Pancreatitis From Pathophysiology to Pharmacotherapy
RELEASE DATE
December 1, 2023
EXPIRATION DATE
December 31, 2025
FACULTY
Hunter Edge, PharmD Candidate 2024
Jeffery Bruni, PharmD Candidate 2024
William Carey University School of Pharmacy
Biloxi, Mississippi
Elina Delgado, PharmD, BCPS
Assistant Professor
William Carey University School of Pharmacy
Department of Pharmacy Practice
Biloxi, Mississippi
FACULTY DISCLOSURE STATEMENTS
Hunter Edge, Jeffery Bruni, and Dr. Delgado have no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT
 Pharmacy
Pharmacy
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-23-140-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients' conditions and possible contraindications or dangers in use, review of any applicable manufacturer's product information, and comparison with recommendations of other authorities.
GOAL
To equip pharmacists with useful and practical information as it relates to pancreatitis and its complexities.
OBJECTIVES
After completing this activity, the participant should be able to:
- Describe the pathology of pancreatitis and the differences between acute and chronic pancreatitis.
- Identify risk factors for the development of pancreatitis and pancreatitis-related complications, and describe appropriate treatment, including pharmacologic agents.
- Explain how pharmacologic agents induce pancreatitis.
- Discuss pharmacologic and nonpharmacologic treatment options for pancreatitis and appropriate durations.
ABSTRACT: Pancreatitis is one of the most common gastrointestinal disease states in the United States and has an associated economic burden of around $2.5 billion annually. For many patients, there is often no one specific cause that is to blame; however, excessive alcohol consumption, smoking, elevated triglycerides, and medications are some of the most common reversible etiologies. Pancreatitis may be acute or chronic in nature, present in a range of severities, and cause secondary complications. The treatment approach should be patient-specific, and patient education is imperative to aid in controlling the disease.
Pancreatitis is a complex and potentially life- threatening inflammatory disorder that affects the pancreas, an essential organ with both endocrine and exocrine functions. This multifaceted disease presents a significant healthcare challenge globally due to its high incidence, associated morbidity, and economic burden. There are two forms of pancreatitis: acute and chronic. Acute pancreatitis (AP) has sudden onset and is a short-term condition.1 AP alone is responsible for roughly 270,000 hospitalizations each year and incurs an inpatient cost upward of $2.5 billion. To quantify it further, the incidence of pancreatitis is somewhere along the lines of 13 to 45 cases per 100,000 persons.2 Chronic pancreatitis (CP) is a long-term condition characterized by a loss of endocrine and exocrine pancreatic function. CP is less common than AP and results in roughly 86,000 hospitalizations each year.1
PANCREATIC ANATOMY AND PHYSIOLOGY
Located transversely in the upper abdomen, inferior to the liver and posterior to the stomach, the pancreas is strategically located to provide endocrine and exocrine functions.3,4 The endocrine activity is performed by the islets of Langerhans; three secretory cell types compose the islets. Alpha cells produce and secrete glucagon, beta cells are responsible for insulin, and delta cells secrete somatostatin. Additional hormones produced by the pancreas include amylin, C-peptide, and pancreatic polypeptide. Once produced, the hormones are released into the bloodstream. Perfusion of blood through the pancreas is complex due to anatomical location and vessel junctions.
In addition to a complex vasculature, the pancreas has an extensive ductal system that carries the pancreatic juices and enzymes made by the exocrine cells of the gland.3,4 The main duct traverses the length of the organ and typically joins with the end of the common bile duct to form the ampulla of Vater, which opens into the duodenum. Pancreatic enzymes, electrolytes, and mucus are the main components of pancreatic juice. The four classes of enzymes—proteolytic, lipolytic, glycolytic, and nucleolytic—play an integral role in the digestion of food and absorption of nutrients. Pancreatic exocrine function is regulated by hormones, regulatory peptides, and neurotransmitters—largely in response to food consumption. Trypsin, chymotrypsin, carboxypeptidase, and elastase digest proteins; lipase, phospholipase, and esterase digest fats; lactase and amylase break down carbohydrates; and ribonuclease and deoxyribonuclease break down nucleic acids into mono- and oligonucleotides. Pancreatic insufficiency can lead to malabsorption of vitamins, minerals, and proteins, leading to malnutrition and associated complications.
