Pharmacologic Management of HIV-Associated Wasting Syndrome
RELEASE DATE:
December 1, 2016
EXPIRATION DATE:
December 31, 2018
FACULTY:
Steven Kheloussi, PharmD
Assistant Professor of Pharmacy Practice
Wilkes University
Wilkes-Barre, Pennsylvania
FACULTY DISCLOSURE STATEMENTS:
Dr. Kheloussi has no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT:
Pharmacy
 Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-16-103-H02-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE:
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@jobson.com
DISCLAIMER:
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients’ conditions and possible contraindications or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
GOAL:
To provide pharmacists with a background on the epidemiology, pathophysiology, and clinical implications of human immunodeficiency virus (HIV)–associated wasting syndrome as well as evidence-based treatment recommendations for this condition.
OBJECTIVES:
After completing this activity, the participant should be able to:
- Define HIV-associated wasting syndrome and recognize the implications of the disease.
- Outline the pathophysiology behind HIV-associated wasting.
- Describe the role of nonpharmacologic and pharmacologic treatment in HIV-associated wasting.
- Educate patients on pharmacologic treatment options’ efficacy, safety, dosage, and rationale for use.
ABSTRACT: Wasting syndrome is a serious and life-threatening complication of HIV infection that is characterized by weight loss, but, more specifically, by a significant loss of lean body mass. The prevalence of this disorder is hard to pinpoint due to changes in the definition of wasting over time and the introduction of highly active antiretroviral therapy (HAART). Various pharmacologic and nonpharmacologic approaches can be used for wasting syndrome, but specific regimens must be based on each individual’s underlying pathophysiology. Nutritional therapy, strength training, appetite stimulation, anabolic therapies, and cytokine inhibitors are all important options in controlling this disorder.
Wasting syndrome is a serious and life-threatening complication of HIV infection that is characterized by significant weight loss. With a prevalence that is hard to estimate, a widely variable clinical presentation, a poorly understood pathophysiology, and limited effective treatment options, wasting syndrome in HIV is a complex topic with vast implications.
DISEASE BACKGROUND
Weight loss has long been a notable complication of HIV and AIDS, and it is associated with severe adverse events, including mortality. Wasting syndrome is specifically characterized by a progressive loss of weight, mostly related to a loss of lean muscle mass, commonly measured by body cell mass (BCM). However, loss of lean muscle tissue and the loss of overall body weight are closely correlated.
Definition
In 1987, the CDC designated HIV-associated wasting as an AIDS-defining condition—a condition that is associated with AIDS and can be used in the making of a diagnosis. The CDC defined it as “an involuntary weight loss of greater than 10% of baseline body weight plus either chronic diarrhea (at least two loose stools per day for at least 30 days), or chronic weakness and documented fever (for at least 30 days, intermittent or constant) in the absence of a concurrent illness or condition other than HIV infection that could explain the findings such as cancer, tuberculosis, cryptosporidiosis, or other specific enteritis.”1
However, because this definition fails to account for several important diagnostic and prognostic aspects of the disease—including the rapidity of weight loss, those patients without a documented baseline weight, and those presenting later in their illness—a more refined definition came to be more widely accepted. This updated description of HIV-associated wasting is summarized in TABLE 1.2,3
Epidemiology
Given the evolution of the definition of HIV wasting and the difficulty faced in differentiating this disorder from other forms of weight loss in HIV, the exact prevalence of this disorder and the change over time have been difficult to evaluate.4 Between 1987 and 1993, at the time of the first CDC definition and before the introduction of highly active antiretroviral therapy (HAART), wasting was estimated to have impacted approximately 20% of newly diagnosed AIDS patients and up to 70% at the time of death.5 However, the original CDC definition may have inadvertently underestimated the actual prevalence.3 Using the updated definition and considering the introduction and impact of HAART, the prevalence is now estimated at between 14% and 38% of all patients with HIV.3,5,6 Though the introduction of HAART has presumably reduced the occurrence of wasting, it continues to be a frequent complication of HIV.7
Clinical Presentation and Impact
Patients with wasting syndrome typically present with decreased total body weight. Unlike simple starvation, this weight loss is primarily related to a loss of lean muscle and is likely associated with increased protein-turnover and other hypermetabolic characteristics.8 This weight loss is complicated, however, by a significant heterogeneity in disease progression and by the introduction of HAART. Antiretroviral therapy is associated with weight gain and fat redistribution away from the arms, legs, and face and toward the abdomen.2 Patients may not necessarily present with weight loss, but instead with stable or even increased weight yet with a loss of lean muscle mass.
