Pharmacist Review of Loperamide Abuse
RELEASE DATE
December 1, 2021
EXPIRATION DATE
December 31, 2023
FACULTY
Austin De La Cruz, PharmD, BCPP
Clinical Assistant Professor
University of Houston College of Pharmacy
Houston, Texas
Aroge Imran, PharmD Candidate Class of 2023
University of Houston College of Pharmacy
Houston, Texas
Raymond Thai, PharmD Candidate Class of 2023
University of Houston College of Pharmacy
Houston, Texas
FACULTY DISCLOSURE STATEMENTS
Dr. De La Cruz, Ms. Imran, and Mr. Thai have no conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT
 Pharmacy
Pharmacy
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-21-141-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients’ conditions and possible contraindications or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
GOAL
To provide pharmacists with comprehensive knowledge about loperamide, loperamide abuse, and how to address the rising cases of loperamide toxicity in the midst of the opioid crisis.
OBJECTIVES
After completing this activity, the participant should be able to:
- Recognize the prevalence and risk factors associated with loperamide abuse.
- Describe the clinical presentation of a loperamide overdose and associated cardiotoxicity.
- Summarize how to screen patients for loperamide abuse.
- Review the pharmacist’s role and potential interventions to mitigate abuse.
ABSTRACT: Loperamide is a common OTC antidiarrheal medication, also known as Imodium, that has been targeted as a drug of abuse by those seeking to relieve opioid withdrawal symptoms or achieve a euphoric high. Loperamide is a synthetic opioid agonist that lacks central nervous system effects when taken as directed. When taken at supratherapeutic doses, however, loperamide can cross the blood-brain barrier and lead to a variety of adverse effects, including cardiovascular-related complications. A concerning rise in overdose cases and fatalities associated with loperamide overdose has prompted the FDA to limit the amount of loperamide available in OTC packages. Pharmacists should be aware of the patterns of loperamide abuse and diversion and understand how to implement effective measures to prevent patient harm within their practice setting.
Loperamide is a well-known, easily accessible, and effective nonprescription antidiarrheal agent that is sold under the brand name Imodium. Loperamide was first manufactured by Janssen Pharmaceuticals in 1969 and then marketed in 1977.1 Over the years, it has consistently been included on the World Health Organization’s list of Essential Medicines due to its safety and effectiveness.1 Most cases of acute, nonspecific diarrhea are self-limiting, but the use of OTC antidiarrheal products such as loperamide may be necessary to help alleviate symptoms. In addition to controlling the symptomatic relief of acute nonspecific diarrhea in patients, it is also indicated in adults for chronic diarrhea associated with irritable bowel syndrome and for reducing the volume of discharge from ileostomies.2 Loperamide is not recommended for all types of diarrhea, however, and may lead to worsened outcomes in patients with invasive bacterial diarrhea caused by enteroinvasive Escherichia coli, Salmonella, Shigella, Campylobacter jejuni, or antibiotic-associated diarrhea, such as Clostridium difficile.3
Loperamide has historically been viewed as safe, and most consumers abide by the labeled instructions and recommended dosing range.3 Recent data, however, denote that there has been a rise in loperamide abuse. Like prescription and illegal drugs, OTC medications can also be abused. In the past, the two most commonly abused OTC medications included dextromethorphan and pseudoephedrine, which could produce psychoactive effects and a high when taken at higher than recommended doses.4 Readily available and found online and on the shelves of most grocery stores, loperamide has been anecdotally touted as the “poor man’s methadone,” as it has allowed users to self-treat opioid-withdrawal symptoms or produce euphoria at supratherapeutic doses.5 With a recommended maximum daily dosage of 8 mg for OTC use, cases of loperamide overdoses have detailed incidents of patients ingesting daily doses ranging from 70 mg up to 1,600 mg, which would involve the ingestion of up to 800 2-mg loperamide tablets in one day.2,6
Overdose deaths continue to be a serious national crisis that has yet to be controlled. In 2019, nearly 50,000 people in the United States died of an opioid overdose.7 That number skyrocketed the next year with more than 81,000 drug overdose deaths in 2020, accounting for the highest number of overdose deaths ever recorded in a 12-month period.1,7 As the opioid epidemic continues to take a devastating toll on our nation and as access to prescription pain products becomes more regulated, more individuals appear to be searching for readily accessible alternatives, such as OTC loperamide.
Pharmacological Properties of Loperamide
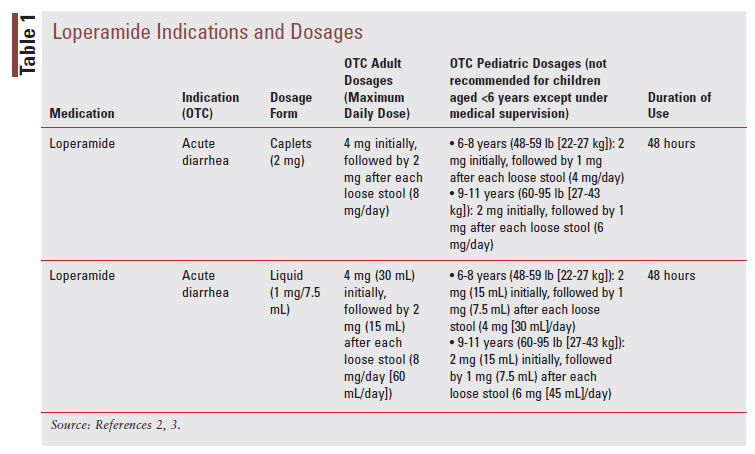
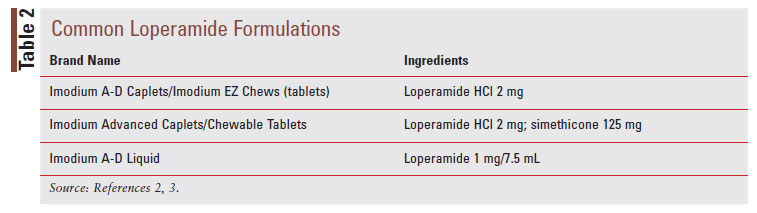
Loperamide, a phenylpiperidine derivative, is classified as an antimotility agent that acts as a peripheral muopioid receptor agonist to provide symptomatic relief of acute, nonspecific diarrhea (TABLE 1).2 The synthetic opioid agonist produces its antidiarrheal effect by stimulating peripheral mu-opioid receptors on the intestinal circular muscles of the myenteric plexus to slow intestinal motility, allowing absorption of electrolytes and water.3,8 Additionally, loperamide inhibits the release of acetylcholine and prostaglandins, which results in reduced propulsive peristalsis and increased intestinal transit time.2,8 Loperamide also has antisecretory effects, possibly mediated via gastrointestinal (GI) micro opioid–receptor stimulation, calmodulin inhibition, and voltage-dependent calcium-channel inhibition.3 Loperamide comes in oral tablet, oral capsule, oral liquid, oral solution, and oral suspension formulations (TABLE 2).2 After the intake of loperamide 2 mg, plasma concentrations of unchanged drugs remain under 2 ng/mL.2 Plasma concentration of the drug is highest 5 hours after administration of the oral capsule and 2.5 hours after administration of the oral liquid.2 Loperamide has high protein binding at about 95%, and the elimination half-life is approximately 10.8 hours, with a range of 9.1 to 14.4 hours.2
