Pediatric Food Allergy Update
RELEASE DATE
August 1, 2021
EXPIRATION DATE
August 31, 2023
FACULTY
Jessica Shue, PharmD
Post-Doctoral Fellow, U.S. Oncology Medical Information
Rutgers Institute for Pharmaceutical Industry Fellowships
Novartis Pharmaceuticals Corporation
East Hanover, New Jersey
Jennifer LaPreze, PharmD, BCACP, CDCES, CPP
Clinical Staff Pharmacist
Atrium Health
Fort Mill, South Carolina
FACULTY DISCLOSURE STATEMENTS
Dr. LaPreze and Dr. Shue have no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT
 Pharmacy
Pharmacy
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-21-084-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients’ conditions and possible contraindications or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
GOAL
To educate pharmacists and pharmacy technicians about the epidemiology, pathophysiology, clinical presentation, prevention, and evidence-based management of food allergies in the pediatric population.
OBJECTIVES
After completing this activity, the participant should be able to:
- Define the prevalence and pathophysiology behind the most commonly encountered pediatric food allergies.
- Identify pediatric patients with the highest risk for food allergies, the clinical presentation, and the basis for diagnosing these allergies.
- Describe the current recommendations for pediatric food-allergy prevention and management.
- Review the role of pharmacists in preventing and managing pediatric food allergies and when to refer to a pediatrician.
ABSTRACT: Multiple risk factors predispose infants to develop food allergies. Food allergies are categorized as IgE-mediated, non-IgE-mediated, and mixed reactions, and each varies by clinical presentation, allergens associated with its development, and diagnostic criteria. Multiple strategies exist to prevent food allergies, but only a few are backed by evidence and proven to reduce the risk of development. The primary treatment for both IgE-mediated and non-IgE-mediated reactions is complete avoidance of the specific food allergen known to elicit the response. The first-line treatment for anaphylactic IgE-mediated reactions is epinephrine. Novel treatment modalities aiming to eliminate food allergies or help speed up the desensitization process are currently being used or investigated.
Infantile and pediatric food allergies have increased in frequency over the past few years in the United States and worldwide. The exact prevalence of infantile and pediatric food allergies is difficult to quantify as values reported in each study vary based on the methodology used to identify food-allergy prevalence (i.e., self-reported vs. confirmed with allergy testing). Reports estimate that food allergies affect about 8% of children in the U.S.1-3 In recent years, it appears that food allergies, especially allergies to peanuts and tree nuts, are becoming more prevalent. The eight most common allergenic foods, not listed in order of prevalence, are cow milk, eggs, fish, crustacean shellfish, tree nuts, peanuts, wheat, and soybeans.4
Pathophysiology
Several factors can increase the risk of developing allergies to specific food allergens or all food allergens. An infant is at the highest risk of developing food allergies if he or she has severe eczema, followed by other known food allergies, mild-to-moderate eczema, and genetic predisposition.2,5 Genetic predisposition is defined as an infant with one or more first-degree relatives who have allergic disease.4 Having severe eczema places an infant at the highest risk for developing an IgE-mediated food allergy.2 Non-Hispanic black children, Hispanic children, and Asian children are at increased risk of developing food allergies along with male children, as compared with females.5 Infants with severe eczema and/or egg allergy are at highest risk of developing a peanut allergy.4 Additionally, infants affected by eczema coupled with a late introduction to peanut products are at increased risk of developing a peanut allergy.5 It is important to remember that even infants without any risk factors can still develop food allergies.2
Another factor that potentially plays a role in the development of food allergies is microbial dysbiosis.1 Dysbiosis is a term that indicates some sort of imbalance within the microbiome and can be associated with specific disease states when it occurs in the human body.6 The term microbiome is used to discuss all of the microorganisms and their unique genomic features and interactions in a specific ecologic site—for example, the human body.6 Microbial diversity refers to the number of species and how they are distributed throughout a sample or site.6 Trillions of microorganisms colonize the linings of the skin and mucosal surfaces of the human body.6 As understanding of the microbiome has expanded over the past few years, multiple studies have looked into the microbiome’s role in developing allergic diseases.6 Most of these studies are heterogeneous, and their importance continues to evolve as we learn more about the role that the microbiome plays in regulating the human immune system.6