Evidence has shown that a greater loss of body weight and lean muscle is associated with poorer outcomes. Given the complexity in diagnosis, however, it is somewhat unclear whether these worse outcomes are seen with overall loss of body weight including adipose tissue or if they are more closely correlated with the loss of BCM seen in wasting. Regardless, wasting syndrome has been linked to disease progression to AIDS; an increased risk of hospitalization, opportunistic infection, and malignancy; a decreased ability to complete activities of daily living; and a decreased quality of life.2,5,9,10 Furthermore, the risk of death is six times higher in patients with 10% weight loss from baseline compared to those with only 5% weight loss from baseline, but the risk of mortality increases significantly with each 1% decrease in weight from baseline.3 Similarly, the loss of lean muscle mass is also associated with increased mortality and decreased quality of life.11,12 It has been suggested that the loss of BCM is more important than a loss of overall body weight in predicting survival, though more evidence is needed to discern the differences.5,11
Pathophysiology
The pathophysiology of wasting syndrome is multifactorial. To date, it appears that there continues to be a lack of full consensus on the true underlying causes of this disorder. While several factors are consistently described in the literature and summarized below, this disagreement regarding the importance of each in the development and worsening of HIV wasting is more related to interpatient variability than to an actual discrepancy in evidence.13
At a high level, total energy balance is made up of total energy intake minus resting energy expenditure (REE) and energy expended through activity. If there is a negative balance between these variables (i.e., total energy intake is less than energy expenditure), the result will be weight loss. Likewise, if energy intake is greater than energy expenditure, the result will be weight gain.11
Decreased Energy Intake: Insufficient caloric intake and malabsorption are the two most universally accepted etiologies of wasting syndrome, and both heavily impact the total energy intake portion of the energy balance equation.5,6,8,10,14,15
Malnutrition can come about through a variety of mechanisms, including anorexia secondary to neurologic disease, illness, or medications; abnormal taste sensation, nausea, or vomiting as a medication-related adverse event; alterations to oral mucosa; fatigue; malaise; mental status changes, including depression and HIV-associated dementia; and illicit substance abuse.3,6,8,10,11,14,15 Additionally, socioeconomic status affecting the ability to procure and prepare foods can lead to malnutrition, as can the absence of a caregiver as well for patients lacking the functional capacity to feed themselves.3,10,14,15
Further increasing the risk for malnutrition is the increased energy intake requirement associated with infection. Secondary to increases in metabolism, even asymptomatic patients with HIV and no active secondary infections must increase their energy intake by approximately 10% to prevent weight loss. These estimates are higher in patients with opportunistic infections, AIDS, or HIV wasting.7
Malabsorption can also be the result of numerous factors and may be related to gastrointestinal (GI) obstruction, malignancy, vomiting, or diarrhea secondary to opportunistic infection, medication use, or pancreatitis.6,8,14 While malabsorption is thought to decrease energy intake, its impact is much more difficult to quantify than that of malnutrition.16 Still, malabsorption is an important underlying cause of HIV wasting.