PANCREATITIS
Pancreatitis is an inflammatory condition that results from the activation of pancreatic zymogens prior to exiting the pancreas.5 Notably, trypsinogen activates early into trypsin, causing a cascade of other pancreatic enzymes to be converted. This premature conversion causes autodigestion of the peripancreatic tissue and pancreatic parenchyma. To guard against this occurring, the pancreas has three defense mechanisms, largely targeted at trypsin. These include the pancreatic secretory trypsin inhibitor, which can bind to and inactivate 20% of trypsin activity; autolysis of prematurely activated trypsin; and nonspecific proteases such as alpha-1 antitrypsin. These defense mechanisms eventually become exhausted, leading to the development of pancreatitis. Despite the form of pancreatitis (AP or CP), patient complaints of abdominal pain are usually consistent. Patients may describe the abdominal pain as knifelike, radiating, and persistent. A patient's description of his or her pain and additional symptoms may vary based on stage and severity of the disease.
Acute Pancreatitis
AP is one of the most common gastrointestinal (GI) disorders requiring acute hospitalizations globally, including in the United States.6 It is an inflammatory condition that is most commonly due to excessive alcohol consumption, the presence of gallstones, or excessive triglycerides.7 According to the 2012 revision of the Atlanta classification and definitions by international consensus, diagnosis of AP requires two of the following three clinical manifestations: abdominal pain consistent with severe, persistent epigastric pain that can radiate to the back, serum lipase or amylase activity at least three times greater than the upper limit of normal, and characteristic findings consistent with pancreatic inflammation shown on a contrast-enhanced CT or an MRI.
AP can further be classified into two categories—interstitial edematous pancreatitis and necrotizing pancreatitis. AP can also present with infection, which is important to diagnose due to the need for antibiotic treatment. Furthermore, AP can be classified into two phases: early and late. Early phase is usually the first week of inflammation, whereas late phase runs from weeks to months after the early phase. The late phase typically presents in patients who have moderately severe or severe AP. Also, within AP, there are three severity stages of this condition: mild, moderately severe, and severe. Mild AP does not present with any organ failure or local/ systemic complications. Patients who present with mild AP typically stay within the early phase of the disease and can be discharged relatively quickly. Moderately severe AP can present with transient organ failure or local/systemic complications. This stage of pancreatitis is still able to resolve without treatment. Severe AP is defined as persistent organ failure that can have a high associated mortality. Patients should be reevaluated on their grading scale at 24 hours, 48 hours, and 7 days after admission.8
Chronic Pancreatitis
CP is a disease characterized by chronic inflammation and fibrosis of the pancreas, coupled with the loss of acinar and islet cells.9 Similar to the severity ranges seen in AP, patients with CP may or may not experience pain or loss of endocrine or exocrine function. This condition can have an increased rate of development if the patient is a chronic alcoholic or smokes, but it can also develop in as much as 10 years or longer after idiopathic pancreatitis.10 The most common etiologic factor for CP ranges is based on the region of the world. In Western countries, approximately 40% to 70% of CP cases are caused by alcohol abuse, whereas in Asian countries, approximately 59% to 80% of CP cases are caused by idiopathic pancreatitis. Approximately 60% of CP cases occur following a diagnosis of AP and recurrent cases of AP. Clinical manifestations of CP can range from abdominal pain, vitamin A/D/E/K deficiencies, malabsorption of protein and carbohydrates leading to malnutrition, and endocrine insufficiencies that can manifest as diabetes mellitus.11
Drug-Induced Pancreatitis