Although loperamide displays agonist activity 50 times more potent than morphine on peripheral muopioid receptors, there are several biologic factors that limit its ability to produce the common central nervous system (CNS) effects associated with opioid analgesics.9 The lack of CNS effects when administered at therapeutic dosages (up to 16 mg/day for prescription use) is attributed to loperamide’s poor oral bioavailability (<2%), extensive first-pass metabolism by cytochrome P450 (e.g., CYP450 3A4 and 2C8) enzymes to inactive metabolites, and low blood-brain barrier (BBB) penetration due to the P-glycoprotein (P-gp) efflux transporter, which prevents it from entering the CNS.10,11 The P-gp system is part of the adenosine triphosphate (ATP)-binding cassette transporter superfamily, which relies on ATP to actively pump substrates across cell membranes.12-14 The P-gp transporter can be found in the luminal membrane of the small intestine and BBB.12-14 The P-gp expression in the BBB plays a vital role in preventing drugs from crossing into the CNS.12-14 The primary in-vivo metabolites are N-desmethylloperamide and N-hydroxymethyl-mono-desmethylloperamide, which have a potency that is two to three times less than that of loperamide.15 As a result, loperamide does not have any clinically significant analgesic activity at therapeutic dosages.10 Due to these factors, loperamide has historically been viewed as a safe drug associated with minimal side effects and a low potential for abuse.10
Prevalence of Loperamide Misuse and Abuse
When loperamide was introduced in 1977, the FDA initially placed it in the Schedule V list of controlled medications, basing their decision on animal data that suggested loperamide had produced opioid-like effects.11,16,17 Loperamide was later removed from the Schedule V list and became a nonprescription product by 1988 due to several volunteer studies and epidemiological data that demonstrated a low risk of physical dependence and abuse.16-19 Although safety was established through its low abuse potential, doses exceeding the recommended prescription daily dose of 16 mg demonstrated a variety of adverse effects.1,20
Throughout the past decade, a concerning number of published case reports and state poison control center calls related to loperamide misuse/abuse have been documented. Epidemiologic trends were reviewed by the National Poison Data System from January 1, 2010 to December 31, 2015, assessing the intentional misuse, abuse, and suspected suicide due to loperamide exposure.21 It found that in the span of 5 years, there was a 91% increase in loperamide exposure, with one-third of cases occurring in teens and young adults.10,21 During this study period, there were 1,736 intentional loperamide exposures.21 Overall reasons for the intentional exposure included intentional abuse (13.1%), intentional misuse (32.8%), suspected suicide (48.8%), and other (5.2%).21 These exposures increased at approximately 38 cases per year and included 15 deaths.21
Alongside the growing number of case reports and poison control center calls, researchers have also observed a rise in online discussion revolving around loperamide. The first post on loperamide’s misuse appeared in 2005, followed by a pronounced rise in discussions around 2010-2011.22 Borron (2017) utilized online tools such as Google Trends to detect a trend in loperamide’s digital mentions.10 Google Trends, a tool that measures Google search popularity, allowed the researchers to assess Internet interest in the search terms “loperamide,” “loperamide withdrawal,” and “loperamide high.”10 The researchers noted a sudden rise in search volume starting in 2011, demonstrating an increased online interest in the drug.10 Bluelight is an online drug forum where people can discuss individual experiences with illicit drugs and ask questions. One member posted, “I was addicted to lope [loperamide]for about 4 years taken daily. At the apex of my usage, my dose was ~400 mg [1 bottle of 200 2-mg pills a day], but I've done even more at times and to get off it I slowly went down 10 mg per day. I think it almost killed me a couple times with crazy pressure in my head and loud pops that felt like someone dropping a piano inside my head. I had to do enemas daily to keep my bowels somewhat normal. It became a pain.”23
There are many posts similar to this one in which users describe taking extremely large doses of loperamide to treat a variety of conditions from heroin to methadone withdrawal. Several posts even go into the pharmacology of using cimetidine or grapefruit juice to help with CYP3A4 inhibition, thus increasing plasma concentrations and the ability of loperamide to cross the BBB.23 Although not without their limitations, studies using Internet tools and online forum communities have highlighted the potential of the Internet as a resource to identify emerging drug-abuse patterns and gain valuable insight on the hard-to-reach population of illicit drug users.
Pathophysiology of Loperamide Misuse and Abuse
At its recommended dosages, loperamide’s effects are limited peripherally to the gut with minimal BBB penetration due to several factors previously mentioned.11 However, with the discovery of loperamide’s ability to produce CNS effects at supratherapeutic doses, a concerning number of people have turned to the historically safe drug in an attempt to self-treat their opioid withdrawal symptoms or seek euphoria.11 In addition to using high doses of loperamide, some individuals are also taking other prescription medications to enhance the CNS-related effects of loperamide. This method of pharmacokinetic manipulation has been employed by users in an attempt to increase loperamide’s systemic absorption and enhance its ability to penetrate the CNS to exert the desired opioid-like effects.11,24 Since loperamide is a known substrate for the efflux protein P-gp, the use of P-gp inhibitors may assist with loperamide’s access to the BBB, thus producing similar effects seen with opioid analgesics.11 Some of the P-gp inhibitors include ketoconazole, fluoxetine, citalopram, omeprazole, quinine, quinidine, and verapamil (TABLE 3).25-27
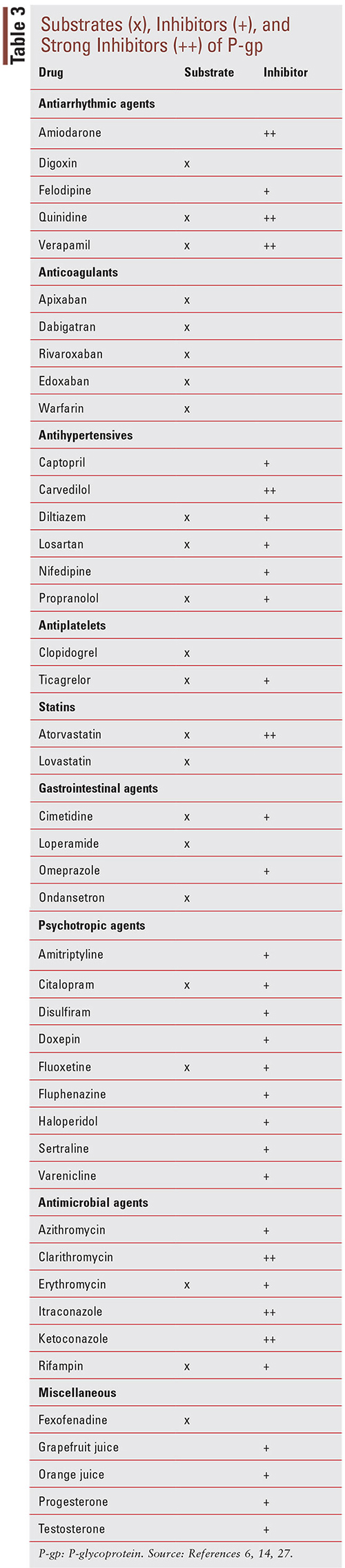
Drugs listed as P-gp inhibitors have been shown in clinical studies to increase the AUC of a sensitive substrate >25% or decrease the clearance of a sensitive substrate >20%, as well as increase the plasma concentration by twofold to threefold.14,28,29
