip/lumbar spine; or T score between –1.0 and –2.5 with a 10-year probability of a hip fracture ≥3% or a 10-year probability of a majority osteoporosis-related fracture ≥20% (based on the U.S.-adapted WHO algorithm).1 The 2010 AACE postmenopausal osteoporosis guidelines recommend selecting pharmacologic agents for the prevention or treatment of osteoporosis based on their efficacy in reducing patient-oriented outcomes (i.e., bone fractures), rather than improvements in BMD or T scores.4 Pharmacologic agents approved for the prevention or treatment of osteoporosis are listed in TABLE 2 and include the following: bisphosphonates (alendronate, ibandronate, risedronate, and zoledronic acid), calcitonin, denosumab, estrogen therapies, raloxifene, and teriparatide. All of the aforementioned agents are efficacious in reducing the risk of vertebral fractures; however, alendronate, risedronate, zoledronic acid, denosumab, and teriparatide have also demonstrated efficacy in reducing nonvertebral fracture risk.1 Of these pharmacotherapies, only alendronate, risedronate, zoledronic acid, and denosumab have shown to reduce the risk of hip fractures.1,4
First-line agents, per the 2010 AACE guidelines, include alendronate, risedronate, zoledronic acid, and denosumab.4 Ibandronate is considered a second-line agent, while raloxifene is recommended as a second- or third-line agent. Calcitonin is reserved for women who are at least 5 years beyond menopause and who have not responded to other agents. Finally, teriparatide is reserved for patients who failed to receive any benefit with bisphosphonate therapy or those with a very elevated fracture risk, left undefined by the NOF Guidelines.1,4 Unlike the 2010 AACE guidelines, the 2014 NOF Clinician’s Guide to Prevention and Treatment of Osteoporosis avoids stratifying pharmacologic agents into first-, second-, and third-line therapies because of the lack of efficacy data from direct-comparison trials at the time of publication.1.4
Calcium
The 2010 AACE guidelines recommend ensuring that patients with osteoporosis receive adequate calcium intake, either from their diet or via external calcium supplementation. Various forms of oral calcium supplementation are available, including calcium carbonate and calcium citrate. Calcium is best absorbed when taken in amounts of 500 mg or less at one time, regardless of the formulation.1 Similarly, calcium is often supplemented with vitamin D. Vitamin D promotes the absorption of calcium in the intestine and is involved in bone remodeling by osteoblasts. Despite the widespread use of calcium supplementation, there is some controversy regarding the benefits of calcium and its potential adverse effects. If administered improperly, calcium supplementation may have decreased bioavailability and fail to compensate for the renal excretion of calcium, thus negating any potential benefits.5 Additionally, it is postulated that increased serum calcium levels may potentiate dangerous adverse effects, such as deposition in the coronary arteries of the heart, leading to myocardial infarction or stroke.5
Furthermore, it is not clear whether calcium supplementation provides a reduced fracture risk or simply improves bone biomarkers. Multiple studies have demonstrated the effect of calcium supplementation on increasing BMD; however, no data exist to support the notion that calcium reduces overall risk of fracture. While the lack of evidence may be due, in part, to issues with study design or high rates of patient noncompliance, the use of calcium supplementation alone is not recommended in either the AACE or NOF guidelines as a means for the prevention or treatment of osteoporosis.4
Calcium Carbonate: Calcium carbonate contains the highest percentage of elemental calcium (40%). Therefore, fewer pills are needed to obtain the recommended daily allowance of calcium (1,200 mg). However, calcium carbonate is also associated with increased prevalence of gastrointestinal (GI) issues, such as constipation, bloating, and abdominal pain.1 Furthermore, calcium carbonate is acid-labile and requires an acidic environment for adequate absorption. As such, it must be taken with meals to increase absorption. Calcium carbonate must be separated from other medications that may increase gastric pH, such as PPIs and histamine-2 receptor antagonists.4 Additionally, calcium carbonate has the potential to chelate with certain classes of antibiotics, such as tetracyclines and quinolones. As a result, patients are advised to separate the administration of calcium and chelating antibiotics by several hours. Specific recommendations exist for varying antibiotics. Therefore, having patients review individual drug recommendations with their pharmacists is imperative.