Drug-induced pancreatitis (DIP), despite only making up 0.1% to 2% of all cases of pancreatitis, has been brought back into the spotlight due to the growing use of glucagon-like peptide 1 inhibitors. The World Health Organization has 525 different medications listed that can cause AP.12 As more medications are brought to market and given to more patients, additional medications will inevitably join the list of agents that can cause DIP. Of course, certain medications are more associated than others with causing pancreatitis. A mnemonic that may aid healthcare providers in remembering some of the main medications that can cause DIP is FATSHEEP (furosemide, azathioprine/asparaginase, thiazides/tetracycline, statins/sulfonamides, hydrochlorothiazide, estrogens, ethanol, and pentamidine).13 This, of course, does not cover all medications leading to DIP. Other common medications that can cause pancreatitis include valproic acid, calcium-channel blockers, 5-aminosalicylic acid, 6-mercaptopurine, isoniazid, and corticosteroids, among many others. How and why medications' mechanism of action can induce pancreatitis remain unknown for many of the common drugs, but theories on how it can occur have been published. These mechanisms include directly affecting the pancreatic tissue with toxins, metabolic effects caused by an increased stimulation of the pancreas, idiosyncratic reactions, changing the amount/rate of bile flow due to pancreatic/biliary duct constriction, accumulation of a toxic metabolite or intermediary, and secondary damage to the pancreas.14
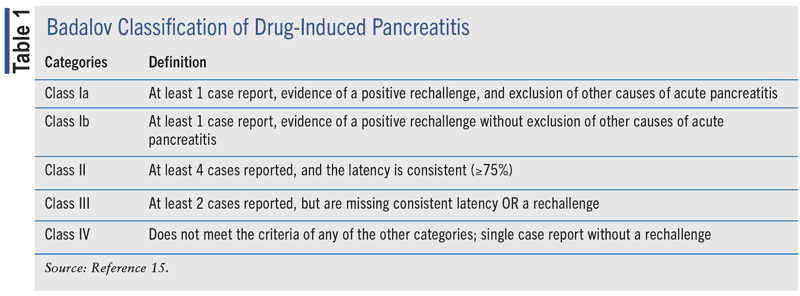
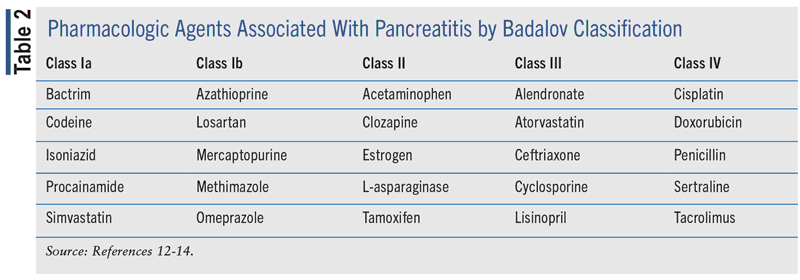
The Badalov classification of DIP is a widely used system to identify and classify medications that can lead to DIP.15 Within this system, it classifies medications that are more prone to induce DIP than others, while also including clinical outcomes to classify them. There are five classes in the Badalov classification of DIP (see TABLE 1). Class I is divided into two subgroups: Ia and Ib. Group Ia was labeled as a medication that had at least one case report with a positive rechallenge and excluded all other causes, such as alcohol, gallstones, etc. Group Ib was labeled as a medication that had at least one case report with a positive rechallenge, but other causes, such as alcohol, gallstones, etc., were not ruled out. Class II medications were labeled as having at least four positive cases in the literature with consistent latency. Class III medications were labeled as having at least two positive cases in the literature and did not have any rechallenges or consistent latency. Class IV medications were those that did not fit into earlier described classes but had at least one positive case in the literature. A noncomprehensive list of medications in each of the classes is listed in TABLE 2.
DIAGNOSTIC TESTS
If a healthcare provider assesses a patient and suspects pancreatitis, laboratory tests that aid in the diagnosis are lipase and amylase.16 Lipase, as mentioned earlier, is a digestive enzyme that the pancreas secretes that serves to help break down dietary fats. Amylase is a digestive enzyme released by the salivary glands and pancreas that serves to break down long-chain carbohydrates into smaller, more easily digested sugars. While the amylase test may help indicate pancreatitis, the most appropriate laboratory test for it is lipase. These two enzymes are elevated when a person experiences pancreatitis because the cells of the pancreas are damaged and can no longer regulate their release. These damaged cells end up releasing more enzymes than normal, and therefore they appear as elevations in the blood. Lipase and amylase levels that correspond with AP are three times the upper limit of normal.7 Additional laboratory tests may include C-reactive protein (CRP), hematocrit (HCT), blood urea nitrogen (BUN), procalcitonin, triglyceride, and calcium levels. CRP, HCT, BUN, and procalcitonin are nonspecific in determining a diagnosis of AP but aid in predicting severity and infected necrosis.
Aside from laboratory value-related indicators, ultrasound, CT scan, and MRI aid in the diagnosis of pancreatitis.16 Multiorgan inflammation may be observed in the pancreas, gallbladder, or liver. This is due to the high amount of lipase in the blood, which feeds the conversion of lipids into fatty acids that work on several inflammatory pathways. Another indicator of pancreatic dysfunction is the loss of pancreatic beta-cell function. As the beta cells of the pancreas become scarred, the release of insulin is reduced and leads to the appearance of elevated blood glucose. This loss of function can be permanent.