Increased Energy Output: Increased REE was long thought to influence weight loss in HIV. However, patients actually tend to have lower levels of total energy expenditure than healthy individuals owing to less energy expended through activity.6,8,10,11,13,16 Patients who are the most ill tend to be more inactive secondary to fatigue and lethargy, which is a survival mechanism intended to offset the improper increase in REE and maintain energy balance.11,16 This highlights the fact that energy imbalance, and therefore weight loss, is not worsened by an increased energy output and instead can theoretically be corrected by improving intake.11,16
Opportunistic Infection: The relationship between opportunistic infection and HIV wasting is complex. It is unclear whether weight loss is directly related to infection or if, instead, weight loss is a secondary outcome to the physiological implications of infection. Patients with an acute infection may experience anorexia and dysphagia, which can decrease food intake, or malabsorption secondary to diarrhea or stress-induced catabolism.11,16 As previously discussed, the risk of opportunistic infections increases with weight loss in HIV. Therefore, a theoretical cycle develops, in which weight loss precipitates secondary infection that can lead to further weight loss, and so on.
Endocrine Dysfunction: Hormonal changes in HIV can also have negative effects on weight and BCM. Low levels of total and free testosterone are commonly found in patients with wasting and may be related to a loss of lean body mass.2,8 These low levels are typically caused by hypogonadotropic hypogonadism, a common sequela of HIV that can affect up to 50% of males with HIV and can also occur in females.7,8
In addition to testosterone, growth-hormone irregularities may lead to wasting. Growth hormone promotes protein synthesis and muscle growth through a cascade of events related to other secondary hormones. In patients with HIV, release of growth hormone may be inhibited, which negates its anabolic effects, in turn decreasing protein synthesis and reducing lean body mass.9
Cytokines: Cytokines are a complex group of immunoregulatory proteins that exert physiological and pathological effects throughout the body. Cytokines are difficult to measure, and specific roles are relatively unknown. The role of cytokines in the development of wasting is unclear. Theories regarding the relationship between interleukin (IL)-1, IL-2, and IL-6, tumor necrosis factor alpha, and interferon gamma and the development of wasting have been postulated due to elevated levels in patients with wasting.9 Specifically, these cytokines may encourage hypermetabolism and anorexia, promote the loss of lean body mass through enhanced protein breakdown, and even accelerate the progression from HIV to AIDS.3,8,16 However, evidence is mixed, and therefore no clear conclusion has been reached about the importance of these cytokines in this disease.8,9,17
TREATMENT
Goals of Therapy
The primary goals of therapy should be not only to gain weight, but also to prevent further reduction and ideally increase lean body mass. Increasing lean body mass can decrease the frequency of opportunistic infections while improving energy, quality of life, and survival.2,11,13 If possible, this should be done without a reliance on pharmacologic agents, and therefore a strong emphasis should be placed on correcting energy imbalance.5
Treatment should be individualized to the specific underlying factors surrounding each patient’s condition.7 For instance, if the patient is found to be malnourished and this is determined to be related to financial barriers limiting access to food, pharmacologic treatment is unlikely to help. Identifying each patient’s specific etiologies will help determine the most appropriate course of action.
Nonpharmacologic Intervention
Nutrition Therapy: As previously mentioned, insufficient caloric intake is the primary driver of weight loss in HIV. As part of a holistic approach to treatment, nutrition counseling and proper supplementation can be highly effective as first-line therapy to improve body weight and lean muscle mass.2,5,13,15 However, inappropriate supplementation it with foods low in protein, vitamins, and minerals but still high in calories can lead to obesity without a regaining of lean muscle mass. Referral to a nutritionist for intervention is recommended.15
Enteral and Parenteral Nutrition: Patients not responding to oral supplementation or those with barriers to oral intake may benefit from enteral or parenteral nutrition. Enteral nutrition—feeding that utilizes the patient’s GI tract—is appropriate for patients with a functional GI tract but poor oral intake due to psychological reasons or physical barriers. Parenteral nutrition—feeding that bypasses the GI tract and is administered IV—is appropriate for patients with malabsorption due to a poorly functioning GI tract or profuse diarrhea or vomiting.5
These forms of supplementation can increase body weight and, in patients with malabsorption or anorexia not related to infection, can increase lean body mass.8,11,18 Most importantly, parenteral nutrition was associated with higher survival rates in patients with severe AIDS.11,18 However, the cost and impact on quality of life limit the use of enteral and parenteral nutrition, as does an inherent risk of infection with parenteral supplementation. As a result, parenteral nutrition should only be used in those unable to feed orally or enterally.18
Exercise: Both aerobic and strength training, alone and in combination, have been associated with improvements in body weight, lean muscle mass, and other important markers, such as depression and quality of life.2,15 It is vital that exercise be implemented as a complementary component of each patient’s regimen.
