Overview of Plaque Psoriasis Treatment
RELEASE DATE:
June 1, 2016
EXPIRATION DATE:
June 30, 2018
FACULTY:
G. Blair Sarbacker, PharmD, BCACP
Associate Professor of Pharmacy Practice
University of the Incarnate Word
Feik School of Pharmacy
San Antonio, Texas
Amy P. Witte, PharmD
Associate Professor of Pharmacy Practice
University of the Incarnate Word
Feik School of Pharmacy
San Antonio, Texas
David F. Maize, PhD, RPh
Associate Dean of Academic Affairs
Professor of Pharmaceutical Sciences
University of the Incarnate Word
Feik School of Pharmacy
San Antonio, Texas
FACULTY DISCLOSURE STATEMENTS:
Drs. Sarbacker, Witte, and Maize have no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT:
Pharmacy
 Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-16-040-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE:
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@jobson.com
DISCLAIMER:
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients' conditions and possible contraindications or dangers in use, review of any applicable manufacturer's product information, and comparison with recommendations of other authorities.
GOAL:
To provide pharmacists with a review of treatment options for the management of plaque psoriasis.
OBJECTIVES:
After completing this activity, the participant should be able to:
- Differentiate between the types of psoriasis.
- Identify pharmacologic and nonpharmacologic treatments and their efficacy and safety.
- Determine which treatments are recommended as first-, second-, or third-line for plaque psoriasis.
- Recommend treatment options for patients with mild, moderate, severe, or refractory disease.
ABSTRACT: Psoriasis is a chronic disease that affects multiple systems in the body, with dominant features of inflammation of the skin and joints. Plaque psoriasis is the most common form of psoriasis, accounting for around 90% of cases. Treatment of plaque psoriasis consists of a variety of options including phototherapy, topical agents, oral agents, and biologics.
Psoriasis is a chronic disease that affects multiple systems in the body. Inflammation of the skin and joints are dominant features of this disease.1 Psoriasis affects 3.2% of the United States population. It occurs primarily in Caucasians, and equally among men and women.2 Despite being primarily considered a skin disease, psoriasis is associated with several comorbidities, and severe disease has been linked with an increase in mortality.1 Symptom onset typically occurs around age 34 years, with diagnosis occurring 3 years later.3
Comorbidities of psoriasis include Crohn’s disease, ulcerative colitis, cardiovascular disease, metabolic syndrome, depression, poor self-esteem, anxiety, and decreased qual ity of life. Patients with psoriasis are shown to have higher rates of smoking and obesity.1
Psoriasis may affect a small portion of the population, but it is not without a substantial economic impact. In 2013, a systematic review noted that the economic burden totaled between $112 billion and $135 billion in the U.S. Patients with one or more comorbidities constituted an additional cost of $2,184 per 6 months in total healthcare costs.4 Among their patients, dermatologists report that patients with psoriasis require more of their time and support.3
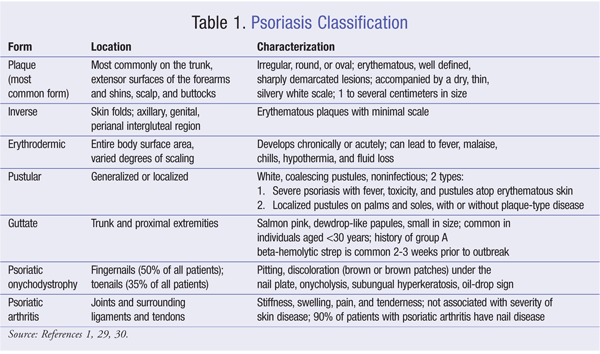
Psoriasis is classified according to the locations and types of lesions present, which are outlined in TABLE 1. Patients report that their classification of disease severity is most impacted by itching (36.1%) and location and size of lesions (21.8%).3 The focus of this lesson, plaque psoriasis, is the most common form of psoriasis; the majority of clinical trials have been conducted on patients with plaque psoriasis.1 Other forms of psoriasis include inverse, erythrodermic, pustular, guttate, nail, and psoriatic arthritis.1 To ensure that treatment is correct, identification of the appropriate disease state is critical.