It is hypothesized that the microbiome plays a key role early in life, especially with immunologic development and response, which means that it could also play a role in whether someone will develop allergic diseases.6 Both human and animal studies have found links between the gut, skin, and respiratory tract microbiome and the development/presentation of allergic diseases.6 Multiple factors can affect the composition of the microbiome, such as age-related changes, diet, disease states, and specific environmental exposures, including medications.6
One large, retrospective, cohort study examined whether an association exists between acid-suppressive medication and antimicrobial use in the first 6 months of life and subsequent development of allergic diseases, such as food allergies.7 This study found an association between histamine-2 receptor antagonist and proton pump inhibitor use within the first 6 months of life and increased risk of food-allergy development with an adjusted hazard ratio (aHR) of 2.18 (95% confidence interval [CI], 2.04-2.33) and an aHR of 2.59 (95% CI, 2.25-3.00), respectively. Additionally, an association was found between use of antimicrobials early in life and an increased risk for developing cow milk and egg allergies, with an aHR of 1.24 (95% CI, 1.15-1.33) and aHR of 1.24 (95% CI, 1.13-1.37), respectively.7 These findings support the judicious use of acid-suppressive medications and antimicrobials during infancy.7
Diet is thought to play a crucial role in regulating the microbiome, because microorganisms within the gut use the food taken in as energy sources.6 The type and quality of food ingested will directly influence which metabolites microorganisms will produce, leading to changes to the microbiota that can be helpful or harmful to human health.6 A diet rich in fiber can help promote a healthy gut microbiome, likely due to the production of short-chain fatty acids (SCFAs), which can have a wide variety of protective effects on the gut.6 When microorganisms in the gut ferment complex carbohydrates, SCFAs are produced.1 Two of the most crucial functions of SCFAs include the induction of differentiation of Treg cells located in the gut and the ability to induce mechanisms that help enforce the integrity of the epithelial barrier.1 A diet that consists of high amounts of less-healthy fats (e.g., saturated fats) can lead to the production of unhelpful metabolites, leading to negative changes in the gut microbiome and potentially leading to tissue damage and inflammation.6
Studies have shown that the window of opportunity to optimize gut health may occur within the first 3 to 6 months of life, because the gut microbiome composition during this time has been associated with milk-allergy resolution.6 Similarly, the gut microbiome composition at age 3 months has been linked to an increased likelihood of food sensitization at age 1 year.6 These studies did not show the same results when looking at older age groups, suggesting the potential importance of the gut microbiome composition during early infancy.6 Additionally, studies suggest avoiding antibiotic exposure early in life and prenatally, choosing vaginal instead of cesarean delivery when possible, and having pets in the home before and after birth to potentially lower the risk for developing allergic diseases linked to microbial dysbiosis.6
Food allergies can be broken down into three main categories: immunoglobulin (IgE)-mediated, non-IgE-mediated, and mixed reactions.5 The typical onset, pathogenesis, and some examples for each reaction are discussed in TABLE 1.3,5, 8-11
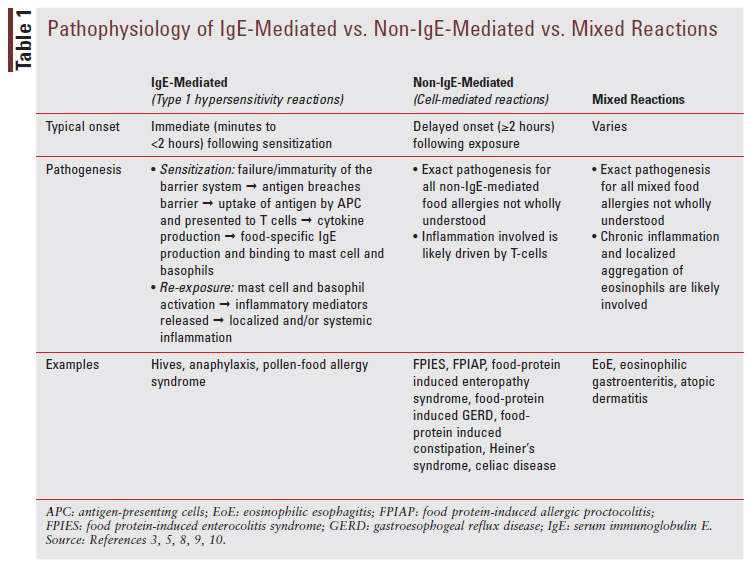
A food allergy is a reproducible immune-mediated reaction to allergenic food exposure.3,5,9 Food intolerance is a nonimmune-mediated reaction caused by pharmacologic agents, food additives/ preservatives, toxic mechanisms, or metabolic/gastrointestinal disorders.3,5,9 Some examples of pharmacologic agents that can cause food intolerance are tyramine, caffeine, and alcohol.5,9 Examples of toxic mechanisms include scombrotoxin poisoning, ciguatera toxin poisoning, and other bacterial toxins.5,9 Examples of metabolic mechanisms include lactose intolerance and fructose intolerance.5,9 Lastly, examples of gastrointestinal mechanisms include gastroesophageal reflux disease.5,9 It is important for pharmacists to recognize the difference between a true food allergy and a reaction indicating food intolerance in order to know when to refer a patient for further evaluation by a pediatrician or allergy specialist.