PROGNOSIS ASSESSMENT
The severity and type of pancreatitis present directly influence the overall prognosis of the patient.17 There are many prognostic tests available to score and determine the outlook of the patient. These tests include the Acute Physiology and Chronic Health Evaluation II (APACHE II) scale, the CT severity index, the Imrie scoring system, and Ranson's criteria. The APACHE II scale looks at many parameters, such as age, mean arterial pressure, heart rate, Glasgow Coma Scale, chronic health status, and a few other parameters to estimate mortality in critically ill patients. A higher score on this scale represents an increase in risk of death.18 Similar to APACHE II is the BISAP (bedside index of severity of acute pancreatitis) score, which is simple to calculate and used to predict severity, risk of death, and organ failure.7 The CT severity index is set up to consider the degree of pancreatic necrosis paired with a Balthazar score.19 The Balthazar score is defined by whether the pancreas is enlarged, presence of inflammatory changes, and extent of pancreatic fluid collections near the pancreas. The sum of these scores constitutes the level of severity of pancreatitis. A score of 3 or less is considered mild, 4-6 is considered moderate, and 7-10 is considered severe. The Imrie scoring system is set up to determine severity of pancreatitis by utilization of blood tests done on admission and then again 48 hours post admission. There are eight components to this blood test, and a cutoff at 3 determines whether someone has mild/moderate pancreatitis or severe pancreatitis. Ranson's criteria is an 11-factor test that assesses severity and predicts mortality for patients with pancreatitis. It is also based on blood- work done upon initial admission and then 48 hours later. In all of these prognostic tests, a higher score correlates with undesirable outcomes and mortality.
TREATMENT
The treatment someone may receive for pancreatitis is dependent on which type of pancreatitis is present. Nonpharmacologic treatments for pancreatitis include reducing alcohol intake, quitting smoking, achieving and maintaining a healthy weight, and regularly eating a healthy diet.
Acute Pancreatitis
Treatment for AP is determined by etiology and severity.6 Mild AP often resolves after a few days of rest and some supportive treatments such as low-fat diets, pain medications, and hydration. One of the most important ways to help a patient with AP of any severity is to maintain adequate hydration via fluid replacement. Patients who are in considerable pain can be expected to eat and drink much less than normal. The International Association of Pancreatology and the American Pancreatic Association guidelines for the management of AP recommend lactated Ringer's at 5 mL/kg/hour to 10 mL/kg/hour until the patient meets rehydration goals. Fluid resuscitation has been accomplished if the patient has met one or more of the three criteria: 1) heart rate <120 beats per minute, mean arterial pressure between 65 and 85 mmHg, and a urinary output >0.5 to 1 mL/kg/hour; 2) invasive clinical targets of stroke volume variation and intrathoracic blood volume determination; 3) biochemical targets of hematocrit 35% to 44%. In comparison, the American Gastrointestinal Association (AGA) recommends use of goal-directed therapy for fluid management but makes no formal recommendation on fluid volume or preference to lactated Ringer's compared with normal saline.20 The AGA does, however, recommend against the use of hydroxyethyl starch fluids. Patients should also be initiated on enteral feedings within the first 24 hours of admission to prevent against gut failure and infectious complications.7,20
Treatment of the underlying cause should always be a goal.19,20 A cholecystectomy (gallbladder removal) is often warranted when gallstones are the root cause of pancreatitis. For patients who present with signs of severe AP related to infected necrosis, antibiotics that penetrate the pancreas should be initiated.7 Empirically, the regimen should include agents that provide coverage against aerobic and anaerobic gram-positive and gram-negative bacteria. Reasonable options would include piperacillin-tazobactam, a carbapenem, or a quinolone for patients with severe allergies to penicillin. Paracentesis can be done to remove fluid from the abdomen and reduce abdominal pressure. Drainage of pseudocysts and removal of necrotic or damaged tissues from the pancreas are other procedures that can help treat AP.