PATHOPHYSIOLOGY
Psoriasis is a disease characterized by skin-cell proliferation and incomplete differentiation caused by cytokines released from infiltrating activated T cells. Evidence points to an immune-mediated, organ-specific, autoimmune disease of the skin. The exact cause of psoriasis is unknown; however, there are several hypotheses that include defects in the skin-cell cycle, genetic disposition, and an immunologic disorder. Inflammatory triggers released from skin cells during trauma or antigen exposure result in recruitment of T cells to the site. The T cells release cytokines, interleukin (IL)-12, IL-17, interferongamma, tumor necrosis factor (TNF)-a, and skin growth factors, which cause vasodilation and new capillary formation (angiogenesis), increased arachidonic acid levels, and further inflammation.5,6 In addition, the cytokines from T cells contribute to the proliferation of the epidermis. In the normal epidermis, skin cells take 13 days to divide and 26 days to mature, migrate to the stratum corneum, and shed. The skin of a patient with psoriasis takes 1.5 days to divide and 4 days to mature and shed, an increase of up to 50-fold.7
CLINICAL PRESENTATION
Psoriasis is a papulosquamous disease and can be highly variable in morphology, distribution, and severity. Papulosquamous diseases are characterized by papules and plaques, both of which are >1cm in diameter. The presentation can range from small tear-shaped papules to pustules.8
Plaque psoriasis presents with sharply circumscribed, round-oval or coin-sized plaques. Initially, the lesions begin as erythematous macules or papules that are flat and <1cm in diameter. Eventually, the macules or papules extend peripherally to form plaques that are one to several centimeters in diameter. A white blanching ring, also known as Woronoff’s ring, is observed surrounding the plaque. Scales can also be present in plaque psoriasis. Scales are silver-white in nature and can vary in thickness.8
DIAGNOSIS AND EVALUATION OF TREATMENT
Diagnosis is typically made on clinical findings. A thorough patient history should be performed along with a complete physical examination. Findings on physical examination depend mostly on the type of psoriasis present. The most common findings on physical examination include skin manifestations such as erythematous macules, papules, and plaques.8 The cornerstone of managing psoriasis is identifying areas of involvement and evaluating the severity of the disease.
Since plaque psoriasis is the most common form, most of the large clinical trials of psoriasis treatment include patients with plaque psoriasis and exclude patients with other less-common forms such as erythrodermic and pustular psoriasis, along with those forms involving the palms, scalp, and soles. The Psoriasis Area and Severity Index (PASI) is defined as a measure of the overall psoriasis severity and coverage. This severity index is commonly used in clinical trials. The PASI score is calculated before, during, and after treatment to determine how well a patient is responding to the treatment.1 Calculating the PASI score consists of two steps: calculating the body surface area (BSA) covered with lesions and assessing the severity of the lesions, such as to redness, thickness, and scaling. The calculations are then combined into a single score from 0 (no lesions) to 72 (most severe case). Overall, the effectiveness of individual treatments in clinical trials is shown by a 75% improvement in the PASI score.1
TREATMENT
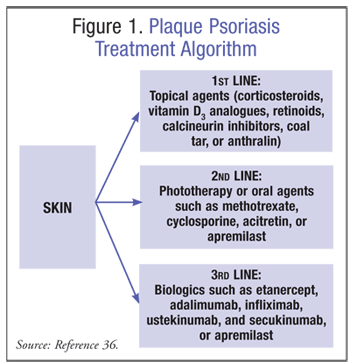 Treatment of plaque psoriasis consists of a variety of options including phototherapy, topical agents, oral agents, and biologics. A summary of the most recent guidelines has been outlined in FIGURE 1.
Treatment of plaque psoriasis consists of a variety of options including phototherapy, topical agents, oral agents, and biologics. A summary of the most recent guidelines has been outlined in FIGURE 1.