Clinical Manifestations
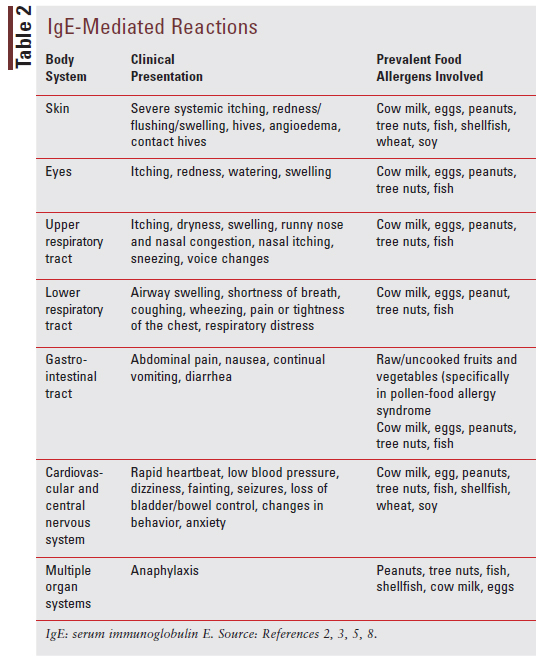
There is much variation in how the different allergic diseases can present clinically. TABLE 2 describes the most common clinical presentation for IgE-mediated reactions based on body system affected and commonly encountered food allergens.
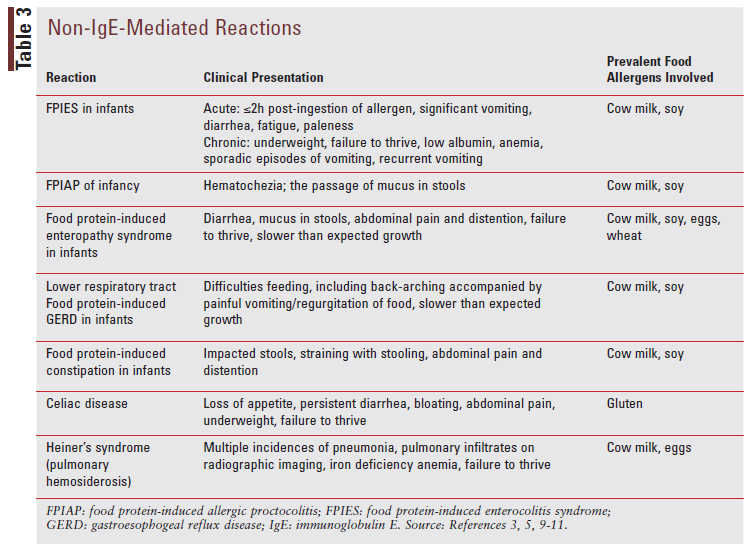
Non-IgE-mediated reactions can present very differently from IgE-mediated reactions, and they commonly affect the gut. The different reactions, clinical presentations for each, and prevalent food allergens involved are listed in TABLE 3.3,5-11
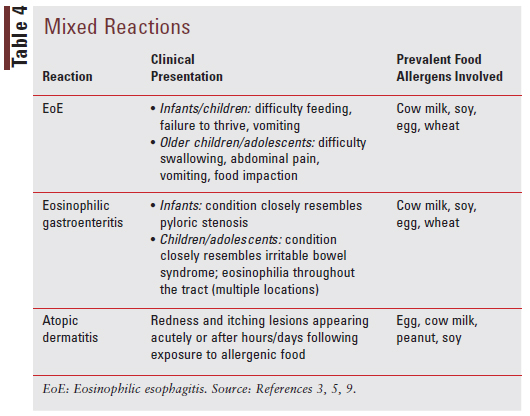
Mixed reactions involve both IgE-mediated and non-IgE-mediated immune processes and present uniquely based on the type of reaction that is occurring. The varied reactions, clinical presentations for each type of reaction, and prevalent food allergens involved for each reaction are described in TABLE 4.3,5,9
Diagnosis
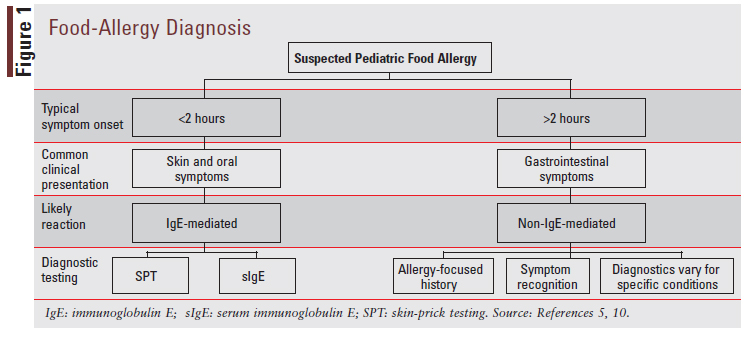
Diagnosis for pediatric food allergies varies based on key factors including the history and physical examination, timing of symptom onset postingestion of allergenic food, clinical presentation, and symptom asperity and extent. When food allergy is suspected to be IgE-mediated, the two first-line diagnostic tests are skinprick testing (SPT) and serum IgE (sIgE) testing.5 IgE testing is generally not recommended for non-IgE-mediated food allergies.10 When food allergy is suspected to be non-IgE-mediated, the diagnosis will generally be made based on the diagnostic criteria listed in FIGURE 1. Due to the heterogeneity of non-IgE-mediated food allergies, diagnosis varies for each type of allergy under this category and will not be discussed in depth. Diagnosis of mixed food allergies can also be quite complex and will not be discussed here. A potential diagnostic pathway for pediatric food allergies is depicted in FIGURE 1.5,10
Prevention
In the past, it has been questioned whether the maternal diet during pregnancy and breastfeeding plays a role in the development of pediatric food allergies. It is now known that restricting common food allergens has not been shown to prevent food allergy in infants and is not recommended.2 Additionally, there is no evidence suggesting that infants who are solely breastfed have an overall lower risk of developing food allergies than infants who are solely formula-fed or occasionally breastfed.2 However, some evidence suggests that solely breastfeeding infants for the first 3 to 4 months after birth can reduce the incidence of atopic dermatitis within the first 24 months of life.4
It has also been debated whether certain types of formula might be able to prevent the development of pediatric food allergies. Currently, there is no evidence to suggest that partially or extensively hydrolyzed formula hinders the development of infantile/pediatric atopic disease regardless of the risk for food allergies.4 Additionally, the American Academy of Allergy, Asthma, and Immunology (AAAAI), the American College of Allergy, Asthma, and Immunology (ACAAI), and the Canadian Society for Allergy and Clinical Immunology (CSACI) recommend against routinely prescribing or recommending the use of hydrolyzed formulas to prevent food allergies or for food sensitization.2