Chronic Pancreatitis
Treatment for CP is slightly different than for AP and should be tailored to each individual patient. For patients displaying signs of exocrine insufficiency, fat-soluble vitamin supplementation (A, D, E, and K) and B12 injections are recommended if there is malabsorption present.9 Enzyme supplementation may be indicated in patients who need GI symptom (diarrheal, flatulence) control and experience weight loss.21 Although approximately 90,000 USP units of lipase are required for appropriate fat absorption, initial pharmacologic lipase supplementation should be 40,000 to 50,000 USP units per meal, and half for snacks.9 The total amount per meal is typically divided into two, one consumed during and one after the meal. Dose escalation is based on symptoms (diarrhea or steatorrhea), weigh gain, and increases in concentrations of fat-soluble vitamins. This may be paired with high-protein diets for patients experiencing protein-energy malnutrition. For patients with CP who develop endocrine insufficiency, glucose control can prove challenging due to the lack of insulin but also glucagon, which defines type 3 diabetes mellitus. Insulin and metformin are used to manage the hyperglycemia, with great caution to not induce hypoglycemia.
Surgery
Surgery may be required to relieve pressure or remove necrotic, damaged, or diseased tissues on or around the pancreas or pancreatic duct.7,21 If pressure due to a clogged duct is a problem, an endoscopic retrograde cholangiopancreatography or endoscopic sphincterotomy may be performed. This procedure allows for dilation of the duct by placement of a pancreatic stent. The open duct will allow for removal of stones and tissues for biopsy, if required. If stones are specifically the problem, the approach could be to utilize extracorporeal shock wave lithotripsy, which blasts the stones into smaller pieces for removal by endoscopy. Historically, pancreatoduodenectomy was the most common operation for CP; however, multiple organ-preserving operations are now first-line when surgical intervention is required. In patients with refractory CP, removal of the pancreas may still be required. If a total pancreatectomy is performed, auto-islet transplantation can be done to help the patient regain some level of hormonal function. If this is done, diabetes management may be integrated in the patient's care for the remainder of his or her life, depending on insulin needs after surgery.
Pancreatic Pain Management
Pain management is crucial for patients who are experiencing pancreatitis, regardless of the diagnosis being acute, chronic, or drug induced. Pain is a common symptom associated with pancreatitis, affecting as many as 90% of patients with CP.22 Pain management will be directed to the specific type of pancreatitis as well as the severity of the diagnosis. Many studies looking at pain management in patients with AP have looked at the use of nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids, such as pethidine, fentanyl, morphine, pentazocine, procaine, and many others. A systematic review of these trials found that there was no significant difference in the change of pain scores for patients when comparing the use of NSAIDs with opioids.23
Pain management for pancreatitis typically follows the World Health Organizations analgesic ladder.24 In terms of treatment options for analgesia, we begin with NSAID use and acetaminophen. If this combination is ineffective, we move to drugs such as tramadol—a low-potency opioid. If a patient does require the use of opioid medications, we start with the lowest effective dose and pair it with selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors, tricyclic antidepressants, or gabapentinoids (e.g., pregabalin and gabapentin). These treatments can last days to months, depending on the patient and his or her response to CP. The patient should be aware that these treatments will not eliminate the pain but instead make the pain much more manageable. Pain management via nerve block may also be done for patients who are in considerable pain for long periods of time.21
Novel techniques for managing pain in pancreatitis patients are currently being researched. One study examined the use of thoracic epidural analgesia (TEA) compared with placebo and found that the use of TEA did not have any major rate of complications, while also establishing effective management of pain.25
SECONDARY COMPLICATIONS
Once patients experience pancreatitis, they become at risk for secondary complications.26 These complications arise in both AP and CP. They vary in severity and are divided into either systemic or local complications.
Systemic Complications
Systemic complications of AP include the following:
Acute Respiratory Distress Syndrome (ARDS): Caused by secretion of inflammatory chemicals into the serum, which eventually distribute to cause inflammation in the lungs.27 ARDS is treated with supportive care by means of oxygen, fluid management, and blood pressure support.
Acute Kidney Injury (AKI): Occurs as the result of a mixture of volume depletion of the vasculature and several other inflammatory, vascular, and humoral factors that follow acute pancreatitis.28 AKI treatment involves IV fluids, alleviating intra-abdominal pressure by draining fluid, and dialysis.
Compartment Syndrome: Refers to a sustained increase in abdominal pressure >20 mmHg coupled with new organ dysfunction.29 Compartment syndrome can cause hypoperfusion to nerve and muscle tissues in the abdomen. Compartment syndrome is remedied by a fasciotomy, which is a surgical procedure that allows built-up pressure to escape through an incision.
Disseminated Intravascular Coagulation (DIC): Can occur from the systemic inflammation that occurs in AP, which causes injury to the endothelium and directly activates clotting factors.30 DIC is treated with plasma and blood transfusions, as well as anticoagulant medication.