Phototherapy
Phototherapy is an efficacious and cost-effective option for patients that has been around since the 1900s. The use of ultraviolet (UV) light allows for the avoidance of systemic immunosuppression. Both UVA and UVB have been utilized. UV light exhibits immunosuppression via several mechanisms, including induction of T-lymphocyte apoptosis and inhibition of epidermal hyperproliferation and angiogenesis, among others. A complete history and physical examination should precede therapy, as lupus erythematosus and xeroderma pigmentosum are contraindications for phototherapy. In addition, caution should be used in patients who have a skin-cancer history or risk, are receiving drugs that cause photosensitivity, or are post organ transplantation.9
UVB phototherapy can be either performed using broadband (BB) or narrowband (NB) therapy. While both are deemed acceptable, NB-UVB is preferred, due to reports of increased efficacy and more rapid clearing. While it should be used with caution, NB-UVB therapy is considered first-line therapy in plaque and guttate psoriasis during pregnancy. Typically, an emollient is applied prior to UV therapy, on the theory that the transmission of UV radiation is increased with emollient use; however, this has not been studied via randomized, controlled trials. There is conflicting evidence reported for the use of UVB phototherapy with topical corticosteroids and vitamin D3 analogues. It is recommended that vitamin D3 analogues be applied after UV exposure, owing to degradation with UV exposure.
When added to UVB treatment, anthralin has shown little benefit, while topical tazarotene can decrease treatment sessions and UVB dosage, and potentially increase the efficacy of the UVB. Methotrexate and the UVB therapy have been used together successfully with a UVB dose of half the dose normally required. Retinoids accelerate the response to phototherapy and allow for a reduced dose of UVB. Additionally, the use of UVB and psoralen plus UVA (PUVA) allowed for more rapid psoriasis clearing than when either therapy was used individually. The combination of UVB with cyclosporine or biologics has not been studied extensively.9
Targeted phototherapy allows for laser targeting of specific lesions. This procedure has good data on its efficacy, but the length of remission of the lesion is unknown.9
Photochemotherapy
PUVA Photochemotherapy is the use of psoralens in UVA therapy. Psoralens are a group of compounds that sensitize cells to the effects of UVA light. In systematic reviews, oral PUVA has shown efficacy in 70% to 100% of patients treated. The typical length of treatment is 24 sessions, with remissions ranging between 3 and 6 months. Topical PUVA is also available, with the UVA being administered 30 minutes after the psoralens are applied. Toxicity includes erythema, pruritus, xerosis, irregular pigmentation, nausea, and vomiting, and can be managed by adjusting the psoralen or UV dose, using emollients, or using antipruritics.9
Topical therapies