Furthermore, adding a CYP450 inhibitor may further increase plasma concentration and the duration of effect by decreasing the metabolism of loperamide. CYP450 inhibitors include grapefruit juice, clarithromycin, ketoconazole, quinidine, ritonavir, and omeprazole and may increase the plasma concentration of loperamide by twoto threefold.25,26,28 One user on a discussion thread of Bluelight stated the she could remain “high” for 12 hours by ingesting 60 mg of loperamide along with cimetidine, grapefruit juice, and an energy drink containing quercetin and quinine.17 Famotidine is an OTC histamine-2 receptor antagonist, similar to cimetidine, that is commonly used to treat gastroesophageal reflux disease and other conditions that cause excess stomach acid. It is possible that at supratherapeutic doses, famotidine and cimetidine can inhibit CYP3A4, resulting in an increase in loperamide serum levels. A case report highlighted this combination of products in a 32-year-old male who presented with severe palpitations and syncope.30 He admitted to taking up to 200 mg of loperamide and up to 500 mg of famotidine daily for 10 consecutive days. The patient had a prior history of alcohol, opiate, and methamphetamine abuse. After 5 days of treatment for QT prolongation and ventricular tachycardia, the patient recovered and was discharged home with a normal ECG reading. Many cases, however, do not always result in patient recovery and subsequent discharge.30
Loperamide-Induced Toxicity
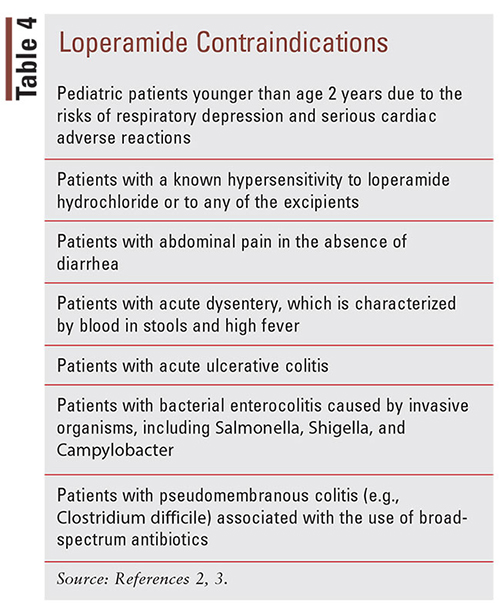
The misuse of loperamide in supratherapeutic doses has been associated with a significant increase in morbidity and mortality.31 Case reports have shown CNS depression, respiratory depression, and cardiotoxicity complications, including QT prolongation, torsades de pointes (TdP), QRS prolongation, ventricular dysrhythmias, syncope, cardiac arrest, and death.11,32-34 The CNS and respiratory depression associated with loperamide overdose present similarly to overdoses typically seen with opioid analgesics, which include the hallmark characteristics of pinpoint pupils, unresponsiveness, and hypoventilation.2 Swank (2017) conducted a study that utilized the FDA Adverse Event Reporting System (FAERS) database to search for postmarketing reports of serious cardiac adverse events associated with loperamide use from 1976 to 2015.6 The researchers noted 48 cases of serious loperamideinduced cardiac adverse events, which resulted in 10 fatalities.6 In the 22 cases that were characterized as drug abuse, the median daily dose was 250 mg, with a range from 70 mg to 1,600 mg, which would involve the ingestion of 35 to 800 2-mg loperamide tablets per day.6 A majority of the cases reviewed exhibited ECG abnormalities of a widened QRS interval (up to 200 ms) and a prolonged QT interval (up to 704 ms), and all developed ventricular dysrhythmias, including monomorphic or polymorphic ventricular tachycardia (TdP).11 These cardiotoxic effects (e.g., cardiac arrest, syncope, and respiratory depression) were also prevalent in postmarketing cases for children younger than age 2 years, making it contraindicated in this patient population (TABLE 4).2
The exact mechanism of loperamide-induced dysrhythmia has not yet been determined, but loperamide has been observed to inhibit potassium and sodium channels in cardiocytes at excessive dosages.35 These aforementioned cardiac channels are not blocked at standard doses but only in the setting of deliberate loperamide overdose, especially with concomitant P-gp or CYP3A4 inhibition.11 QRS complex widening has been associated with inhibition of cardiac sodium channels, and excessively prolonged QTc intervals are associated with drugs that block voltage-dependent cardiac potassium channels, namely the human ether-a-go-go– related gene (hERG) K+ channel that underlies the delayed rectifier current, which is crucial for repolarization of cardiac action potentials.36,37 In addition, the N-desmethyl metabolite of loperamide can contribute secondarily to the cardiotoxicity.38
Kang (2016) explored loperamide’s ability to inhibit cardiac ion channels and found that loperamide exhibited high-affinity dose-dependent inhibition of the cardiac sodium channel, Nav1.5, and even higher inhibition of the cardiac hERG potassium channel, potentially resulting in the QRS and QTc interval prolongations observed with overdose.36 These findings support the hypothesis that loperamide-induced cardiac transmembrane ion channel inhibition is the underlying cause of cardiotoxicity following excessive ingestion.36
As mentioned previously, the elimination half-life of loperamide is 10.8 hours, with a range of 9.1 to 14.4 hours.2 There have been rare reports of half-lives extending to 40.9 hours with 16-mg dosages in healthy volunteers. Given the standard half-life, most poison control centers estimate 4 to 5 days before cardiac stabilization; however, one case report described a patient who remained symptomatic for 11 days postingestion with recurrent TdP and ventricular arrhythmias.39
Screening
Loperamide-induced cardiotoxicity is an under-recognized clinical presentation that has only started appearing more frequently throughout the past decade. Since its transition from prescription drug to OTC status, loperamide had not been suspected to be a drug of abuse. A challenge that healthcare establishments may face is lack of awareness surrounding loperamide abuse and its life-threatening complications by both providers and patients. Loperamide is not detected in routine drug screens, and concentrations must be specifically tested for through special laboratories. Tests, therefore, would only be ordered if there is a reasonable suspicion of abuse.40 Patients may also not be forthcoming regarding their medication history, which adds to the challenge of proper diagnosis. For example, Ahmed et al describe a patient case in which a 23-year-old woman was referred for evaluation of wide complex tachycardia.41 The care team did not initially suspect loperamide toxicity until the patient’s mother informed the nursing staff that “hundreds” of empty Imodium boxes were found in her daughter’s car.41 The patient did not consider this discovery to be clinically relevant enough to mention, repeatedly answering “no” when asked if she took any nonprescription or OTC medications.41 This case demonstrates the need for greater awareness of the potentially life-threatening toxicity associated with loperamide so that proper diagnosis, management, and patient education can be provided in a timely manner.