It is not completely understood what role the microbiome plays in developing food allergies, but a few suggestions can be implemented to potentially decrease the risk of developing food allergy.1,6 When possible, reducing elective cesarean section deliveries may decrease the risk of microbial dysbiosis, potentially decreasing the infant’s risk for developing allergic diseases.6 Also, reducing the use of antimicrobials and acid-reducing agents when possible during the perinatal period and throughout infancy may decrease the risk of microbial dysbiosis.6 Throughout all stages of life, encouraging adequate intake of fermentable fiber in the diet to promote microbial production of SCFAs may also contribute to reducing risk for developing allergic diseases.6 It is hypothesized that the early introduction of the most common food allergens can help develop tolerance to them, potentially by coexisting with the transition from breast milk to a solid diet and imprinting lasting allergen-specific iTreg cells, which may help to foster long-term tolerance into adulthood.1
Early introduction to formulations of peanut products deemed safe for infants can decrease the risk of developing peanut allergies, with the timing of introduction determined by the risk factors for peanut allergy development.4 The American Academy of Pediatrics recommends that high-risk infants with severe eczema and/or egg allergy be introduced to peanut products at age 4 to 6 months based on the Learning Early About Peanut Allergy (LEAP) study and guidance from the National Institute of Allergy and Infectious Diseases-sponsored expert panel.4,5,12 Additionally, they recommend introducing peanut products to infants with mild-to-moderate eczema at age 6 months and low-risk infants without eczema or other food allergies based on family preference.4,5
The AAAAI, ACAAI, and CSACI recommend introducing peanut products and cooked egg products to all infants regardless of allergy risk starting at age 4 to 6 months, once infants show that they are ready for complementary food introduction.2 Complementary foods are foods or drinks given to an infant/ young child for nutritional support (micronutrients, macronutrients, energy) that are not derived from breast milk, cow milk, or infant formula.4
Additionally, these organizations recommend against postponing the introduction of other potentially allergenic foods with the goal of achieving a diverse diet once infants show that they are ready for complementary-food introduction, between ages 4 and 6 months.2 General screening for peanut, egg, or other food allergies is unnecessary, and families can introduce infants to peanuts at home instead of in the healthcare provider’s office.2 At the family’s request, screening for peanut, egg, or other food allergies can be performed, and a