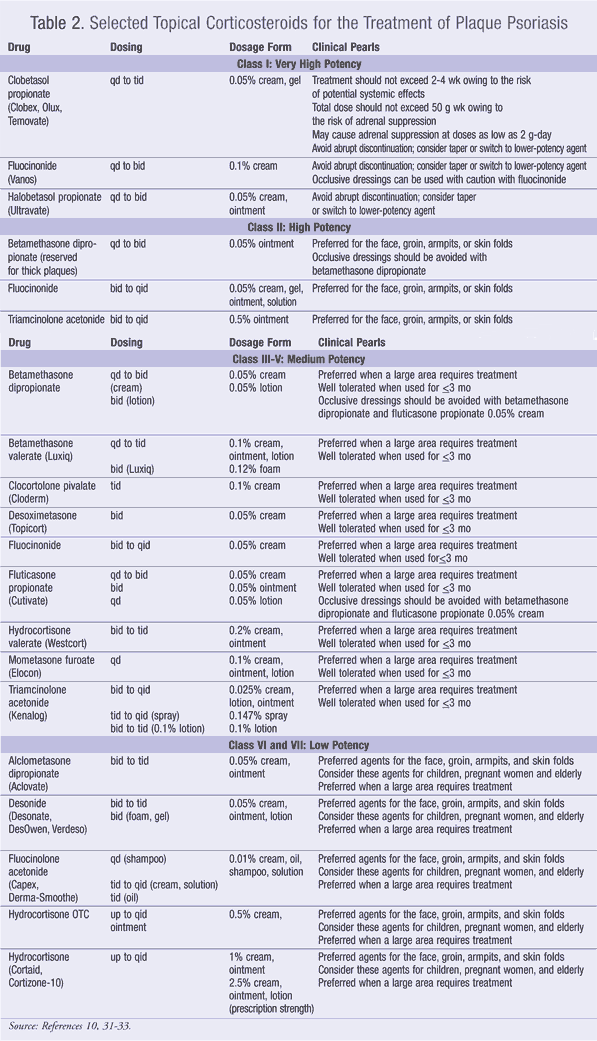
The majority of patients with moderate-to-severe plaque psoriasis use topical therapy.3 Topical corticosteroids (TABLE 2) are the mainstay of treatment for plaque psoriasis. They can be used as monotherapy or combined with other therapies. They are typically dosed one to two times daily. The more potent steroids should be used for no longer than 4 weeks in duration, with a gradual tapering off. The more potent steroids have a higher efficacy.10
When compared to the vitamin D3 analogues, a 2013 Cochrane review showed that corticosteroids had a lower incidence of local side effects and performed similarly.11 Another systematic review conducted in 2013 showed that high-potency topical corticosteroids were the most effective topical agents. Coal tar was one of the least effective of the topical agents. Coaltar shampoo was similar in efficacy to placebo. Vitamin D3 analogues were also one of the least effective topical treatments.12
The steroids, retinoids, and vitamin D3 analogues bind to receptors located in the cytoplasm instead of membrane-bound receptors or ion channels. Each drug has its own receptor in the cytoplasm. There are two glucocorticoid receptors (GR1 and GR2). Typically, anti-inflammatory steroids bind to the GR2 receptor in the cytoplasm. Retinoids bind to two receptor types, retinoic acid receptor (RAR) and retinoid X receptor (RXR). The RAR receptor has three subtypes, alpha, beta, and gamma. The RAR-gamma receptor is found most prevalently in skin cells. The vitamin D analogue binds to only one receptor, the vitamin D receptor (VDR). After the drugs bind to the c-terminal of their respective cytoplasmic receptor, heat-shock proteins dissociate from the area of the receptor called the zinc fingers. Once the zinc fingers are exposed, they have increased affinity for promoter regions on the DNA. These promoter regions are called response elements and may direct the action of the nucleus to alter transcription for proteins. Thus, when a drug-receptor complex binds to a response element, the mechanism of action of these drugs is either for the cell to produce fewer inflammatory proteins or more anti-inflammatory proteins.13-16 The pharmacologic effects are to decrease inflammation of the psoriatic plaques, reduce proliferation, and promote differentiation of skin cells.
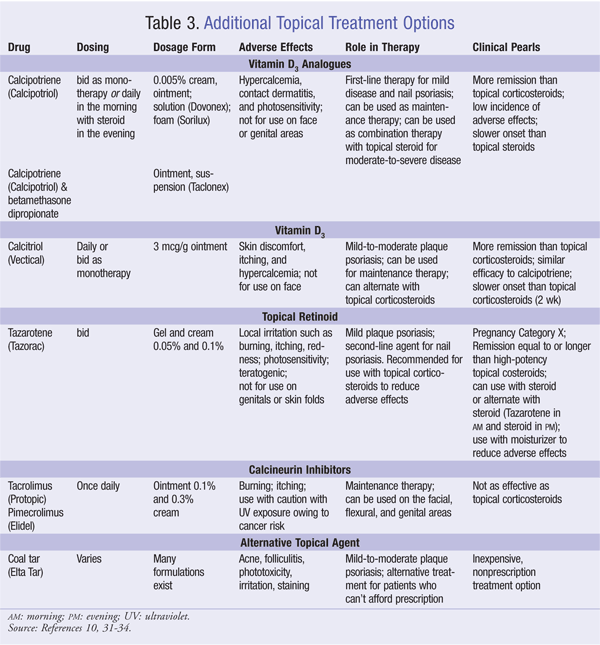
The most common side effects of topical steroids are burning, stinging, and itching at the application site. Prolonged use can lead to atrophy of the dermis and epidermis and the masking of skin infections. With long-term use around the eyes, steroids have been reported to produce cataracts and glaucoma. Topical steroids can be absorbed in sufficient quantities to produce systemic side effects when applied to large areas of the skin, or if high-potency steroids are used.17,18 TABLE 2 and TABLE 3 summarize the dosing, toxicities, laboratory monitoring, role in therapy, and clinical pearls for the topical agents.