Situational Interventions: Several easy-to-implement interventions should be considered for certain patient groups suffering from malnutrition. If anorexia is occurring secondary to an individual’s inability to afford food, the patient should be referred to social services for help in applying for food stamps. If available, the patient should be provided information on local charitable groups that provide meals to impoverished individuals.2 Patients who smoke should be encouraged to quit, as smoking can depress appetite.2 And finally, patients who are still active IV drug abusers must be referred to addiction specialists, since these patients are at a high risk of malnutrition.
Pharmacologic Treatment Options
HAART: Antiviral medications are very effective in controlling markers of disease progression including viral load and CD4t count. Since their introduction, the prevalence of wasting has changed considerably. However, their true effects on wasting are unclear.9-11 To properly examine the relationship between HAART and weight change, several factors must be considered.
Disease severity, measured by viral load, appears to be inversely correlated with changes in weight, such that patients with higher viral loads tend to experience greater weight loss and vice versa.3 However, this appears to be the case only in patients who are antiretroviral treatment-naïve. In those patients currently on therapy, CD4t count is more closely associated with weight loss.19 Regardless, HIV disease severity appears to be related to weight loss, as the most severe cases of wasting are seen in those patients with the most advanced disease.10
Interpatient variability in response to HAART is another important factor that must be considered in the relationship between antiretroviral medications and weight loss. Some patients present with no change in weight after beginning HAART, especially those early in their disease. Others will present with weight gain primarily composed of body fat with no associated gain in muscle, while still others will experience weight gain and an increase in BCM.10 This may be related in part to nonpharmacologic considerations, such as diet and exercise, or perhaps it is related to an inherent variability in drug-specific effects.9
Finally, drug-specific adverse events, such as diarrhea and vomiting, are common occurrences with several antiretroviral drug classes. Nucleoside reverse transcriptase inhibitors, protease inhibitors, integrase strand transfer inhibitors, and, to a lesser extent, the non-nucleoside reverse transcriptase inhibitors can cause these unwelcome effects and may lead to reduced intake or malabsorption.20
HAART has changed the landscape of all aspects of HIV, including wasting. While the relationship between HAART and weight loss is somewhat unclear, it is certain that HIV wasting and the poor outcomes associated with this condition are not a thing of the past. Antiretroviral therapy may not prevent weight loss and may lead to fat redistribution, but the positive effects on mortality and quality of life of HAART significantly outweigh the drawbacks.20 It is strongly recommended to start therapy for treatment-naïve patients or intensify regimens for those currently uncontrolled on therapy to prevent future weight loss and worsening HIV infection.2,13,19 Pharmacists can have the biggest impact by removing barriers to adherence with HAART or identifying and working to correct drug-related adverse events that may lead to complications like malabsorption.
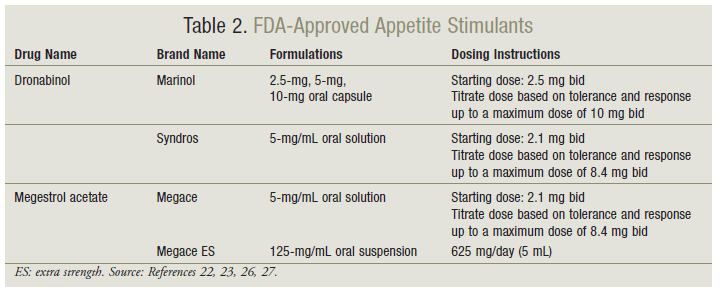
Appetite Stimulants: For patients experiencing anorexia, appetite stimulation is an important therapeutic mechanism. Two appetite stimulants are approved for use by the FDA—dronabinol and megestrol acetate.