LOCAL COMPLICATIONS
Local complications of AP include the following:
Pancreatic Fluid Collections (PFCs): A complication that entails a sequestration of pancreatic fluids that can develop into either pseudocysts or walled-off pancreatic necrosis.31 The management of PFCs involves nutritional support: oral if tolerated for mild pancreatitis or enteric for moderate pancreatitis. As well, drainage may be required depending on the presence or not of symptoms.
Walled-Off Pancreatic Necrosis (WOPN): A complication of pancreatitis in which solid necrotic tissues are encapsulated. There are instances wherein WOPN resolves spontaneously but this is not usually the case, and instead drainage is necessary. Drainage in WOPN can be done by means of surgical debridement of the pancreatic bed, percutaneously by utilization of a catheter, or endoscopically by using coiled wire to expand the size of the lumen to allow entry of the endoscope for mechanical removal of necrotic tissues.
Pancreatic Pseudocyst: A fluid-filled pseudocyst near the pancreas that is often benign.32 A pseudocyst refers to a collection of fluid surrounded by a nonepithelialized wall, as opposed to a true cyst that has a capsule lined with epithelial tissue. Treatment of a pseudocyst involves utilization of endoscopic ultrasound (EUS) and transmural drainage. The EUS grants endoscopists insight into which vascular structures may be present around the pancreatitis so that they may prevent any possible damage while setting up to drain the pseudocyst.
CP COMPLICATIONS
Complications of CP include the following:
Diabetes: Can occur in the presence of both AP and CP; however, it is more common in CP.33 Somewhere between 25% and 40% of patients who experience AP will develop diabetes within 3 to 5 years after the event. Somewhere between 25% and 80% of patients with CP will develop type 3c diabetes. The reason for the development of diabetes is that the pancreas becomes scarred and fibrosed over time.9 This leads to malfunction and decreased secretion of the important pancreatic hormones and enzymes necessary for proper function. Diabetes management will be required for the remainder of the patient's life.
Progression to Pancreatic Cancer: Can occur after a prolonged period of pancreatic inflammation.34 Pancreatic cancer is the fourth-highest cause of cancer death globally. Pancreatic cancer has a complex pathology that is beyond the scope of this article, but this complication is worth keeping in mind.
Pseudocyst Formation: Can occur in CP just as it does in AP.9
Pancreatic Pseudoaneurysms: These are quite rare in CP and are slightly different than a typical aneurysm.35 In a typical aneurysm, the arterial wall becomes weaker/thinner due to erosion and eventually bulges out and causes damage. In pancreatic pseudoaneurysms, this same basic principle remains but involves fibrous tissues instead. Pancreatic pseudoaneurysms are also different in that the weakening of the fibrous wall happens due to proteolytic enzymes that escape from the damage done by chronic inflammation. Pancreatic pseudoaneurysm is treated by embolization in patients who are extensively bleeding, and if the conditions are right, thrombin can also be utilized.36 Aside from these options, surgery may be required.
Splenic Vein Thrombosis (SVT): A complication that occurs in both AP and CP and is often treated with anticoagulation medication.37 There are a few pancreas-specific factors that lead to SVT, such as changes in thrombotic inflammation near the pancreas, compression of splenic veins by a pseudocyst, or a lack of adequate blood flow to the pancreas. MRIs, CT scans, and ultrasounds can all be used to identify if a patient has an SVT. SVT is treated with anticoagulation therapy, such as low-molecular-weight heparin.38
RISK MITIGATION/PREVENTION
Risk factors for pancreatitis are similar to the causes previously discussed; therefore risk mitigation may overlap.4 To prevent recurrent AP or progression/exacerbation of CP, patients should be counseled on lifestyle modifications. Avoidance of tobacco and alcohol abuse is a must. For tobacco cessation, this will help to decrease the probability of developing pancreatic carcinoma secondary to pancreatitis. Dietary changes can be recommended to these patients for supportive care. Patients with history of pancreatitis should consume low-fat, small meals and maintain adequate hydration. The recommended energy distribution is 15% from protein, 20% from fat, and 65% from carbohydrates. Vegetable fats are recommended over saturated and trans fats; to do this, patients should decrease consumption of red meats, fried foods, and processed food. Additionally, they should avoid food high in sugar or spicy items. All of this, coupled with an increase in fruits, vegetables, and foods rich in omega fatty acids, will help with pancreatitis. This dietary change will also help with triglyceride control—a separate risk factor for pancreatitis. Vitamin D and calcium supplementation may be recommended as well if malabsorption is present to prevent against the development of osteopenia.