According to the National Poison Data System, male subjects were more likely to intentionally abuse loperamide, whereas female subjects more often used it in reported suicidal attempts.21 Even though single-agent exposure is more frequent than polysubstance exposures, providers should still be mindful that both patterns contribute to increasing exposure rates. Singleagent loperamide exposures showed an increase of 24.7 exposures per year (95% CI 21.3-28.0; P <.001), whereas polysubstance loperamide exposures increased at a rate of 13.1 exposures per year (95% CI 10.7-15.4; P <.001).21 Polysubstance exposure can also put patients at a higher risk for medical complications. Approximately one-third of polysubstance exposure patients were admitted to a critical care unit compared with 14.1% of patients with single-agent exposures. Common coingestions included antihistamines (13.7%), sedatives, hypnotics, antipsychotics (12.0%), antidepressants (11.1%), alcohol (7.5%), opioids (6.9%), and cough or cold medications (7.1%).21 Due to the lack of guideline-directed therapy for loperamide overdose, clinicians need to be aware of signs and symptoms associated with loperamide toxicity and should be cognizant of when to refer the patient to a psychiatrist or cardiac electrophysiologist.42 These signs might include GI complications, such as nausea, vomiting, constipation, and a paralyzed intestine.42 Loperamide overdose should be considered in the differential diagnosis of patients with a history of opioid abuse or recent ingestion of unknown drugs presenting with unexplained cardiotoxicity and/or signs of opioid overdose.40 Even though a patient may be asymptomatic, physicians should consider obtaining a consultation with a medical toxicologist and order an ECG, given the high incidence for loperamide-related cardiotoxicity.28 An extensive medication history, including both prescription and nonprescription drugs, should be obtained, either through the patient or their family and friends, so that any offending agents can be identified.40 For example, when patients are being treated for opioid withdrawal, common treatment options include clonidine and methadone, which both have the potential to worsen cardiac conduction and should be used with caution.12 Many common psychotropic medications are also known to worsen QT prolongation, which could potentially worsen outcomes.43
Acute Treatment of Loperamide Overdose
Management of a loperamide overdose can be quite challenging due to the unclear understanding of loperamide’s mechanism of action and the resulting effect on the cardiovascular system. The majority of cardiac adverse events were reported in patients who intentionally misused and abused supratherapeutic doses of loperamide to achieve euphoria or analgesia and to attempt to self-treat opioid withdrawal symptoms that resulted from the discontinuation of chronic opioid therapy.6 While there is information throughout the medical literature on clinical presentation and treatment options of loperamide overdose, current data are mostly limited to case reports.11 There is currently no guideline-directed therapy for the treatment of loperamide overdose.30
Upon diagnosis of loperamide-induced toxicity, any offending agents should be discontinued, and supportive care should be initiated.30 Selection of supportivecare treatment options is determined by individual patient factors and can include electrolyte replacement, heart rate support with isoproterenol or temporary overdrive pacing, avoidance of QT-prolonging agents, and defibrillation.30 Presentation during the early stages of ingestion can be treated similarly to other ingestions with the use of activated charcoal for patients presenting within 2 to 4 hours after ingestion.11,44,45 Activated charcoal is an adsorbent that is widely used to treat overdoses of a variety of medications and may reduce absorption in acute loperamide overdoses by up to ninefold.11,30 Activated charcoal is typically administered as a single dose, and it possesses a large surface area that allows the molecule to bind many drugs and toxins in the GI lumen, with the goal of decreasing their absorption into the systemic circulation.46 When used to treat overdoses of other toxic compounds, activated charcoal is effective within 1 hour post ingestion, but the extended administration window of 2 to 4 hours post ingestion is justified with loperamide due to its ability to reduce peristalsis.11,47
If respiratory depression is observed during a loperamide overdose, then administration of naloxone is recommended, given loperamide’s opioid-related effects in the CNS and naloxone’s competitive inhibitor action on mu-opioid receptors in the CNS.30,33 However, it is important to note that naloxone administration will not affect the cardiotoxicity effects since they are mediated by alterations in the cardiac ion channel activity.11,30,44,45 Naloxone administration can be repeated at 2- to 3-minute intervals if the patient’s overdose status does not improve.2 Because naloxone has a shorter serum half-life (approximately 60 minutes) compared with loperamide (approximately 10.8 hours), vital signs for patients receiving naloxone to treat loperamide overdose should be closely monitored, and if signs and symptoms of opioid toxicity reappear, naloxone should be readministered.2,48
Standard advanced cardiovascular life support measures have been employed by clinicians to treat cardiac arrest and dysrhythmias secondary to loperamide overdose.24 It has been recommended that synchronized cardioversion should be performed for patients presenting with ventricular tachycardia and hemodynamic instability.24 Synchronized cardioversion involves the delivery of a low-energy shock, which is timed or synchronized to be delivered at a specific point in the QRS complex.49 For patients who are pulseless and experiencing ventricular fibrillation or ventricular tachycardia, asynchronous cardioversion (defibrillation) should be used.24 Patients experiencing TdP or polymorphic ventricular tachycardia without spontaneous resolution of their dysrhythmia should also be treated with asynchronous cardioversion.24 Once there is a perfusable rhythm established, IV magnesium should be considered.24 Amiodarone or transvenous pacing are options for patients with recurrent dysrhythmias.24