supervised oral food challenge should be performed in the appropriate healthcare setting for positive SPT or sIgE test.2 It is recommended that common food allergens be integrated into the infant’s diet repeatedly following the initial introduction.2 While the data are not robust, they do suggest possibly harmful results when introduction to other potentially allergenic foods is delayed.2 Data do not suggest that introducing these foods prior to age 1 year is either harmful or beneficial.2
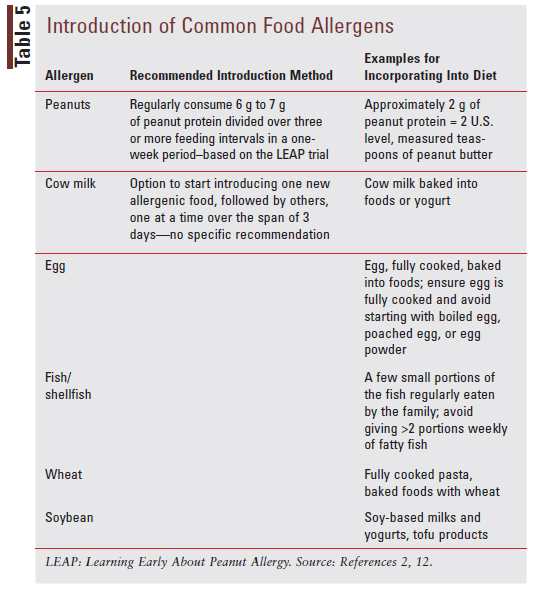
The AAAAI, ACAAI, and CSACI suggest introducing allergenic foods other than peanuts following the introduction of complementary solid foods and the successful introduction of peanut-containing foods without reaction. For specific recommendations and examples of how to introduce peanuts and other common food allergens to infants, see TABLE 5.2,12
Management
The primary treatment modality for both IgE-mediated and non-IgE-mediated reactions consists of avoiding the specific food allergen known to trigger the reaction. If the food allergy occurs during infancy while the baby is still being breastfed, it is suggested that the mother avoid the food allergen to prevent the allergen from potentially being present in the breast milk. Formula-fed infants known to be allergic to cow milk can switch to an extensively hydrolyzed or amino acid–based formula or switch to a soy-based formula.5
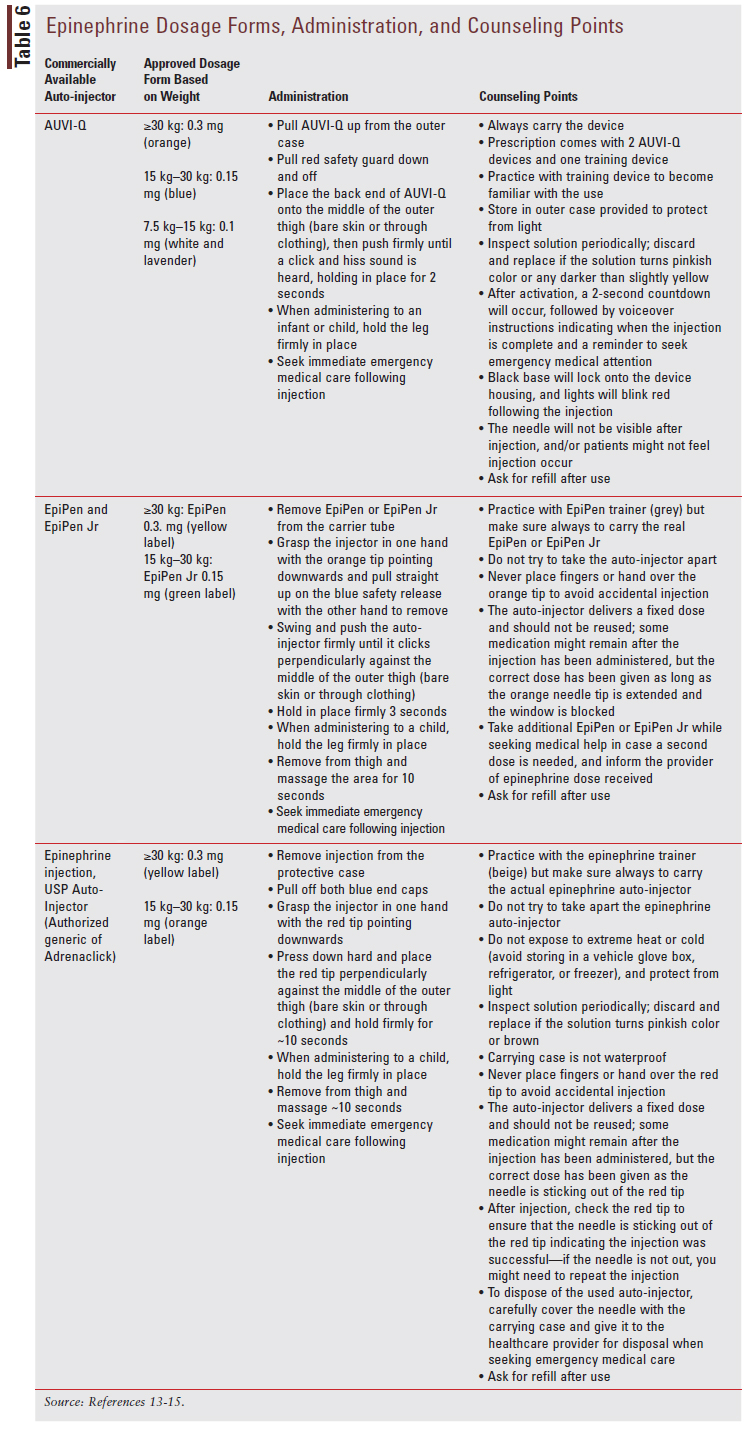
For patients who experience mild IgE-mediated reactions such as itchy skin and/or rash, antihistamine medications can be used to treat acute symptoms.5 Patients experiencing severe IgE-mediated anaphylaxis (SIDEBAR 1) should be treated with epinephrine and seek immediate medical care following the injection.5 All patients with a history of anaphylactic reaction should be prescribed an epinephrine auto-injector kit, and parents and patients should be trained on how to use this kit.5 The commercial dosage form of epinephrine depends on body weight and is discussed further in TABLE 6.13-15 Other medications can be used along with epinephrine as adjuvants in the treatment of anaphylaxis, but epinephrine is the only first-line treatment option available and delaying its use has been linked to an increase in mortality.5 Medications that can be used in conjunction with epinephrine for anaphylaxis include antihistamines, glucocorticoids, and beta-agonists.5 There is no role for antihistamines, glucocorticoids, and beta-agonists as monotherapy for anaphylaxis.

Medical management for non-IgE-mediated and mixed reactions varies for each type of reaction, and the specific medical treatments for each will not be discussed in depth here. For one specific type of non-IgE-mediated reaction, food protein-induced enterocolitis syndrome (FPIES), supportive care may be warranted depending on the severity of symptoms. In severe and prolonged FPIES cases, patients may present with signs and symptoms of extreme dehydration warranting referral and sometimes IV fluid resuscitation.5
Investigational Therapies