Conventional Systemic Therapies
Conventional therapies such as methotrexate, cyclosporine (CSA), and acitretin are commonly used when psoriasis is refractory to topical therapy and phototherapy and when the disease is too extensive to use topical therapy. A minimum of 10% of body surface area is used as a marker to start systemic therapy. Even with the newer agents that are available, such as biologics, traditional systemic therapies continue to play an integral part in the treatment process. Oral administration and low cost compared to other treatment options are two advantages to making systemic therapies an important treatment alternative in certain patient populuations.19 A survey by Lebwohl et al noted that half of patients using conventional oral therapy found the treatment to be burdensome due to adverse effects and required laboratory monitoring.3 TABLE 4 summarizes the dosing, toxicities, laboratory monitoring, role in therapy, and clinical pearls for the systemic agents.
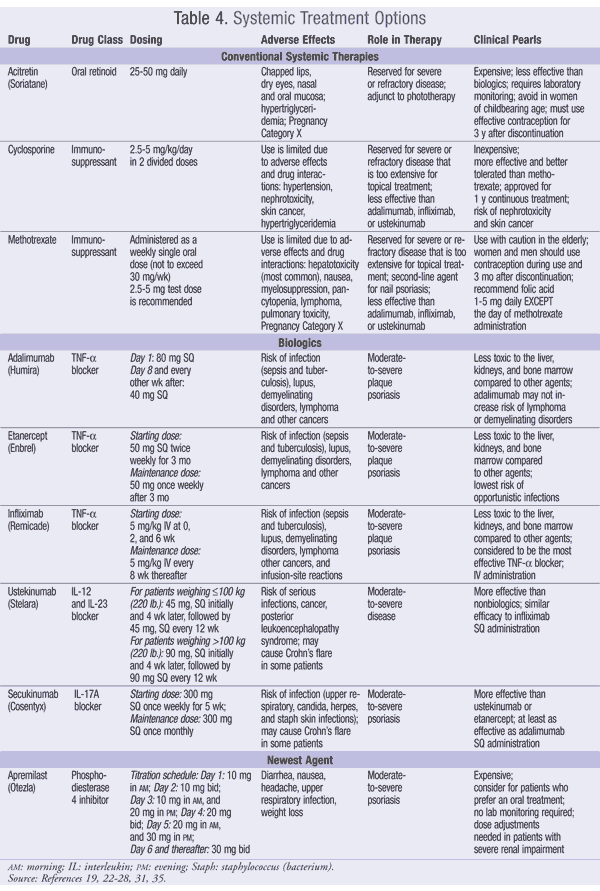
Methotrexate (MTX, Rheumatrex) reduces skin cells and T-cell proliferation by inhibiting dihydrofolate reductase. This antifolate drug inhibits DNA synthesis, promoting the apoptosis of activated T-cells. Methotrexate has serious side effects that are listed in a black box warning. Potential fatal bone marrow, liver, lung, and kidney toxicities; malignant lymphomas; and opportunistic infections have occurred in patients. Diarrhea and ulcerative stomatitis have required temporary discontinuation of treatment. Close monitoring of liver, lung, and kidney function, as well as blood count, is necessary. The more common side effects that are not life-threatening are temporary hair loss, nausea and vomiting, and loss of appetite.20
Cyclosporine (Sandimmune) is an oral calcineurin inhibitor, but it binds to the cyclophilin A immunophilin, unlike the topical calcineurin inhibitors. In addition to its use in psoriasis, it is more commonly used to prevent organ rejection during transplantation. Since it is an oral medication, careful monitoring is required for serious systemic side effects and drug interactions. Cyclosporine has a black box warning for nephrotoxicity and hypertension along with warnings against its use after light therapy, methotrexate, other immunosuppressants, and coal tar. Along with nephrotoxicity, hepatotoxicity is possible, and kidney and liver function monitoring should be done. Other less serious side effects are abdominal discomfort, nausea and vomiting, diarrhea, increase in triglycerides, hirsutism, and gingival hyperplasia.21
Acitretin (Soriatane) is an oral retinoid that reduces proliferation and enhances differentiation of skin cells. It does not suppress sebum production as effectively as isotretinoin (Accutane), which is used in the treatment of acne, but the two drugs have similar side effects. The dermatologic side effects are quite common. Patients may see inflammation of the lips and itchy, dry skin. Other common side effects are nosebleeds and dry eyes and mouth. Less common side effects are skin rashes, peeling of skin from palms and soles, photosensitization, and alopecia. Some systemic side effects are pain or stiffness of muscles, bones, and joints as a result of calcification and hyperostosis. Headache may occur with visual changes, nausea, and vomiting. Other central nervous system (CNS) side effects are lethargy and fatigue, depression, psychosis, and suicidal ideation. A change in blood lipids with an increase in triglycerides and plasma cholesterol levels and a decrease in HDL levels has been seen in some patients. Liver enzymes may also rise. Like the other retinoids, acetretin is teratogenic and in Pregnancy Category X. Women of childbearing potential need to have a negative pregnancy test and use two forms of birth control before starting treatment, during treatment, and for 3 years posttreatment when using acitretin for psoriasis.22
Biologics