Patients who have diseases that cause systemic inflammation, such as cystic fibrosis, are at increased risk for the development of pancreatitis.4 Patients with diseases that are associated with inflammation or that indirectly impair pancreatic function (e.g., celiac disease) should receive medical management aimed at those conditions to reduce pancreatic insult. Vigilance regarding prescribing of medications that are associated with pancreatitis is also important. While history of pancreatitis is not a specific contraindication to many drugs associated with the disease, a risk-benefit analysis should be conducted; if a medication is utilized, it should be done with close follow-up monitoring.15 If a patient's pancreatitis has been due to gallstones in the past, removing the gall-bladder can be a viable option for these patients. Obesity is a risk factor for developing gallstones, so making sure that patients are aware of their ideal body weight range can help to avoid the issue of gallstones.
CONCLUSION
As the landscape of pharmacy continues to evolve, we must continue to strive to meet the needs of the contemporary patient. For pancreatitis, notable ways pharmacists could play a role in care include patient education, drug-drug and drug-disease assessment, supplementation dosing, and pain management. Many patients are not informed consumers; they do not completely understand the risk factors for development of pancreatitis, including medications or approaches to mitigate the risk once they have had pancreatitis. When able, pharmacists can serve as a source of information for patients and also advise physicians when patients are on multiple medications known to increase the risk of pancreatitis. Once pancreatitis has already occurred, ensuring proper pain management is required acutely. For patients affected by CP, ensuring that they receive adequate enzyme supplementation to mitigate symptoms and prevent mal-absorption is imperative. Additionally, people must remain diligent in confirming that they are receiving the necessary vitamin and mineral supplementation required, as malabsorption of these may not cause obvious symptoms but can lead to poor clinical outcomes.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
1. National Institute of Diabetes and Digestive and Kidney Diseases. Definition & facts for pancreatitis. November 2017. www.niddk.nih.gov/health-information/digestive-diseases/pancreatitis/definition-facts. Accessed November 14, 2023.
2. Yadav D, Lowenfels AB. The epidemiology of pancreatitis and
pancreatic cancer. Gastroenterology. 2013;144(6):1252-1261.
3. Mahadevan V. Anatomy of the pancreas and spleen. Surgery
(Oxford). 2019;37(6):297-301.
4. Karpińska M, Czauderna M. Pancreas—its functions, disorders, and physiological impact on the mammals' organism. Front Physiol. 2022;13:807632.
5. Jones MR, Hall OM, Kaye AM, Kaye AD. Drug-induced acute
pancreatitis: a review. Ochsner J. 2015;15(1):45-51.
6. Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/
APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13(4 Suppl 2):e1-e15.
7. Leppäniemi A, Tolonen M, Tarasconi A, et al. 2019 WSES
guidelines for the management of severe acute pancreatitis.
World J Emerg Surg. 2019;14(1):27.
8. Banks PA, Bollen TL, Dervenis C, et al. Classification of acute
pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102-111.
9. Forsmark CE. Management of chronic pancreatitis. Gastroenterology. 2013;144:1282-1291.
10. Roberts-Thomson IC. Progression from acute to chronic pancreatitis. JGH Open. 2021;5(12):1321-1322.
11. Gardner TB, Alder DG, Forsmark CE, et al. ACG clinical
guideline: chronic pancreatitis. J Gastroenterol. 2020;115:322-339.
12. Conti Bellocchi MC, Campagnola P, Frulloni L. Drug-induced acute pancreatitis. Pancreapedia: Exocrine Pancreas
Knowledge Base. 2015. www.pancreapedia.org/reviews/drug-induced-acute-pancreatitis.
13. Kaufman MB. Drug-induced pancreatitis: a potentially serious and underreported problem. P T. 2013;38(6):349-351.
14. Weissman S, Aziz M, Perumpail RB, et al. Ever-increasing
diversity of drug-induced pancreatitis. World J Gastroenterol.
2020;26(22):2902-2915.
15. Zheng J, Yang Q, Dang F, Yang J. Drug-induced pancreatitis:
an update. Arab J Gastroenterol. 2019;20(4):183-188.