A variety of new therapies are being investigated and aim to help eradicate food allergies, including food-allergy allergen immunotherapy (FA-AIT) such as oral immunotherapy (OIT), sublingual immunotherapy (SLIT), or epicutaneous immunotherapy (EPIT), and the adjunctive use of monoclonal antibody drugs.5 FA-AIT consists of recurring exposure to a food allergen at routine intervals, in order to modify immune reaction and thereby decrease symptoms and medication use as well as prevent the development of new food allergies.16 The goal of these new therapies is to achieve tolerance, which is the ability to ingest a food allergen without symptoms occurring regardless of when the child was last exposed to the food allergen.16 A potentially more realistic goal to aim for with FA-AIT is desensitization.16 This refers a child’s capacity to safely ingest the foods containing the food allergen while on FA-AIT, which is dependent on continued regular exposure to the food allergen.16 With desensitization, once the allergen exposure is discontinued, the clinical symptoms and reactivity previously experienced to said allergen can return.16
Although not recommended by allergy organizations in the U.S., The European Academy of Allergy & Clinical Immunology (EAACI) strongly recommends OIT as a treatment option to increase the threshold of reaction for children with persistent cow milk and peanut allergy at age 4 to 5 years.16 The same recommendation exists for egg allergy but is graded as moderate instead of strong due to less supporting evidence.16 The Academy also recommends that these children have evidence of IgE sensitization to the known food allergen before OIT is initiated.16 Patients should be monitored carefully for local and systemic reactions throughout OIT, especially during the up-dosing phase.16 Some tips listed by the EAACI to help patients be as successful as possible with OIT are remembering to take the dose daily after a meal; avoiding going to bed less than 1 hour following a dose; avoiding exercise 2 to 3 hours following a dose; and holding or decreasing the dose while sick, experiencing an asthma exacerbation, having gastrointestinal diseases, or during menstruation.16 The use of nonsteroidal anti-inflammatory drugs (NSAIDs) while on OIT appears to be associated with increased frequency of adverse effects.5 Patients and caregivers can be counseled to avoid the use of NSAIDs while on OIT to potentially decrease the incidence of adverse effects.5 Additionally, the EAACI states that combining OIT or any other form of FA-AIT with monoclonal antibody drugs such as omalizumab has the potential to increase the overall safety of the process, and that more research is needed in this area to fully understand its place in clinical practice.16 They also emphasize that FA-AIT should only be implemented in a clinical setting that is adequately prepared to manage all types of allergic reactions, including anaphylaxis, which can be deadly.16 The 2014 AAAAI, ACAAI, and Joint Council of Allergy, Asthma & Immunology practice-parameter update on food allergies strongly recommended against the implementation of OIT into clinical practice at this time due to a lack of evidence supporting the benefits versus risks of immunotherapeutic approaches for food-allergy treatment.17 The various novel treatment modalities, risks, and benefits are summarized in TABLE 7.5,16,18-22