To prevent further QT prolongation, electrolyte abnormalities including potassium, calcium, and magnesium should be identified and corrected.24 QRS-interval widening associated with sodium channel blockade can be addressed by using a trial of IV sodium bicarbonate, although the efficacy of this treatment option has not yet been established.11 If using sodium bicarbonate, there should be close monitoring of serum potassium, sodium, and pH due to sodium bicarbonate’s ability to induce hypokalemia, which can further prolong the QT interval.24
Another option to treat sodium channel blockade is with the Class 1B antiarrhythmics, notably lidocaine, which have rapid binding and dissociation properties compared with other drugs.50 Several case reports have used IV isoproterenol if the patient is experiencing significant QT prolongation associated with hemodynamic instability.11,44,45 For more severe cases of loperamideinduced cardiotoxicity, IV lipid emulsion therapy, molecular adsorbent recirculating system, and venoarterial extracorporeal membrane oxygenation have been used in a few published cases after traditional methods have been exhausted, although their uses have not been frequently documented throughout the clinical literature.11
A study reviewed 48 cases of serious adverse events associated with loperamide use, using the FAERS database.6 The most frequently reported cardiac adverse events were syncope (n = 24), cardiac arrest (n = 13), electrocardiographic QT-interval prolongation (n = 13), ventricular tachycardia (n = 10), and TdP (n =7).6 Of the 48 cases, the most commonly reported reasons for use can be characterized as drug abuse (n = 22) and diarrhea treatment (n = 17).6 Medical intervention was reported in 16 of the 22 drug abuse cases and consisted of one or more medications or procedures: sodium bicarbonate (n = 6), IV magnesium (n = 6), placement of an implantable cardioverter-defibrillator or pacemaker (n = 5), cardioversion (n = 4), IV potassium (n = 4), amiodarone (n = 3), isoproterenol (n = 3), lipid emulsion (n = 3), and lidocaine (n = 2).6
Long-Term Treatment of Loperamide Abuse
Postacute care and follow-up evaluation are critical in this patient population, as some case reports have detailed instances where patients were successfully treated but readmitted to the hospital following complications of recurrent loperamide abuse.51 Since many users have reported ingesting loperamide in large amounts to ameliorate opioid-withdrawal effects, it is possible that there could be an underlying opioid-use disorder (OUD), which should be screened for and addressed prior to discharge to prevent another overdose event.11 Referral to substance-use disorder treatment programs may be warranted in patients with OUD, and patient education should be provided with the goal of cessation.28
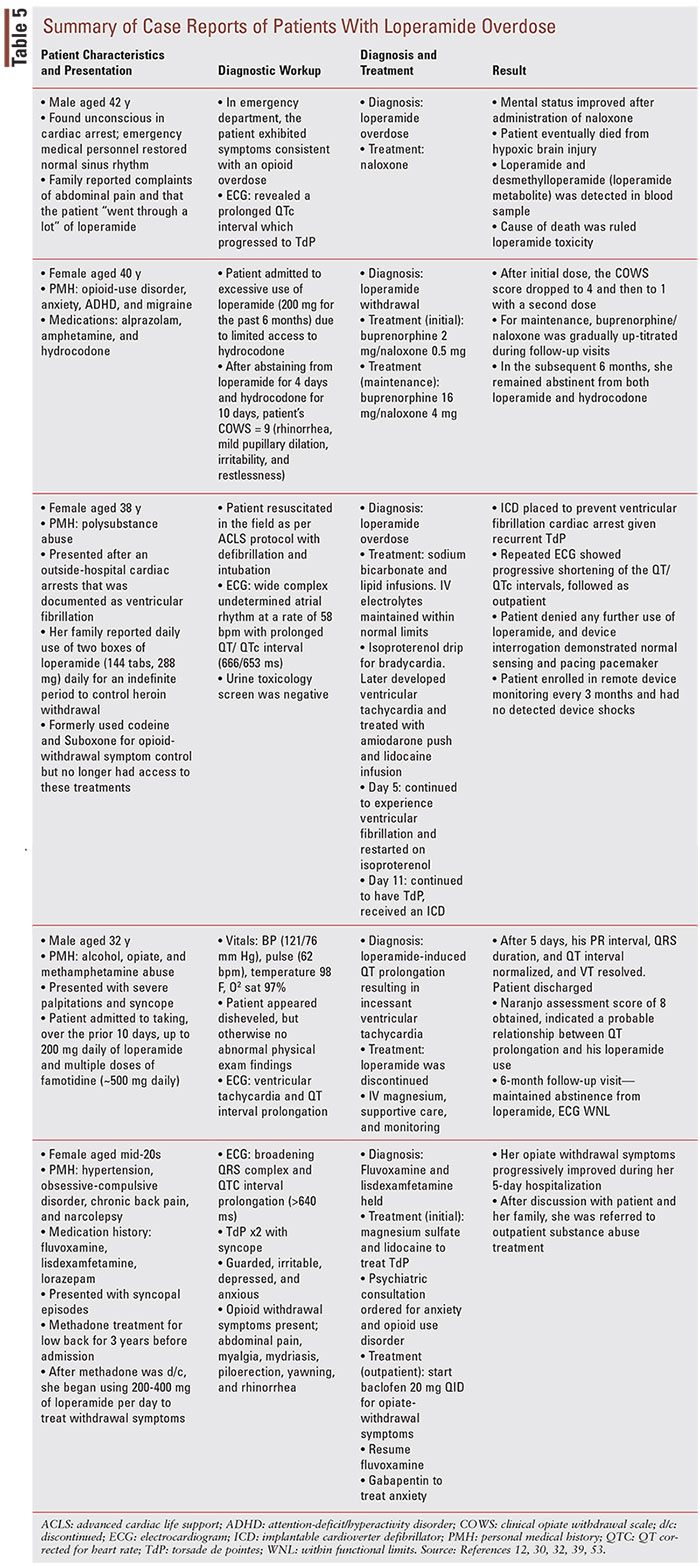
A long-term treatment option that is being explored for loperamide-abuse postdischarge is buprenorphine, either as monotherapy or coformulated with naloxone as buprenorphine/naloxone (BUP/NAL). Buprenorphine and BUP/NAL are FDA-approved drugs used to treat OUD, in addition to methadone.52 The medication-assisted treatment (MAT) strategy for OUD often combines these drugs with behavioral therapy and psychosocial support.52 Although MAT is typically reserved for treating OUD associated with narcotics, there have been promising reports of successful treatment using MAT in patients who were previously hospitalized for loperamide abuse, although the data are still limited. Varghese (2019) described a case of a 40-year-old female with a history of OUD who reported that she had been taking 200 mg (100 2-mg tablets/day) of loperamide every day for 6 months due to reduced effectiveness of hydrocodone (TABLE 5).53 After assessment, the patient was initiated on BUP 2 mg/NAL 0.5 mg, and the dose was gradually titrated up during follow-up visits to BUP 16 mg/NAL 4 mg daily.53 In the following 6 months, she remained abstinent from both loperamide and hydrocodone.53 Similarly, Brar (2020) describes a case series where three patients with loperamide-associated OUD were successfully treated with on-going buprenorphine treatment.54
Proposed Changes
The FDA has taken several steps to help decrease the misuse and abuse of loperamide. In June 2016, the FDA released a Drug Safety Communication stating that loperamide was causing serious and fatal cardiac events (e.g., QT-interval prolongation, TdP, syncope, and cardiac arrest).55 Patients were also warned not to exceed prescribed or OTC doses, as doing so could lead to severe heart rhythm complications or death.56 Additionally, the FDA Center for Drug Evaluation and Research approved a safety-related drug labeling change in November 2016.55 A black box warning was added to Imodium’s package insert stating that “Cases of Torsades de Pointes, cardiac arrest, and death have been reported with the use of a higher than recommended dosages of IMODIUM.”55 It also prompted loperamide consumers to notify their healthcare provider about all the medications they are taking, especially if they are taking Class 1A or Class III antiarrhythmics, antipsychotics, antibiotics, or any drugs known to prolong the QT interval to reduce the likelihood of known drug-drug interactions that could predispose individuals to a potentially fatal overdose.55 This black box warning was based on 48 case reports the FDA received over the course of 39 years, involving 31 required hospitalizations and 10 deaths.21,57,58 In January 2018, the FDA issued an updated Drug Safety Communication reporting that it was working with manufacturers to develop abuse-resistant packaging with fewer doses.21,57,58
In September 2019, the FDA approved new packaging for brand-name OTC loperamide intended to increase the safe use of loperamide products without limiting OTC access for consumers who use loperamide for its approved indication.59 The packaging changes were applied to Imodium A-D tablets (Janssen), Imodium Multi-Symptom Relief tablets (Janssen), and Be Health Loperamide HCl capsules (Bionpharma).59 The changes limit each package to no more than 48 mg of loperamide, in addition to requiring unit-dose blister packaging.59 Liquid formulations will continue to be sold in 4-ounce and 8-ounce containers, with no more than 32 mg of loperamide in 8 fluid ounces.59 The FDA continues to work with other loperamide manufacturers and online distributors to support the safe use of loperamide.59