Dronabinol: Dronabinol is a synthetic form of delta-9-tetrahydrocannabinol, more commonly referred to as THC. It exerts its effects in the emetic center in the brain by primarily targeting one of two cannabinoid receptors.6,7 Therefore, dronabinol is most effective for anorexia caused by nausea.7 Changes in total body weight with dronabinol tend to be modest, with average gains of up to 3.2 kg seen in clinical trials. While some contradictory evidence actually showed weight loss in dronabinol-treated patients, the majority of data suggest that dronabinol may be useful to maintain current body weight, rather than to promote weight gain.2,15,21 There are no long-term data available regarding changes in survival or dronabinol’s impact on lean muscle mass.6
Dronabinol is generically available in 2.5-mg, 5-mg, and 10-mg oral capsules. The FDA also approved a new formulation in July 2016. Branded under the name Syndros, dronabinol is now available as a 5-mg/ mL oral solution.22 See TABLE 2 for dosing instructions. Dronabinol capsules should be stored in a cool environment (46°F-59°F) or be refrigerated, while the oral solution must be refrigerated until opening. Once opened, the oral solution can be kept at room temperature and should be discarded after 28 days.22,23
Adverse events appear to be dose-related and typically affect the central nervous system.6 The most common side effects include euphoria, paranoia, somnolence, and dizziness. Notably, caution should be used in patients with a history of substance abuse, as tolerance and dependence may develop with prolonged use. Dronabinol may also lower seizure threshold and may cause occasional hypotension. No major drug interactions are expected, though dronabinol is a minor substrate of the CYP2C9 and -3A4 pathways.22,23
Megestrol Acetate: Megestrol acetate was historically indicated only for breast cancer, until it was found that it had the unwelcome adverse reaction of weight gain.7 However, what is considered a side effect in one disease can be utilized as the desired therapeutic effect in another. Such is the case in wasting. Megestrol acetate has shown increases in total body weight of 4 kg to 5 kg over a period of 12 weeks.6 But while the drug is very effective in increasing total body weight, the majority of weight gain is fat. This is likely related to megestrol acetate’s hypogonadic effects, which promote fat accumulation over lean muscle mass.2,15 Use of megestrol acetate is also associated with increased appetite and improved overall sense of well-being, though no effect on survival has been studied.24,25
Megestrol acetate is available as an oral tablet and as a regular-strength or concentrated oral suspension. Only the oral suspensions are FDA-approved for use in AIDS-associated wasting after treatable causes of weight loss are sought and addressed. Unlike dronabinol, megestrol acetate suspensions are to be stored at room temperature.26,27 See TABLE 2 for dosing instructions. The lesser volume required with the concentrated formulation can be beneficial for patients with dysphagia or anorexia.6
Megestrol acetate suspension is typically well tolerated, but cases of new-onset diabetes and adrenal suppression have been reported. It is contraindicated in pregnancy and in patients taking dofetilide, an interaction that may result in QT prolongation and torsades de pointes.26,27
Anabolic Therapies
While appetite stimulants target malnutrition, their gains are primarily in total body weight and not lean mass. Other potential therapies, however, provide a more balanced weight gain between lean muscle mass and fat.11 The underlying causes of wasting previously discussed with targeted therapies include testosterone, growth hormone, and cytokines. Additionally, anabolic steroids can be utilized.