Currently, biologics, which include monoclonal antibody therapy, are changing the treatment of psoriasis. This class of drugs provides us with additional treatment options that are potentially less toxic to the liver, kidneys, and bone marrow and are not teratogenic. However, patients report injection-related anxiety and fear, in addition to side effects, as the most common reasons they consider biologics to be burdensome. Concerns over safety and tolerability, lack or loss of effectiveness, and cost are some of the most common reasons cited for discontinuation of biologic therapy.3
Monoclonal antibody therapy can be used in the treatment of moderate-to-severe plaque psoriasis. All of the antibodies are produced by recombinant DNA technology and are partly human. Etanercept (Enbrel), adalimumab (Humira), and infliximab (Remicade) bind free TNF-a. Ustekinumab (Stelara) binds IL-12 and IL-23, and secukinumab (Cosentyx) binds IL-17A. The binding of TNF-a and the interleukins (12, 23 and 17A) will reduce skin thickness and infiltration of inflammatory cells into plaques. The result will be a reduction in the number and size of the plaques. The medications share similar side effects. These drugs are injected and can produce injection-site reactions like chills, site pain, and inflammation. Since part of the antibody is a nonhuman protein, a clinically significant hypersensitivity reaction may occur. These drugs are strong inhibitors of the immune system. Upper respiratory tract infections are common side effects. black box warnings for increases in the incidence of serious infections like sepsis, tuberculosis, invasive fungal infections, and opportunistic bacterial and viral diseases along with increased malignancies, particularly lymphomas, are associated with these monoclonal antibody therapies. Secukinumab has been shown to worsen Crohn’s disease. Ustekinumab may cause reversible posterior leukoencephalopathy syndrome (RPLS) presenting as headache, seizures, confusion and visual disturbances.23-27 TABLE 4 summarizes the dosing, toxicities, laboratory monitoring, role in therapy, and clinical pearls for these agents.
Newest Agent
Apremilast (Otezla), approved in 2014, is a relatively new drug for the treatment of psoriasis and psoriatic arthritis. It is classified as a phosphodiesterase (PDE) inhibitor. Other phosphodiesterase inhibitors include theophylline and sildenafil, but apremilast inhibits a different subtype of the enzyme. Apremilast inhibits the PDE 4 enzyme. The PDE 4 enzyme is responsible for breaking down cyclic adenosine monophosphate (cAMP). When the enzyme is inhibited, cAMP increases in the cell. It is unknown at this time how this mechanism reduces psoriatic symptoms, but clinically, the drug has been shown to reduce psoriatic plaques and inflammation in swollen joints. Some patients have experienced a 5% to 10% weight loss while on the drug as well as worsening depression symptoms. Deteriorating mood and suicidal ideations should be monitored when taking this drug. Other more common side effects include diarrhea, nausea, and headache.28
CONCLUSION
Psoriasis is a complex systemic inflammatory disease that affects multiple systems in the body, with inflammation of the skin and joints being dominant features in this disease. Plaque psoriasis is the most common form and is typically classified according to the location and types of lesions present. Treatment of plaque psoriasis consists of a variety of options including phototherapy, topical agents, oral agents and biologics. Topical agents are the most effective at treating mild-to-moderate disease; the conventional systemic therapies, along with other treatment options such as biologics, can be used to treat severe or refractory disease. Factors to consider when selecting therapy include cost, ease of use, adverse effects, patient allergies, and comorbid conditions. Pharmacists play a crucial role in providing education on the various treatment options described above to patients with plaque psoriasis.
REFERENCES
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58(5):826-850.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70(3):512-516.