The federal government previously took steps to limit another OTC product, pseudoephedrine, which is used to make illicit methamphetamine. Congress passed the Combat Methamphetamine Epidemic Act (CMEA) in 2005, which requires pharmacies and other retail stores to keep logs of pseudoephedrine purchases and limit the amount that an individual can purchase in one day.60 The CMEA limited daily sales of pseudoephedrine to 3.6 grams, and sales within a 30-day period were limited to 9 grams.61 Pseudoephedrine-containing products are now located behind the pharmacy counter, and patients must present valid photo identification prior to purchase, which allows pharmacies to track whether or not a pseudoephedrine sale would be over the patient’s allotted limit.61 These restrictions dramatically reduced domestic production of the drug, dropping domestic methamphetamine laboratory incidents over 80% from 15,256 in 2010 to 3,036 in 2017.60
Although the FDA has limited the amount of loperamide available in packages, there is currently no policy in place to deter abusers from purchasing an unlimited number of packages. A major reason contributing to loperamide’s abusive capability is its accessibility as an OTC product that can be purchased in large volumes throughout multiple channels outside of a pharmacy, such as online retailers and convenience stores. One individual reported that they were able to purchase 2,400 capsules of loperamide from Amazon, an amount equal to 100 fatal doses.62 Despite FDA limitations, individuals can still purchase large quantities/in bulk from online retailers without pharmacovigilance or any regulatory barriers. Unfortunately, ease of accessibility still remains a significant limitation towards reducing the chances of loperamide misuse and abuse.
It will be up to the FDA if they wish to make loperamide a prescription product, which would give the tightest control over availability and ensure that all prescribers are aware of its use. Regulation of loperamide sales in a manner similar to that of pseudoephedrine and dextromethorphan could prove to be an effective measure to reduce further loperamide overdoses, but careful consideration must be taken so that patients who use loperamide as indicated are not inadvertently limited.
Pharmacist’s Role
Prevention in the outpatient setting is key to reducing unnecessary harm and death associated with loperamide abuse. Greater awareness regarding this issue among pharmacists is warranted to minimize further adverse events. Pharmacists can implement risk-stratification strategies and should provide effective patient and community education to ensure that individuals are being properly informed.63 In addition, through community outreach about appropriate pain management and medication use, pharmacists can play an active role in addressing the opioid crisis.63 However, how pharmacists do this is pivotal. Pharmacists need to ask the right questions and choose their words carefully when discussing proper loperamide use.42 When counseling a patient, pharmacists should be cautious with which words they use and what information they share to avoid inadvertently informing a patient that loperamide can be used to achieve euphoria or manage withdrawal.42 Examples of questions to ask include “Have you been taking loperamide?, “How often do you take loperamide and how much?,” “What do you take loperamide for?,” and “Are you aware of the risks associated with taking too much loperamide?”
By being cognizant of the signs and symptoms of loperamide misuse and abuse, pharmacists can be vigilant and identify patterns of diversion among consumers. Since routine drug tests do not look for the presence of loperamide and because there are no established treatment guidelines for loperamide overdose, pharmacists need to be especially mindful of identifying patients at risk.64 Oftentimes, the first point of contact between patients and the healthcare system, pharmacists are in a position to play a crucial role in combating loperamide abuse by identifying patients at risk of this atypical OUD, providing education on the dangers of nonmedical loperamide use to both clinicians and patients, and referring patients to an appropriate source of treatment for substance-use disorder.
In a national assessment of pharmacists’ awareness of loperamide abuse and their ability to restrict sales if abuse is suspected, 72% of 153 pharmacies reported that they were aware of how loperamide abuse occurred and felt that they could decrease the quantity purchased or deny the sale if abuse was suspected.65 However, only 3.2% of pharmacists had taken measures by placing loperamide behind the counter.65 With this in mind, there is substantial room for pharmacists to make interventions. As loperamide abuse has been growing over the years, it is vital that measures are taken to avert misuse. Multiple studies have provided significant evidence that pharmacists can make an impact in the opioid crisis by providing opioid overdose–prevention training, medication reviews, and counseling.63 With the rising cases of loperamide abuse, steps need to be taken to address this issue. Every member of the healthcare team has a role to play, whether it is providing appropriate pain management, providing education, or making changes at the state level. To prevent patient harm, the healthcare team needs to work quickly and effectively to circumvent this rising concern.
It is important to note that many of the numbers reported by the poison centers may not be a true representation of ongoing incidents. Much of the loperamide overdose data we rely on has limitations since reporting to poison centers is voluntary and symptom driven, relying on the validity of the information provided by the caller.10 Experienced physicians who are comfortable handling loperamide overdose cases on their own may not refer out to a poison control center for consultation, which could decrease the number of actual cases. Given the widespread availability of loperamide and its low cost, the number of individuals misusing and abusing loperamide may be far greater than we predict.10 Further research and education for healthcare providers and patients should continue to reduce the trends for loperamide abuse and misuse.22 Patients will unfortunately continue to get their loperamide information from online forums, but pharmacists can provide up-to-date education on the dangers of loperamide abuse.