Prognosis and Resolution
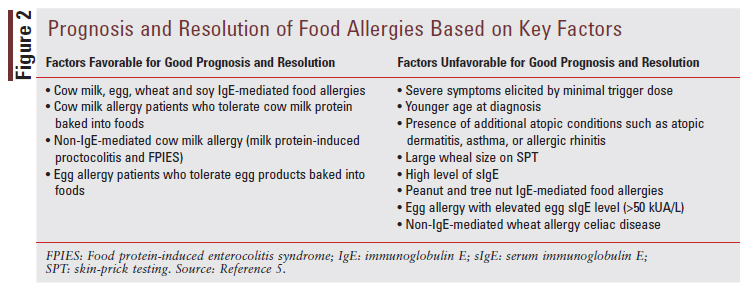
Prognosis and resolution of food allergies are determined by patient-specific clinical characteristics, including severity of symptoms; size of trigger dose; age at diagnosis, and comorbid allergic conditions, combined with allergic-sensitization results, including wheal size on SPT and food-allergen sIgE levels.5 Some of the most common IgE-mediated food allergies have a high likelihood of dissipating throughout childhood, such as cow milk, egg, wheat, and soy allergies.5 Other common IgE-mediated food allergies, such as peanut and tree nut allergies, are generally much less likely to dissipate by childhood and will likely persist into adulthood.5 Additionally, FIGURE 2 discusses the critical factors used to predict the prognosis and potential for resolving common food allergies.
Pharmacist’s Role in Food Allergy Management
Whenever a patient is encountered who appears to have symptoms consistent with the food allergies discussed above, referral to the pediatrician is warranted as he or she may need further evaluation by an allergy specialist to diagnose the allergy properly. Promptly identifying patients who need to be referred to a physician for further evaluation is key to avoiding the dangerous consequences that can occur when a child or infant has an undiagnosed food allergy. Pharmacists can also help to identify the differences between food allergy and food intolerance, helping patients know when to see the pediatrician. When there are questions regarding food-allergy prevention in infancy, the pharmacist can help counsel on the current recommendations as well as refer to the pediatrician if more information is warranted.
For patients with already established food allergies, pharmacists can help recommend adjuvant OTC medications like histamine-1 receptor antagonists for mild IgE-mediated reactions, such as itchy skin or rash. They can also help to counsel on which dosage form and pediatric dose are most appropriate for OTC medications available for adjuvant use. For patients with established food allergies and a history of severe IgE-mediated anaphylaxis, pharmacists can help educate patients and guardians on the IM administration techniques for each specific epinephrine auto-injector along with other device-specific counseling points, adverse effects, and more. They can also emphasize the importance of ensuring the epinephrine auto-injector has not expired and that it will need to be refilled in the event of its use.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
1. Stephen-Victor E, Crestani E, Chatila TA. Dietary and microbial determinants in food allergy. Immunity. 2020;53(2):277-289.