- Lebwohl MG, Kavanaugh A, Armstrong AW, Van Voorhees AS. US Perspectives in the management of psoriasis and psoriatic arthritis: patient and physician results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) survey. Am J Clin Dermatol. 2016;17(1):87-97.
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151(6):651.
- Boehncke W-H. Etiology and pathogenesis of psoriasis. Rheum Dis Clin N Am. 2015;41(4):665-675.
- Kumar V, Abbas A, Fausto N, Aster J, eds. Robbins and Cotran Pathologic Basis of Disease. 8th ed. Philadelphia, PA: Saunders Elsevier; 2010.
- Schön MP, Boehncke WH. Psoriasis. N Engl J Med. 2005;352(18):1899-1912.
- Langley RG. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(suppl_2):ii18-ii23.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62(1):114-135.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60(4):643-659.
- Mason A, Mason J, Cork M, et al. Topical treatments for chronic plaque psoriasis: an abridged Cochrane systematic review. J Am Acad Dermatol. 2013;69(5):799-807.
- Samarasekera EJ, Sawyer L, Wonderling D, et al. Topical therapies for the treatment of plaque psoriasis: systematic review and network meta-analyses. Br J Dermatol. 2013;168(5):954-967.
- Mangelsdorf DJ, Thummel C, Beato M, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83(6):835-839.
- O'Malley B. The steroid receptor superfamily: more excitement predicted for the future. Mol Endocrinol. 1990;4(3):363-369.
- Fisher GJ, Voorhees JJ. Molecular mechanisms of retinoid actions in skin. FASEB J Off Publ Fed Am Soc Exp Biol. 1996;10(9):1002-1013.
- Haussler MR, Whitfield GK, Haussler CA, et al. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 1998;13(3):325-349.
- Temovate (clobetasol propionate) package insert. Melville, NY: Fougera Pharmaceuticals Inc; 2012.
- Ultravate [package insert]. Jacksonville, FL: Ranbaxy; 2013.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61(3):451-485.
- Methotrexate [package insert]. Eatontown, NJ: Roxane Laboratories, Inc; 2015.
- Sandimmune (cyclosporine) package insert. East Hanover, NJ: Novartis Pharmaceuticals Corp; 2015.
- Soriatane (acitretin) package insert. Research Triangle Park, NC: Stiefel Laboratories, Inc; 2015.
- Enbrel (etanercept) package insert. Thousand Oaks, CA: Amgen; 2013.
- Humira (adalimumab) package insert. North Chicago, IL: AbbVie Inc; 2016.
- Remicade (infliximab) package insert. Horsham, PA: Janssen Biotech, Inc; 2013.
- Stelara (ustekinumab) package insert. Horsham, PA: Janssen Biotech, Inc; 2013.
- Cosentyx (secukinumab) package insert. East Hanover, NJ: Novartis Pharmaceuticals Corp; 2015.
- Otezla (apremilast) package insert. Summit, NJ: Celgene Corporation; 2015.
- Gottlieb A, Korman NJ, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 2. Psoriatic arthritis: overview and guidelines of care for treatment with an emphasis on the biologics. J Am Acad Dermatol. 2008;58(5):851-864.
- Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386(9997):983-994.
- Canadian Psoriasis Guideline Commitee. Canadian guidelines for the management of plaque psoriasis. Can Dermatol Assoc. 2009. www.dermatology.ca/wp-content/uploads/2012/01/cdnpsoriasisguidelines.pdf. Accessed March 17, 2016.
- Guenther L, Koo J, Choi J. Psoriasis treatment. www.skintherapyletter. com/treat/psoriasis/. Accessed March 17, 2016.
- Kim GK. The rationale behind topical vitamin D analogs in the treatment of psoriasis: where does topical calcitriol fit in? J Clin Aesthetic Dermatol. 2010;3(8):46-53.
- Vectical (calcitriol) package insert. Melville, NY: Fougera Pharmaceuticals Inc; 2012.
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis—results of two Phase 3 trials. N Engl J Med. 2014;371(4):326-338.
- Coates LC, Kavanaugh A, Mease PJ, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015: treatment recommendations for psoriatic arthritis 2015. Arthritis Rheumatol. 2016;68:1060-1071.