The Consumer Healthcare Products Association has launched a Loperamide Safety education campaign to increase healthcare provider awareness of loperamide abuse, providing fact sheets, resources, and peerreviewed studies for members of the healthcare team who may encounter this throughout their profession, including pharmacists.66 The education campaign materials can be accessed at www.loperamidesafety.org.66
Conclusion
The number of loperamide-induced overdose cases have risen, seemingly coinciding with the restrictions placed on prescription opioids, throughout the past decade. Healthcare providers should be aware of the abuse potential of loperamide, an easily accessible OTC medication that allows users to self-treat opioid withdrawal or experience euphoric opioid effects at large doses. Patients with loperamide overdose typically present with signs and symptoms similar to an opioid overdose in addition to cardiac rhythm disturbances. There are currently no guideline-directed therapies for loperamide overdose. Management of loperamide overdose is supportive therapy, which can include naloxone, advanced cardiovascular life support, and correction of electrolyte imbalances. Close follow-up and monitoring are warranted in patients with an underlying substance-use disorder due to a likelihood of recurrence, and referral to a rehabilitation center should be considered. The FDA has recognized loperamide’s abuse potential and has taken steps to minimize misuse by limiting the amount of loperamide per package as well as requiring unit-dose blister packaging. Pharmacists play a critical role in prevention of further overdoses by identifying patterns of abuse and diversion, providing education on the dangers of loperamide overdose, and referring at-risk patients to substance-use treatment.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
- Reinert JP, Dunn RL. Management of overdoses of loperamide, gabapentin, and modafinil: a literature review. Expert Rev Clin Pharmacol. 2019;12(9):901-908.
- Imodium (loperamide) [package insert]. Raritan, NJ: Janssen Pharmaceuticals; 2016.
- Handbook of Nonprescription Drugs: An Interactive Approach to Self-Care. 19th ed. American Pharmacists Association.
- Reissig CJ, Carter LP, Johnson MW, et al. High doses of dextromethorphan, an NMDA antagonist, produce effects similar to classic hallucinogens [published correction appears in Psychopharmacology (Berl). 2013 Aug;228(3):513]. Psychopharmacology (Berl). 2012;223(1):1-15.
- Stanciu CN, Gnanasegaram SA. Loperamide, the "poor man's methadone": brief review. J Psychoactive Drugs. 2017;49(1):18-21.
- Swank KA, Wu E, Kortepeter C, et al. Adverse event detection using the FDA post-marketing drug safety surveillance system: cardiotoxicity associated with loperamide abuse and misuse. J Am Pharm Assoc. 2017;57(2S):S63-S67.
- CDC/NCHS, National Vital Statistics System, Mortality. CDC WONDER, Atlanta, GA: US Department of Health and Human Services, CDC; 2019. https://wonder.cdc.gov.
- Van Nueten JM, Helsen L, Michiels M, Heykants JJ. Distribution of loperamide in the intestinal wall. Biochem Pharmacol. 1979;28(8):1433-1434.
- Baker DE. Loperamide: a pharmacological review. Rev Gastroenterol Disord. 2007;7(Suppl 3):S11-S18.
- Borron SW, Watts SH, Tull J, et al. Intentional misuse and abuse of loperamide: a new look at a drug with "low abuse potential." J Emerg Med. 2017;53(1):73-84.
- Wu PE, Juurlink DN. Clinical review: loperamide toxicity. Ann Emerg Med. 2017;70(2):245-252.
- Caro MA, Shah SA, Jerry JM, et al. Loperamide abuse and lifethreatening arrhythmias: a case report and literature review. Psychosomatics. 2017;58(4):441-445.
- Schinkel AH, Wagenaar E, Mol CA, van Deemter L. P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest. 1996;97(11):2517-2524.
- Wessler JD, Grip LT, Mendell J, Giugliano RP. The P-glycoprotein transport system and cardiovascular drugs [published correction appears in J Am Coll Cardiol. 2014;63(20):2176. Dosage error in article text]. J Am Coll Cardiol. 2013;61(25):2495-2502.
- Kim KA, Chung J, Jung DH, Park JY. Identification of cytochrome P450 isoforms involved in the metabolism of loperamide in human liver microsomes. Eur J Clin Pharmacol. 2004;60(8):575-581.
- Jaffe JH, Kanzler M, Green J. Abuse potential of loperamide. Clin Pharmacol Ther. 1980;28(6):812-819.
- MacDonald R, Heiner J, Villarreal J, Strote J. Loperamide dependence and abuse. BMJ Case Rep. 2015;2015:bcr2015209705.
- Korey A, Zilm DH, Sellers EM. Dependence liability of two antidiarrheals, nufenoxole and loperamide. Clin Pharmacol Ther. 1980;27(5):659-664.
- DEA Office of Diversion Control. DEA Orange Book Lists of Scheduling Actions. US Dept of Justice (2021). www.deadiversion. usdoj.gov/schedules/orangebook/orangebook.pdf. Accessed September 3, 2021.
- De Vera J, Kim HB, Sakr AE. A case report of loperamide-induced ventricular storm. J Investig Med High Impact Case Rep. 2021;9:2324709621990768.
- Vakkalanka JP, Charlton NP, Holstege CP. Epidemiologic trends in loperamide abuse and misuse. Ann Emerg Med. 2017;69(1):73-78.
- Daniulaityte R, Carlson R, Falck R, et al. "I just wanted to tell you that loperamide WILL WORK": a Web-based study of extra-medical use of loperamide. Drug Alcohol Depend. 2013;130(1-3):241-244.
- Bluelight. Loperamide (Imodium) Megathread v. 2. www.bluelight. org/xf/threads/loperamide-imodium-megathread-v-2.717682. Accessed September 3, 2021.
- Eggleston W, Palmer R, Dubé PA, et al. Loperamide toxicity: recommendations for patient monitoring and management. Clin Toxicol (Phila). 2020;58(5):355-359.
- Powell JW, Presnell SE. Loperamide as a potential drug of abuse and misuse: fatal overdoses at the Medical University of South Carolina. J Forensic Sci. 2019;64(6):1726-1730.
- Bishop-Freeman SC, Feaster MS, Beal J, et al. Loperamide-related deaths in North Carolina. J Anal Toxicol. 2016;40(8):677-686.
- Inhibitors and Inducers of P-glycoprotein. Drug monographs. Access Pharmacy. https://accesspharmacy-mhmedical-com.ezproxy.lib. uh.edu/drugs.aspx?gbosID=410405. Accessed September 6, 2021.
- Palkar P, Kothari D. Bradycardia and syncope in a patient presenting with loperamide abuse. Cureus. 2018;10(5):e2599.
- Zhang Y, Benet LZ. The gut as a barrier to drug absorption: combined role of cytochrome P450 3A and P-glycoprotein. Clin Pharmacokinet. 2001;40(3):159-168.
- Larsen TR, McMunn J, Ahmad H, AlMahameed ST. Ventricular tachycardia triggered by loperamide and famotidine abuse. Drug Saf Case Rep. 2018;5(1):11.
- Sahi N, Nguyen R, Santos C. Loperamide. In: StatPearls. Treasure Island (FL): StatPearls Publishing; August 3, 2021.