16. National Institute of Diabetes and Digestive and Kidney Diseases. Diagnosis of pancreatitis. November 2017. www.niddk.nih.gov/health-information/digestive-diseases/pancreatitis/diagnosis. Accessed November 14, 2023.
17. Carroll JK, Herrick B, Gipson T, Lee SP. Acute pancreatitis:
diagnosis, prognosis, and treatment. Am Fam Physician.
2007;75(10):1513-1520.
18. Medscape. APACHE II. https://reference.medscape.com/calculator/12/apache-ii. Accessed November 24, 2023.
19. Cuete D, et al. CT severity index in acute pancreatitis. September 20, 2021. Radiopaedia. www.radiopaedia.org/articles/ct-severity-index-in-acute-pancreatitis-3?lang=us. Accessed November 14, 2023.
20. Crockett SD, Wani S, Gardner TB, et al. American Gastroenterological Association Institute Guideline on Initial Management of Acute Pancreatitis. Gastroenterology. 2018;154(4):1096-1101.
21. Cohen SM, Kent TS. Etiology, diagnosis, and modern management of chronic pancreatitis: a systematic review. JAMA Surg. 2023;158(6):652-661.
22. Goulden MR. The pain of chronic pancreatitis: a persistent
clinical challenge. Br J Pain. 2013;7(1):8-22.
23. Cai W, Liu F, Wen Y, et al. Pain management in acute pancreatitis: a systematic review and meta-analysis of randomised controlled trials. Front Med (Lausanne). 2021;8:782151.
24. Gapp J, Tariq A, Chandra S. Acute pancreatitis. In: Stat-Pearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan-.
25. Bachmann KA, Trepte CJ, Tomkötter L, et al. Effects of thoracic epidural anesthesia on survival and microcirculation in
severe acute pancreatitis: a randomized experimental trial. Crit Care. 2013;17(6):R281.
26. Mohy-ud-din N, Morrissey S. Pancreatitis. In: StatPearls
[Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan-.
27. Brady V. What you need to know about pancreatitis. UChicagoMedicine. October 31, 2019. https://www.uchicagomedicine.org/forefront/gastrointestinal-articles/what-you-need-to-know-about-pancreatitis#:~:text=With%20severe%20pancreatitis%20there%20are. Accessed November 14, 2023.
28. Nassar TI, Qunibi WY. AKI associated with acute pancreatitis. Clin J Am Soc Nephrol. 2019;14(7):1106-1115.
29. Zarnescu NO, Dumitrascu I, Zarnescu EC, Costea R.
Abdominal compartment syndrome in acute pancreatitis: a narrative review. Diagnostics. 2023;13(1):1.
30. Abu-Abaa M, Al-Qaysi G, Abdulsahib A, Acob T. Acute pancreatitis and disseminated intravascular coagulopathy in COVID-19 infection: a case report. Cureus. 2023;15(1):e34104.
31. Tyberg A, Karia K, Gabr M, et al. Management of pancreatic
fluid collections: a comprehensive review of the literature. World
J Gastroenterol. 2016;22(7):2256-2270.
32. Misra D, Sood T. Pancreatic pseudocyst. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan-.
33. The Ohio State University Wexner Medical Center. Unlocking
the mysteries surrounding acute pancreatitis and diabetes. https://wexnermedical.osu.edu/departments/innovations/gastroenterology/pancreatitis-diabetes-study. Accessed October 1, 2023.
34. Kandikattu HK, Venkateshaiah SU, Mishra A. Chronic pancreatitis and the development of pancreatic cancer. Endocr Metab
Immune Disord Drug Targets. 2020;20(8):1182-1210.
35. Gurala D, Polavarapu AD, Idiculla PS, et al. Pancreatic pseudoaneurysm from a gastroduodenal artery. Case Rep Gastroenterol. 2019;13(3):450-455.
36. Hoilat GJ, Mathew G, Ahmad H. Pancreatic pseudoaneurysm. In: StatPearls [Internet]. Treasure Island, FL: StatPearls
Publishing; 2023 Jan-.
37. Benjamin C, Bryant M, Tran T, et al.
The need to anticoagulate patients with splenic vein thrombosis.
Cureus. 2023;14(7):e26488.
38. Sissingh NJ, Groen JV, Timmerhuis HC, et al. Therapeutic
anticoagulation for splanchnic vein thrombosis in acute pancreatitis: a national survey and case-vignette study. World J Gastroenterol. 2023;29(21):3328-3340.