Calcium Citrate: Calcium citrate contains 21% elemental calcium. Therefore, more tablets are needed to obtain the same amount of elemental calcium as in calcium carbonate. This may be inconvenient for older patients or patients with a high pill burden. An additional disadvantage is cost: calcium citrate is typically more expensive than calcium carbonate, which may sway some patients away from this formulation. Because of its lower percentage of elemental calcium, calcium citrate is less associated with GI issues, such as constipation, compared with calcium carbonate.1 It also has the added benefit of reduced acid lability. As such, calcium citrate is less dependent on an acidic environment for absorption and may be taken without regard to meals.1 It may be more appropriate for patients with gastroesophageal reflux disease or ulcers, who require therapy with PPIs.
Calcitriol: Calcitriol is a synthetic active metabolite of vitamin D, which effectively stimulates calcium absorption. It is FDA-approved for the treatment of hypocalcemia, hypoparathyroidism, and mineral and bone disorder in patients with chronic kidney disease. Calcitriol’s effects are believed to be due to its ability to increase the peripheral uptake of calcium from the GI tract and renal tubule.4 It also stimulates osteoblasts to release RANKL, which in turn activates osteoclasts and promotes bone resorption. These mechanisms have the net effect of increasing calcium concentration.4
Although there is contention as to whether calcitriol has enough reliable evidence to demonstrate its efficacy in reducing fracture risk, there are data supporting its use.4 A systematic qualitative review of 16 double-blind, randomized, clinical trials evaluated the effects of calcitriol monotherapy on various bone biomarkers in postmenopausal women with primary or secondary osteoporosis.6 While a majority of the trials in the review lasted approximately 1 year, the duration of these trials ranged from 6 months to 8 years. Seven of the 16 trials utilized a two-arm design, comparing the efficacy of calcitriol with that of calcium therapy or placebo.7 Three of the seven trials found that there was no difference in bone biomarkers between women treated with calcitriol and those receiving placebo.6 The other four studies found that calcitriol resulted in statistically significant increases in at least one of the bone biomarkers but did not lead to any reduction in fracture risk.6 Ultimately, the validity of these trials is limited by small population sizes. Despite the fact that there is some evidence suggesting calcitriol may increase bone biomarkers, there are no data supporting the efficacy of calcitriol in reducing fracture risk. As such, calcitriol may not be an optimal monotherapy for the prevention or treatment of osteoporosis.
Bisphosphonates
Bisphosphonates are the most commonly prescribed class of medications for osteoporosis.1 They function by inhibiting farnesyl diphosphate synthase in the HMG-CoA reductase pathway, which prevents the formation of proteins needed for osteoclast cell membranes and results in osteoclast apoptosis. Generally, bisphosphonates require a “drug holiday” given their long-term adverse effects, including possible fractures. The guidelines note that there is considerable controversy regarding the optimal duration of said holiday. There is some evidence to suggest that patients may benefit from a 3- to 5-year drug holiday after 5 to 10 years of therapy.1,4 The drug holiday may be continued until there is a significant loss of BMD or the patient experiences a fracture, whichever occurs first.1,4 After the drug holiday, bisphosphonate therapy may be restarted as deemed appropriate by the prescribing physician. When administered orally, bisphosphonates must be taken first thing in the morning with a full 8-oz glass of water to lower the risk of the tablet lodging in the esophagus. Patients must wait at least 30 minutes after taking alendronate and risedronate and at least 60 minutes after taking ibandronate before eating or drinking. Taking bisphosphonates within 2 hours of any meal or beverage (other than water during administration) significantly reduces bioavailability. Even when administered ideally, it is estimated that less than 1% of oral bisphosphonates is absorbed systemically.1