- Strzyzewski L, Key P, Jones P, Prahlow JA. Loperamide abuse and its sequelae. Am J Forensic Med Pathol. 2020;41(3):207-210.
- Wu PE, Juurlink DN. Clinical review: loperamide toxicity. Ann Emerg Med. 2017;70(2):245-252.
- Schifano F, Chiappini S, Corkery JM, Guirguis A. Abuse of prescription drugs in the context of novel psychoactive substances (NPS): a systematic review. Brain Sci. 2018;8(4):73.
- Akel T, Bekheit S. Loperamide cardiotoxicity: a brief review. Ann Noninvasive Electrocardiol. 2018;23(2):e12505.
- Kang J, Compton DR, Vaz RJ, Rampe D. Proarrhythmic mechanisms of the common anti-diarrheal medication loperamide: revelations from the opioid abuse epidemic. Naunyn Schmiedebergs Arch Pharmacol. 2016;389(10):1133-1137.
- Thomas D, Karle CA, Kiehn J. The cardiac hERG/IKr potassium channel as pharmacological target: structure, function, regulation, and clinical applications. Curr Pharm Des. 2006;12(18):2271-2283.
- Vaz RJ, Kang J, Luo Y, Rampe D. Molecular determinants of loperamide and N-desmethyl loperamide binding in the hERG cardiac K+ channel. Bioorg Med Chem Lett. 2018;28(3):446-451.
- Salama A, Levin Y, Jha P, Alweis R. Ventricular fibrillation due to overdose of loperamide, the "poor man's methadone." J Community Hosp Intern Med Perspect. 2017;7(4):222-226.
- Schifano F, Chiappini S. Is there such a thing as a 'lope' dope? Analysis of loperamide-related European Medicines Agency (EMA) pharmacovigilance database reports. PLoS One. 2018;13(10):e0204443.
- Ahmed A, Freeland S, Steinberg L, Prystowsky EN. Wide complex tachycardia and cardiomyopathy: What would you do? J Cardiovasc Electrophysiol. 2018;29(8):1171-1173.
- Understanding loperamide abuse. Prevent loperamide abuse. www. loperamidesafety.org/home-1?fbclid=IwAR3wPdndkGasTXUqifLDimS pJzUYU_xor3gauxb-7n96h2xMdd_R7TCpzjE. Accessed September 25, 2021.
- Nakka S, Riverdale, Reddy S, Mehta N. Re: dysrhythmias with loperamide used for opioid withdrawal. J Am Board Fam Med. 2018;31(3):488-489.
- Miller H, Panahi L, Tapia D, et al. Loperamide misuse and abuse. J Am Pharm Assoc. 2017;57(2S):S45-S50.
- Ali M, Mujahid A, Bulathsinghala CP, Surani S. Cardiac arrhythmia secondary to loperamide abuse and toxicity. Cureus. 2020;12(2):e6936.
- Juurlink DN. Activated charcoal for acute overdose: a reappraisal. Br J Clin Pharmacol. 2016;81(3):482-487.
- Green R, Grierson R, Sitar DS, Tenenbein M. How long after drug ingestion is activated charcoal still effective? J Toxicol Clin Toxicol. 2001;39(6):601-605.
- Rzasa Lynn R, Galinkin JL. Naloxone dosage for opioid reversal: current evidence and clinical implications. Ther Adv Drug Saf. 2018;9(1):63-88.
- Becoming familiar with synchronized cardioversion. ACLS resources. https://resources.acls.com/free-resources/knowledge-base/ tachycardia/about-synchronized-cardioversion. January 27, 2021.
- Betting DJ, Chenoweth JA, Jarman AF. A case report of cardiogenic syncope due to loperamide abuse: acute presentation and novel use of buprenorphine. Clin Pract Cases Emerg Med. 2021;5(2):214217.
- Marraffa JM, Holland MG, Sullivan RW, et al. Cardiac conduction disturbance after loperamide abuse. Clin Toxicol (Phila). 2014;52(9):952-957.
- Toce MS, Chai PR, Burns MM, Boyer EW. Pharmacologic treatment of opioid use disorder: a review of pharmacotherapy, adjuncts, and toxicity. J Med Toxicol. 2018;14(4):306-322.
- Varghese SP, Kumari P, Wijegunaratne H, et al. Loperamide addiction: atypical opioid use disorder treated with buprenorphine/naloxone. Prim Care Companion CNS Disord. 2019;21(4):19102446.
- Brar JK, Broyan VR, Allgaier JT, et al. Long-term buprenorphine treatment for loperamide use disorder: a case series. J Addict Med. 2020;14(6):e378-e381.
- Drug Safety-Related Labeling Changes (SrLC). FDA. www.accessdata.fda.gov/scripts/cder/safetylabelingchanges/index. cfm?event=searchResult.page. Accessed September 25, 2021.
- FDA drug safety communication. www.fda.gov/drugs/drug-safetyand-availability/fda-drug-safety-communication-fda-warns-about-serious-heart-problems-high-doses-antidiarrheal. January 29, 2018. Accessed September 11, 2021
- Shastay A. Dangerous abuse of a 40-year-old over-the-counter mmedication, loperamide. Home Healthc Now. 2020;38(3):167-168.
- White CM. Loperamide: a readily available but dangerous opioid substitute. J Clin Pharmacol. 2019;59(9):1165-1169.
- FDA media inquiries. www.fda.gov/news-events/fda-brief/fda-brieffda-approves-new-packaging-brand-name-over-counter-loperamidehelp-curb-abuse-and-misuse. 2019 September 20, 2019. Accessed September 21, 2021.
- NIDA. How is methamphetamine manufactured? National Institute on Drug Abuse. www.drugabuse.gov/publications/research-reports/ methamphetamine/how-methamphetamine-manufactured. April 13, 2021. Accessed September 26, 2021.
- CMEA (Combat Methamphetamine Epidemic Act of 2005). U.S. Department of Justice Diversion Control Division. www.deadiversion. usdoj.gov/meth/index.html. Accessed September 26, 2021.
- Haigney MC, Klein MG, Flagg TP, et al. Reply: labeling and drug safety of loperamide: time for a proactive approach? JACC Clin Electrophysiol. 2017;3(4):422-423.
- Chisholm-Burns MA, Spivey CA, Sherwin E, et al. The opioid crisis: origins, trends, policies, and the roles of pharmacists. Am J Health Syst Pharm. 2019;76(7):424-435.
- Terrie YC. Antidiarrheal is latest candidate for abuse. Pharmacy Times. 2020;88(3).
- Feldman R, Everton E. National assessment of pharmacist awareness of loperamide abuse and ability to restrict sale if abuse is suspected. J Am Pharm Assoc. 2020;60(6):868-873.
- Understanding loperamide abuse. Loperamide safety. www.loperamidesafety.org/home-1. Accessed September 27, 2021.