Testosterone: Although the link between low testosterone and HIV wasting is unclear, replacement therapy in hypogonadal males has been shown to aid in the restoration of total body weight, lean body mass, and quality of life.5,13 The addition of strength training to testosterone was associated with additional gains in lean muscle.2 On the downside, clinical trials have been short in duration and have not consistently reviewed a specific formulation; while testosterone consistently restores weight and muscle mass loss, results vary with regard to the extent of these effects.15 Although testosterone is not FDA-approved for use for HIV wasting, its use in men is still recommended by wasting treatment guidelines, particularly for those with hypogonadism and resulting low testosterone levels.2
Women, on the other hand, have typically been underrepresented in clinical trials for testosterone. Given the limited, conflicting data available and the inherent risks and adverse events associated with testosterone in this population, testosterone’s specific role in women with HIV wasting is unclear.13,15
Testosterone is available in a variety of formulations, including a transdermal cream, ointment, or gel; an intranasal gel; a buccal system; a pellet for implantation; and a solution for intramuscular injection. No overwhelming evidence has proven one specific formulation more efficacious than another. Fittingly, then, it is recommended to take into account patient preference, tolerability, and adherence when choosing a testosterone product.7 Pharmacists can educate patients on the available formulations and can help in switching patients to a more appropriate medication if they are unable to adhere to therapy or are unwilling to utilize certain formulations. Many products are still brand-name only and thus are quite expensive, so payer restrictions must also be taken into account. It bears repeating that, to date, no testosterone formulation is FDA-approved for use in HIV wasting.
The use of testosterone is associated with numerous serious adverse events, including cardiovascular events, prostate cancer, and venous thromboembolism. Other side effects include dyslipidemia, gynecomastia, application-site reactions, hirsutism, and changes in libido.7,28 Testosterone must be kept out of the reach of children, and use in pregnant women is contraindicated. Finally, different formulations and products are not necessarily interchangeable owing to varying concentrations, so care must be taken when altering therapy.
Growth Hormone: As previously mentioned, growth hormone promotes protein synthesis and muscle growth, but its effects may be dulled in HIV. Recombinant human growth hormone (rhGH) works as a replacement to endogenous growth hormone and has been shown to be an effective therapeutic option by increasing total body weight, lean muscle mass, and exercise capacity.2,9 Most evidence points to a single course of therapy of 12 weeks, with some sources, including the FDA-approved labeling, suggesting one additional 12-week course of therapy for some patients.9,29 Unfortunately, unknowns remain, including the ideal length of therapy and the extent to which wasting returns upon discontinuation.14 Guidelines recommend rhGH as a suitable treatment option for three groups of patients—male patients with normal testosterone levels, hypogonadal male patients who tried and failed therapy with testosterone, and female patients with HIV wasting.2
rhGH (somatropin) is available in a number of products; however, the only FDA-approved product for HIV wasting is Serostim. Serostim is available in a 4-mg multiuse vial or a 5-mg or 6-mg single-use vial. It is dosed at 0.1 mg/kg once daily, which works out to be approximately 6 mg daily for patients weighing over 55 kg, 5 mg daily for those weighing between 45 and 55 kg, or 4 mg daily for those patients weighing less than 45 kg.29
The major drawback of therapy is that this product is only available as a daily SC injection. Patients should be counseled on the benefits of therapy and that the length of therapy is not open-ended. Another important consideration is that patients who are at a high risk for glucose intolerance or who have a strong family history of diabetes should receive an appropriate weight-based dose of somatropin every other day rather than daily.9 Less serious adverse events seen in the clinical trials include peripheral edema, myalgia, arthralgia, and paresthesia. Serostim has a Pregnancy Category B rating, indicating that it is likely safe to use in pregnancy, and while it is not FDA-approved for use in pediatric patients, two small studies have shown positive results.29 Notably, however, Serostim contains benzyl alcohol, which, at high doses may cause gasping syndrome in pediatric patients, particularly neonates.29
Anabolic Steroids: Oxandrolone is FDA-approved for use as an adjunctive therapy to help promote weight gain after weight loss following extensive surgery, chronic infections, or severe trauma. However, its use in HIV-associated wasting is considered off-label. Unfortunately, evidence supporting the use of oxandrolone in wasting is sparse. Oxandrolone with or without strength training improves total body weight and lean body mass, but it has not been shown to be more effective than nutritional intervention alone.2,3
Oxandrolone is available in 2.5-mg and 10-mg oral tablets, and doses between 2.5 mg daily and 20 mg daily in two to four divided doses can be used for weight gain. It has been suggested, however, that doses over 15 mg per day are required for weight gain in HIV.30 The use of oxandrolone is limited by mixed reports of changes in liver function tests and lipids.2
Cytokine Inhibitors: As previously mentioned, cytokines may promote the loss of lean muscle mass and weight loss through a variety of mechanisms. Thus, inhibition of cytokine activity seems to be a straightforward therapeutic target. While the cytokine inhibitor thalidomide has shown some limited benefits in clinical trials, improvements in lean muscle mass were consistently seen.2,14,15 However, thalidomide is not FDA approved for use in this condition.