There are several contraindications to bisphosphonate therapy, including hypocalcemia or hypersensitivity to the bisphosphonate or any of its ingredients. Bisphosphonates should be used cautiously in patients with renal impairment (typically a glomerular filtration rate less than 30 mL/min for risedronate and ibandronate or less than 35 mL/min for alendronate and zoledronate). While these conditions are not a contraindication, patients with active upper-GI diseases or esophageal abnormalities should also avoid bisphosphonate therapy.1
Bisphosphonates are available in both oral and IV formulations. While oral bisphosphonates may be more appealing to patients, many women report GI side effects, such as constipation or diarrhea. Similarly, bisphosphonates may cause first-dose infusion reactions, characterized by fever and muscle aches, when administered intravenously. Administering acetaminophen along with the bisphosphonate therapy may minimize the infusion reaction. Despite this, intravenous bisphosphonates have the added benefit of being longer-acting, allowing for intermittent administration. This may be beneficial in patients who struggle with adherence. Some patients may experience arthralgias, myalgias, or bone pain that resolves upon discontinuation of therapy. Another adverse effect is osteonecrosis of the jaw (ONJ). While ONJ is extremely serious, it is also quite rare. Risk factors for ONJ include existing dental conditions, renal impairment, invasive dental procedures, and poor dental hygiene.1,4
Alendronate: Alendronate (Fosamax) is approved for the prevention and treatment of postmenopausal osteoporosis. Alendronate is dosed as 5 mg by mouth daily or 35 mg by mouth weekly for prevention. For treatment, alendronate may be dosed as 10 mg by mouth daily or 70 mg by mouth weekly. Alendronate is considered a first-line bisphosphonate therapy based on the number of studies supporting its efficacy in reducing fracture risk.1
The 2000 Fracture Intervention Trial (FIT) evaluated the efficacy of alendronate, dosed at 5 mg/day for 2 years, in reducing verbal fracture risk. The dosage of alendronate could be increased to 10 mg/day at the second annual visit based on clinician discretion. The study included women with existing vertebral fractures or with osteoporosis (T score <–2.5). The primary endpoint was the relative risk of fracture. Ultimately, it was determined that women with both existing fractures and osteoporosis experienced significant reductions in fracture risk (P <.001). The reduction in fracture risk was seen relatively early (within 12 months) in the trial. This trial is just one of many demonstrating that alendronate may reduce the risk of fractures in patients with existing fractures or osteoporosis.9
Further, the 2009 eValuation of IBandronate Efficacy (VIBE) study was a head-to-head 12-month observational fracture study that compared the fracture rates of over 64,000 patients newly treated with a bisphosphonate medication. Patients were randomized to receive one of the following therapies: monthly oral ibandronate, weekly oral alendronate, and weekly oral risedronate. The primary endpoint was the rate of any clinical fracture. After the 12-month observational period, it was determined the incidence of any clinical fracture was lower in patients receiving ibandronate therapy (P = .052) compared with patients receiving weekly oral alendronate or risedronate. However, in the intent-totreat analysis, relative risk of fracture was not found to be significantly different between monthly oral ibandronate, weekly oral alendronate, and weekly oral risedronate. The VIBE study suggests that weekly oral alendronate or risedronate reduces risk of fracture at a similar rate compared with monthly oral ibandronate but that the ibandronate regimen may be preferred in certain patients struggling with adherence.10
Risedronate: Risedronate (Actonel) is approved for the prevention and treatment of postmenopausal osteoporosis as well. Risedronate is dosed as 5 mg by mouth daily, 35 mg by mouth weekly, or 150 mg by mouth monthly for prevention. For treatment, risedronate may be dosed as 5 mg by mouth daily, 35 mg by mouth weekly, or 150 mg by mouth monthly. Risedronate is also considered first-line bisphosphonate therapy based on evidence demonstrating reduced fracture risk.1