2. Fleischer DM, Chan ES, Venter C, et al. A consensus approach to the primary prevention of food allergy through nutrition: guidance from the American Academy of Allergy, Asthma, and Immunology; American College of Allergy, Asthma, and Immunology; and the Canadian Society for Allergy and Clinical Immunology. J Allergy Clin Immunol Pract. 2021;9(1):22-43.e4.
3. Calvani M, Anania C, Caffarelli C, et al. Food allergy: an updated review on pathogenesis, diagnosis, prevention and management. Acta Biomed. 2020;91(11-S):e2020012.
4. Greer FR, Sicherer SH, Burks AW; Committee on Nutrition; Section on Allergy and Immunology. The effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, hydrolyzed formulas, and timing of introduction of allergenic complementary foods. Pediatrics. 2019;143(4):e20190281.
5. Cosme-Blanco W, Arroyo-Flores E, Ale H. Food allergies. Pediatr Rev. 2020;41(8):403-415.
6. Huang YJ, Marsland BJ, Bunyavanich S, et al. The microbiome in allergic disease: current understanding and future opportunities—2017 PRACTALL document of the American Academy of Allergy, Asthma & Immunology and the European Academy of Allergy and Clinical Immunology. J Allergy Clin Immunol. 2017; 139 (4) :1099-1110.
7. Mitre E, Susi A, Kropp LE, et al. Association between use of acid-suppressive medications and antibiotics during infancy and allergic diseases in early childhood . JAMA Pediatr. 2018;172(6):e180315.
8. Pajno GB, Fernandez-Rivas M, Arasi S, et al. EAACI Guidelines on allergen immunotherapy: IgE-mediated food allergy. Allergy. 2018;73(4):799-815.
9. Cianferoni A. Non-IgE mediated food allergy. Curr Pediatr Rev. 2020;16(2):95-105.
10. Meyer R, Chebar Lozinsky A, Fleischer DM, et al. Diagnosis and management of Non-IgE gastrointestinal allergies in breastfed infants—An EAACI Position Paper. Allergy. 2020;75(1):14-32.
11. Spergel JM. Nonimmunoglobulin E-mediated immune reactions to foods. Allergy Asthma Clin Immunol. 2006;2(2):78-85.
12. Togias A, Cooper SF, Acebal ML, et al. Addendum guidelines for the prevention of peanut allergy in the United States: summary of the National Institute of Allergy and Infectious Diseases–sponsored Expert Panel. J Acad Nutr Diet. 2017;117(5):788-793.
13. AUVI-Q® package insert. Richmond VA. Kaleo Inc; 2017.
14. EpiPen® and EpiPen Jr® package insert. Columbia, MI: Mylan Inc; 2016.
15. Epinephrine injection, USP auto-injector package insert. Hayward, CA: Impax Laboratories, Inc; 2016.
16. Pajno GB, Fernandez-Rivas M, Arasi S, et al. EAACI Guidelines on allergen immunotherapy: IgE-mediated food allergy. Allergy. 2018;73(4):799-815.
17. Sampson HA, Aceves S, Bock SA, et al. Food allergy: a practice parameter update—2014. J Allergy Clin Immunol. 2014;134(5):1016-25.e43.
18. Omalizumab. In: DRUGDEX System Micromedex 2.0. Greenwood Village, CO: Thomson Reuters (Healthcare) Inc. micromedexsolutions. com.micromedex2/4.85.0/WebHelp/Document_help/Drug_Eval_ document.htm. Accessed July 10, 2021.
19. Sampson HA, Leung DY, Burks AW, et al. A phase II, randomized, double-blind, parallel-group, placebo controlled oral food challenge trial of Xolair® (omalizumab) in peanut allergy. J Allergy Clin Immunol. 2011;127(5):1309-10.e1.
20. Nadeau KC, Schneider LC, Hoyte L, et al. Rapid oral desensitization in combination with omalizumab therapy in patients with cow's milk allergy. J Allergy Clin Immunol. 2011;127(6):1622-1624.
21. Xolair® (omalizumab) package insert. South San Francisco, CA: Genentech, Inc., A Member of the Roche Group; Revised April 2021.
22. Dupixent® (dupilumab) package insert. Tarrytown, NY: Regeneron Pharmaceuticals, Inc.; Revised June 2021.