Thalidomide is associated with significant adverse events, including a transient increase in HIV viral load, irreversible peripheral neuropathy, and fetal toxicity.6,7 Related to the potential for birth defects, thalidomide is available only through a Risk Evaluation and Mitigation Strategy program. Given its restricted availability, limited efficacy, significant risk of toxicities, and lack of FDA approval for use in this condition, thalidomide should be used only in patients with profound wasting that is unresponsive to other therapies.7,31
Combination Therapy: Combination therapies have been tried with megestrol acetate, including testosterone, dronabinol, and oxandrolone. Unfortunately, both dronabinol and testosterone were not found to provide any additional benefit over therapy with megestrol acetate alone.7,15,32 Conversely, oxandrolone in combination with megestrol acetate and nutritional counseling was associated with significant increases in total body weight and lean muscle mass.7,12
Conclusion
HIV-associated wasting is a dense topic with unclear pathophysiology and a complex treatment algorithm filled with conflicting evidence and a number of unanswered questions. The true impact of all of the discussed treatment options on survival is questionable. Further, the ideal length of treatment has not been defined for any agent, comparative data are sparse, and the true benefit of combination therapy is unknown. Still, strides have been made since this disease’s inception that have altered its course. Improved quality of life has been shown with several agents, and HAART has played a big role in survival.
A new diagnosis of HIV can be crushing for patients. Awareness of the relationship and implications of wasting is likely limited. It is important that healthcare professionals work collaboratively to prevent and correct this treatable aspect of the disease. Pharmacists can play an important role in counseling patients about maintaining their weight, the consequences of weight loss, and the availability and importance of nonpharmacologic and pharmacologic intervention.
REFERENCES
- Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(RR-17):1-19.
- Polsky B, Kotler D, Steinhart C. HIV-associated wasting in the HAART era: guidelines for assessment, diagnosis, and treatment. AIDS Patient Care STDS. 2001;15(8):411-423.
- Mangili A, Murman DH, Zampini AM, Wanke CA. Nutrition and HIV infection: review of weight loss and wasting in the era of highly active antiretroviral therapy from the nutrition for healthy living cohort. Clin Infect Dis. 2006;42(6):836-842.
- Coodley GO, Loveless MO, Merrill TM. The HIV wasting syndrome: a review. J Acquir Immune Defic Syndr. 1994;7:681-694.
- Salomon J, De TP, Melchior JC. Nutrition and HIV infection. Br J Nutr. 2002;87(suppl 1):S111-119.
- Badowski ME, Perez SE. Clinical utility of dronabinol in the treatment of weight loss associated with HIV and AIDS. HIV AIDS (Auckl). 2016;8:37-45.
- Badowski M, Pandit NS. Pharmacologic management of human immunodeficiency virus wasting syndrome. Pharmacotherapy. 2014;34(8):868-881.