The 2013 Monthly Intravenous Ibandronate Versus Daily Oral Risedronate (MOVER) trial, a randomized, double-blind study, evaluated the efficacy and safety of IV ibandronate compared with oral risedronate. The study included ambulatory women aged 60 years and older. Eligible patients were assigned to receive 0.5 or 1.0 mg/month of IV ibandronate plus oral daily placebo or 2.5 mg/day oral risedronate plus IV placebo. The primary endpoint was noninferiority of ibandronate versus risedronate for new or existing vertebral fracture over 3 years. A total of 1,134 patients underwent randomization. Ultimately, it was found that both ibandronate doses were noninferior to risedronate (95% CI 0.61-1.27). The rate of new vertebral fracture over 3 years was 16.8% for 0.5-mg ibandronate, 11.6% for 1-mg ibandronate, and 13.2% for risedronate. Despite these results, both doses of ibandronate had higher rates of adverse events than risedronate (96.8%), with the 0.5-mg dose demonstrating the highest rate (98.8%) of all three groups. Ibandronate 1.0 mg/month also had the highest rate of serious adverse events leading to withdrawal compared with risedronate (6.8% vs. 6.7%).11
Further, a 2003 meta-analysis evaluated the efficacy of risedronate on several factors, namely bone density and fracture risk reduction, in postmenopausal women. Eight trials were identified, randomizing women to either risedronate or an alternative (either placebo or calcium with/without vitamin D). The primary endpoint was the rate of vertebral and nonvertebral fractures. Ultimately, 11% of women who received risedronate experienced a vertebral fracture compared with 17% of women who received placebo (95% CI 0.52-0.77). Similarly, 3% of patients in the risedronate group experienced a nonvertebral fracture compared with 4.6% of those in the placebo group. Further, the weighted mean difference for the percent change in BMD from baseline was 4.54% for the lumbar spine (P <.01), 2.75% for the femoral neck (P <.01), and 4.38% for the trochanter (P <.01). As a result, this meta-analysis demonstrated risedronate’s efficacy in reducing the risk of both vertebral and nonvertebral fractures.12
Ibandronate: Ibandronate (Boniva) is approved for the prevention and treatment of postmenopausal osteoporosis. Ibandronate is dosed as 2.5 mg by mouth daily or 150 mg by mouth monthly for prevention. For treatment, ibandronate may be dosed as 2.5 mg by mouth daily, 150 mg by mouth monthly, or 3 mg IV every 3 months. Ibandronate is considered second-line bisphosphonate therapy, as it has the least amount of data supporting its efficacy for fracture reduction.1
Monthly Oral Ibandronate in Ladies (MOBILE), a randomized, phase III, noninferiority study from 2006, evaluated the efficacy and safety of once-monthly ibandronate with daily ibandronate, which has previously demonstrated benefit in reducing verbal fracture risk. The study included women aged 55 to 80 years that were at least 5 years postmenopause with a diagnosis of osteoporosis (T score between -2.5 and -5.0). Eligible patients were assigned to receive ibandronate 2.5 mg daily, ibandronate 50 mg given on 2 consecutive days, 100 mg once monthly, or 150 mg once monthly. The primary endpoint was the percent change from baseline in lumbar spine BMD at 1 year. Ultimately, it was found that the once-monthly regimens were as effective as daily treatment. Between the monthly regimens, the 150-mg dose resulted in significant increases in femur BMD (P <.001). The study concluded that once-monthly ibandronate is just as effective as daily treatment; once-monthly dosing may also increase adherence and patient compliance. Despite these results, the findings of the MOBILE study are limited by the fact that the patient outcome was BMD rather than fracture risk.7
Further, the 2004 Ibandronate Osteoporosis trial in North America and Europe (BONE), a randomized, double-blind, placebo-controlled, parallel-group study, evaluated the efficacy and safety of once-daily and intermittent oral ibandronate in 2,946 osteoporotic women with vertebral fractures. Eligible patients were randomized to receive ibandronate 2.5 mg by mouth once daily, ibandronate 20 mg by mouth every other day for 12 doses every 3 months, or placebo. The primary endpoint was the rate of patients with new vertebral fractures at 3 years. After 3 years, it was determined that daily and intermittent oral ibandronate significantly reduced the risk of new vertebral fractures by 62% (P = .0001) and 50% (P = .0006), respectively. Further, a statistically significant relative risk reduction (RR) in clinical vertebral fractures was produced with both once-daily (49%) and intermittent (48%) ibandronate as well. The findings of the BONE study are significant in that this was the first study to prospectively suggest that intermittently dosed bisphosphonates possess antifracture efficacy.8
Zoledronic Acid: Zoledronic acid (Reclast) is approved for the prevention and treatment of postmenopausal osteoporosis. Zoledronic acid is dosed as 5 mg IV every second year for prevention or 5 mg IV once yearly for treatment. Zoledronic acid is administered via IV infusion over 15 minutes. The decreased frequency of administration may provide added convenience for patients who struggle with nonadherence. Zoledronic acid is renally adjusted; prior to each dose, a serum creatinine level should be obtained and creatinine clearance calculated using the Cockcroft-Gault equation. The use of zoledronic acid for prevention or treatment of osteporosis is contraindicated in patients with a creatinine clearance less than 35 mL/minute.13
Estrogens/Hormone Therapy
Hormone therapy is indicated for the prevention of postmenopausal osteoporosis in patients who may also seek relief from menopausal symptoms. There are various forms of hormone therapy, including estrogen-only therapy or combined hormonal therapy. For women with a uterus (no hysterectomy), combination hormone therapy is recommended. Women who have had a hysterectomy would benefit from estrogen-only therapy. Despite its efficacy, hormone therapy is associated with various adverse effects, such as deep venous thrombosis, pulmonary embolism, myocardial infarction, and stroke.1
Estrogen Agonists/Antagonists
Selective estrogen receptor (ER) modulators, such as raloxifene, tamoxifen, and toremifene, act as agonists of ERs in bone tissue while acting as an ER antagonist in reproductive tissue. This dual mechanism of action allows for decreased bone resorption in bone tissue while also minimizing the risk of ER activation in peripheral reproductive tissues. Raloxifene is dosed as 60 mg by mouth daily for prevention and treatment of osteoporosis. Raloxifene is associated with various adverse effects, such as an increased risk of thromboembolism, peripheral edema, hot flashes, arthralgias, and infections.1
Raloxifene Use for the Heart (RUTH) trial is a randomized, placebo-controlled trial from 2008 that compared the fracture rates of 10,101 postmenopausal women. Inclusion criteria were age at least 55 years and documented coronary heart disease. Patients were placed on raloxifene 60 mg by mouth daily or matching placebo and followed for a median of 5.6 years. The prespecified secondary endpoints were nonvertebral and vertebral fractures. After the follow-up period, no difference in rate of nonvertebral fractures was found between the raloxifene and placebo groups (428 events vs. 438 events, respectively; 95% CI 0.84-1.10). However, women treated with raloxifene did have a lower risk of vertebral fractures (64 events vs. 97 events; 95% CI 0.47-0.89). This effect was consistently seen in women of all ages, smoking status, BMI, and family history of fracture. There was no statistically significant difference between the treatment groups in the number of women with one or more reported adverse events (93% in both groups; P = .71). Women taking raloxifene experienced a higher number of adverse events versus placebo with regard to hot flushes, leg cramps, peripheral edema, and gallbladder issues. Ultimately, while raloxifene failed to reduce risk of nonvertebral fractures, women treated with raloxifene for 5 years did experience a reduced risk of vertebral fractures.14
Denosumab