- Strawford A, Hellerstein M. The etiology of wasting in the human immunodeficiency virus and acquired immunodeficiency syndrome. Semin Oncol. April 1998; 25(2 suppl 6):76-81.
- Gelato M, McNurlan M, Freedland E. Role of recombinant human growth hormone in HIV-associated wasting and cachexia: pathophysiology and rationale for treatment. Clin Ther. 2007;29(11):2269-2288.
- Wheeler DA. Weight loss and disease progression in HIV infection. AIDS Read. 1999;9(5):347-353.
- Macallan DC. Wasting in HIV infection and AIDS. J Nutr. 1999;129(1 suppl): 238S-242S.
- Mwamburi DM, Wilson IB, Jacobson DL, et al. Understanding the role of HIV load in determining weight change in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;40(1):167-173.
- Wanke C, Kotler D. Collaborative recommendations: the approach to diagnosis and treatment of HIV wasting. J Acquir Immune Defic Syndr. 2004;37(suppl 5): S284-S288.
- Balog DL, Epstein ME, Amodio-Groton MI. HIV wasting syndrome: treatment update. Ann Pharmacother. 1998;32(4):446-458.
- Keithley JK, Swanson B. HIV-associated wasting. J Assoc Nurses AIDS Care. 2013;24(1 suppl):S103-S111.
- Macallan DC, Noble C, Baldwin C, et al. Energy expenditure and wasting in human immunodeficiency virus infection. N Engl J Med. 1995;333(2):83-88.
- Grinspoon SK, Bilezikian JP. HIV disease and the endocrine system. N Engl J Med. 1992;327:1360-1365.
- Ockenga J, Grimble R, Jonkers-Schuitema C, et al. ESPEN guidelines on enteral nutrition: wasting in HIV and other chronic infectious diseases. Clin Nutr. 2006;25(2):319-329.
- Mwamburi DM, Gerrior J, Wilson IB, et al. Combination megestrol acetate, oxandrolone, and dietary advice restores weight in human immunodeficiency virus. Nutr Clin Pract. 2004;19(4):395-402.
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. 2016. https://aidsinfo.nih.gov/contentfiles/lvguidelines/ adultandadolescentgl.pdf. Accessed August 20, 2016.
- Beal JE, Olson R, Laubenstein L, et al. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage. 1995;10:89-97.
- Syndros (dronabinol oral solution) package insert. Chandler, AZ: Insys Therapeutics; July 2016.
- Marinol (dronabinol) package insert. Marietta, GA: Unimed Pharmaceuticals, Inc; July 2006.
- Oster MH, Enders SR, Samuels SJ, et al. Megestrol acetate in patients with AIDS and cachexia. Ann Intern Med. 1994;121:400-408.
- Von Roenn JH, Armstrong D, Kotler DP, et al. Megestrol acetate in patients with AIDS-related cachexia. Ann Intern Med. 1994;121:393-399.
- Megace (megestrol acetate) package insert. Princeton, NJ: Bristol-Myers Squibb Company; March 2012.
- Megace ES (megestrol acetate extra strength) package insert. Spring Valley, NY: Par Pharmaceutical Co, Inc; September 2014.
- Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010 JunE;95(6):2536-2559.
- Serostim (recombinant human growth hormone, somatropin) package insert. Rockland, MA: EMD Serono, Inc; October 2015.
- Berger JR, Pall L, Simpson D, et al. Oxandrolone in AIDS wasting/myopathy [abstract]. J Neurovirol. 1996;2:32.
- Gunzler V. Thalidomide in human immunodeficiency virus (HIV) patients. A review of safety considerations. Drug Saf. 1992;7:116-134.
- Timpone JG, Wright DJ, Li N, et al. The safety and pharmacokinetics of single-agent and combination therapy with megestrol acetate and dronabinol for the treatment of HIV wasting syndrome. The DATRI 004 Study Group. Division of AIDS Treatment Research Initiative. AIDS Res Hum Retroviruses. 1997;13(4):305-315.