Denosumab (Prolia) is a RANKL inhibitor indicated for the treatment of osteoporosis. It is dosed as 60 mg SC every 6 months and must by administered by a healthcare professional because of the risk for infusion reactions. It functions by binding to RANKL and preventing it from activating osteoclasts, thus decreasing bone resorption and increasing the structural integrity of the bone. Some precautions to consider include skin reactions, increased risk of infections, hypocalcemia, and (rarely) ONJ, as with bisphosphonates. Denosumab is also associated with nausea, vomiting, diarrhea, and constipation, as well as fatigue and hypercholesterolemia.15
Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) trial from 2009 is a placebo-controlled, randomized, controlled trial of 7,868 postmenopausal women. Inclusion criteria consisted of patients aged 60 to 90 years and those with a BMD T score of less than –2.5 but not less than –4.0. The primary endpoint was new vertebral fracture. Compared with placebo, denosumab lowered the risk of new vertebral fracture. Patients receiving denosumab had a 2.3% incidence of vertebral fracture versus 7.2% in the placebo group (P <.001). This represents an RR reduction of 68%. The study results found no increase in the risk of cancer, infection, cardiovascular disease, and hypocalcemia. There were also no incidences of ONJ and no adverse effects due to the denosumab itself.16
Teriparatide
Teriparatide (Forteo) is a recombinant parathyroid hormone (PTH) with a unique mechanism of action as an anabolic agent. Administration of this drug, with its extremely short half-life, shifts the PTH balance toward osteoblast activity, stimulating the creation and fortification of bone. Once teriparatide is discontinued, the patient may experience rapid bone loss. As a result, patients tapering off teriparatide therapy should be transitioned to a bisphosphonate or alternative therapy (such as denosumab) to preserve the BMD gained, owing to teriparatide’s effects. Teriparatide should not be used for more than 2 years owing to the black box warning of osteosarcoma. Teriparatide is dosed as 20 mcg SC every day and is indicated only for severe osteoporosis, as indicated by the patient experiencing a fracture while taking another pharmacologic therapy for osteoporosis. Some precautions to consider include first-dose orthostasis and renal or hepatic impairment.
Further, in a 2012 meta-analysis by Han and Wan, eight randomized, controlled trials were identified. All trials were aimed at evaluating the efficacy of daily teriparatide, injected SC in women with postmenopausal osteoporosis. While the outcomes examined by the trials varied, the main endpoints were fracture risk and percent change in BMD from baseline. It was found that teriparatide increased bone mass by 8.14% (95% CI 6.72-9.55) in the trials that reported BMD as an outcome. Further, in the three trials that reported fracture as an outcome, teriparatide was associated with a 70% RR in vertebral fractures (RR 0.30, 95% CI 0.21-0.44) and a 38% RR in nonvertebral fractures (RR 0.62; 95% CI 0.44-0.87). Ultimately, this evidence supports the assertion that teriparatide is effective in treating postmenopausal women with osteoporosis at risk for a fracture.17
Calcitonin
Calcitonin (Miacalcin, Fortical) is indicated for the treatment of osteoporosis in postmenopausal women. It is available in IM, SC, and intranasal forms. Given that calcitonin is derived from salmon, it should be avoided in patients with a history of a seafood or fish allergy. Calcitonin is associated with adverse effects such as rhinitis, epistaxis, and allergic reactions, especially when administered intranasally. When administered IM or SC, calcitonin is dosed as 100 units daily. Intranasal administration provides 200 units (1 spray) in one nostril once daily.1,4
THE ROLE OF THE PHARMACIST
As there are various treatment options for the management of osteoporosis and many individuals may be unaware that they have the disease, pharmacists can play a pivotal role in treatment management and patient education. Statistics show that only 23% of women aged 67 years or older who have osteoporosis-related fracture receive either a BMD test or a prescription for a drug to treat osteoporosis in the 6 months following a fracture.1 This introduces a role for pharmacists in encouraging their patients to adhere to their medication regimen and to educate patients on the proper pharmacologic and nonpharmacologic management of their osteoporosis.
Pharmacists can also perform a crucial service in determining adequate treatment for patients with osteoporosis and identifying drug-drug and drug-disease interactions. As most calcium supplements are available OTC, the pharmacist is an accessible healthcare professional for those with osteoporosis who are experiencing confusion over choosing the best drug therapy for their disease state.5 Identifying drug-drug interactions is also a critical role that pharmacists can fulfill in the management of osteoporosis. Pharmacists may be able to identify and tailor therapy for the patient based on the patient’s current medication profile. Some studies have shown that long-term PPI use may put patients at a higher risk for osteoporosis-related fracture, although the use of PPIs is not contraindicated in those with osteoporosis. However, if a pharmacist notices that a patient with a prescription for a PPI is also looking for a calcium supplement, the pharmacist should counsel and encourage the patient on the use of calcium citrate rather than calcium carbonate, as calcium citrate can be absorbed in the absence of the acidic environment when PPI use has lowered the acidity of the GI tract.4 As pharmacists are a constant and easily accessible healthcare provider, various opportunities are available to properly educate and counsel patients with osteoporosis on the most appropriate use of their medications. Patients need to be made aware of possible side effects of the medications they are taking for their osteoporosis. Specifically, bisphosphonates present an important potential need for patient counseling. Pharmacists can assist patients in determining if and when a drug holiday from a bisphosphonate may be needed.5 Bisphosphonates also have specific instructions for administration and when they must be taken with regard to meals. Pharmacists can also provide counseling on spacing bisphosphonates from specific antibiotics that a patient may be taking. Finally, pharmacists can also assist patients by examining their medication profiles and determining possible iatrogenic causes of osteoporosis.2
REFERENCES
- Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381.
- Compston J. Direct effects of glucocorticoids on bone. www.nature.com/nrrheum/journal/v6/n2/fig_tab/nrrheum.2009.259_f1.html. Accessed May 30, 2016.
- Bethel M. Medscape. Osteoporosis: practice essentials, background, pathophysiology. http://emedicine.medscape.com/article/330598-overview#a3. Accessed June 13, 2016.
- Watts NB, Bilezikian JP, Camacho PM, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract. 2010;16(3):1-37.
- Paziana K, Pazianas M. Calcium supplements controversy in osteoporosis: a physiological mechanism supporting cardiovascular adverse effects. Endocrine. 2015;48(1):776-778.
- Peppone LJ, Hebl S, Purnell JQ, et al. The efficacy of calcitriol therapy in the management of bone loss and fractures: a qualitative review. Osteoporos Int. 2010;21(7):1133-1149.
- Reginster JY, Adami S, Lakatos P, et al. Efficacy and tolerability of oncemonthly oral ibandronate in postmenopausal osteoporosis: 2 year results from the MOBILE study. Ann Rheum Dis. 2006;65(5):654-661.
- Chestnut CH, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19(8):1241-1249.
- Black DM, Thompson DE, Bauer DC, et al. Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab. 2000;85(11):4118-4124.
- Harris ST, Reginster JY, Harley C, et al. Risk of fracture in women treated with monthly oral ibandronate or weekly bisphosphonates: the eValuation of IBandronate Efficacy (VIBE) database fracture study. Bone. 2009;44(5):758-765.
- Nakamura T, Nakano T, Ito M, et al. Clinical efficacy on fracture risk and safety of 0.5 mg or 1 mg/month intravenous ibandronate versus 2.5 mg/day oral risedronate in patients with primary osteoporosis. Calcif Tissue Int. 2013;93:137-146.
- Cranney A, Waldegger L, Zytaruk N, et al. Risedronate for the prevention and treatment of postmenopausal osteoporosis. Cochrane Database Syst Rev. 2003;(4):CD004523.
- Boonen S, Reginster JY, Kaufman JM, et al. Fracture risk and zoledronic acid therapy in men with osteoporosis. N Engl J Med. 2012;367:1714-1723.
- Ensrud KE, Stock JL, Barrett-Connor E, et al. Effects of raloxifene on fracture risk in postmenopausal women: the Raloxifene Use for the Heart Trial. J Bone Miner Res. 2008;23(1):112-120.
- Capozzi A, Lello S, Pontecorvi A. The inhibition of RANK-ligand in the management of postmenopausal osteoporosis and related fractures: the role of denosumab. Gynecol Endocrinol. 2014;30(6):403-408.
- Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765.
- Han SL, Wan SL. Effect of teriparatide on bone mineral density and fracture in postmenopausal osteoporosis: meta-analysis of randomised controlled trials. Int J Clin Pract. 2012;66(2):199-209.
- Clifford J, Rosen MD. Postmenopausal osteoporosis. N Engl J Med. 2005;353:595-603.