Prescribing Oral Contraceptives: A New Pharmacist Role
RELEASE DATE:
September 1, 2018
EXPIRATION DATE:
September 30, 2020
FACULTY:
Cortney M. Mospan, PharmD, BCACP, BCGP
Assistant Professor of Pharmacy
Wingate University School of Pharmacy
Wingate, North Carolina
FACULTY DISCLOSURE STATEMENTS:
Dr. Mospan has no potential or actual conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT:
Pharmacy
 Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-18-041-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE:
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER:
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients' conditions and possible contraindications or dangers in use, review of any applicable manufacturer's product information, and comparison with recommendations of other authorities.
GOAL:
To provide participants with knowledge of oral contraceptive products including mechanism of action, side effects, contraindications, differences between products, and other relevant prescribing information, and review key contraception resources and prescribing considerations as more states gain practice authority for pharmacist prescribing of contraception.
OBJECTIVES:
After completing this activity, the participant should be able to:
- Identify necessary patient assessments before provision of oral contraceptives.
- Recall key differences between oral contraceptive types, drug interactions, and contraindications for use.
- Employ the medical eligibility criteria and patient factors for oral contraceptive selection.
- Provide effective counseling for safe and appropriate use of pharmacist-provided oral contraceptives.
ABSTRACT: Pharmacists have an emerging role as contraceptive providers as more and more states pass legislation allowing pharmacist prescribing of various contraceptive agents. With this new opportunity, pharmacists must be prepared to properly assess and screen patients prior to contraceptive initiation, select the best product for the patient, provide effective counseling, and make necessary adjustments when patients experience adverse effects and other challenges.
Over half of pregnancies in the United States are unintended, and HealthyPeople 2020 places strategic importance on increasing access to contraceptive services.1 One out of every four women in the U.S. who are at risk for unintended pregnancy experiences challenges in obtaining her chosen contraceptive (e.g., difficulty obtaining an appointment with her physician, high copays, inconvenient clinic hours, or not desiring a pelvic exam).2 With longer service hours and convenient locations, pharmacists provide a potential solution to these barriers. The American College of Obstetricians and Gynecologists (ACOG) supports OTC access to hormonal contraception, but there are significant concerns that this would jeopardize insurance coverage of contraception.1,3
Currently, at least five states allow pharmacists to prescribe contraception, with at least four other states currently working on legislation.4,5 However, pharmacist participation in and patient access to pharmacist-prescribed contraception remains low owing to lack of patient awareness, store policy limitations, out-of-pocket consultation fees, and lack of pharmacist comfort or interest in offering contraception.6,7 The Direct Access study established that community pharmacists can efficiently screen women for safe use of contraceptive products and select the appropriate product for patients.8 Contraceptive recommendations of family medicine physicians were found to be inconsistent with CDC guidelines 23% of the time for oral contraceptives (OCs) and 40% of the time for intrauterine devices (IUDs), presenting an opportunity for pharmacists to improve patient outcomes and adherence to CDC guidelines to further justify their role as contraception providers.9
As more states allow pharmacists to prescribe, pharmacists must be prepared with adequate knowledge of necessary patient-assessment processes, pharmacotherapy products, and rules and regulations surrounding their prescribing activities. A systematic review and meta-analysis by Jebara and colleagues in 2018 found overall positive experiences with pharmacist prescribing, which may provide further support and demand for pharmacist-prescribing roles (e.g., to ease patient access, improve patient outcomes, and reduce physician workload).10 Pharmacists interested in prescribing contraception must be mindful of patients' perception of their role on the healthcare team and must work to create the expectation and desire that pharmacists are contraception providers. Consumers' relationships with pharmacists have been found to be important in determining patient acceptance of pharmacists' prescribing roles.10 Patients have previously reported a desire to obtain contraception at their local pharmacy if able to do so, with 41% of these patients not currently using contraception.2 In the Direct Access study, community pharmacists did offer contraceptive services that patients were satisfied with and willing to pay for.8
Necessary Patient Evaluation
While contraceptives are generally safe and effective, they are not without health risks. Specific algorithms for patient evaluation will vary based on Board of Pharmacy requirements, but the following should be included in the screening process before a contraceptive is prescribed: 1) screening to rule out pregnancy via symptoms and menses history; 2) review of patient-completed self-screening in a private area; 3) blood pressure screening; and 4) discussion of contraceptive history and preferred method. The patient self-screening should include information to identify whether the patient might be pregnant or have comorbidities (to determine if a contraceptive is indicated or contraindicated, and if the patient requires referral) and a medication history to avoid potentially clinically significant drug interactions.11 Patients who may be pregnant, have uncontrolled blood pressure (>140/90 mmHg), or have contraindications should be referred to their primary care provider and/or obstetrician-gynecologist (ob-gyn) for follow-up.
Patients do not need to have a negative pregnancy test before contraception is initiated, because the the benefits of contraception likely exceed any risks. A patient should be "reasonably not pregnant," which means she lacks any symptoms of pregnancy and meets one of the following criteria: 1) has not had sexual intercourse since last menses; 2) last menses started within 7 days; 3) 7 days or less have passed since spontaneous or induced abortion; 4) is using a reliable form of contraception correctly and consistently; 5) is 4 weeks postpartum or breastfeeding 85% or more feeds, or is amenorrheic and less than 6 months postpartum.12 A follow-up pregnancy test can be recommended 2 to 4 weeks after starting hormonal contraception.13
Owing to the cardiovascular risks of contraception, blood pressure should be assessed before prescribing and before any prescription for contraception is renewed. Patients with blood pressure >140/90 mmHg should be referred to their primary care provider for blood pressure management, despite the fact that it is possible to safely use contraception in patients with blood pressure <160/110 mmHg.12 Pap smears are recommended by the United States Preventive Services Task Force every 3 years in patients aged 21 to 65 years and every 5 years if the patient is aged 30 to 65 years and also gets human papillomavirus screening with the Pap.12 Thus, Pap smears are not a requirement for contraception prescriptions. BMI screening is not necessary for contraception prescribing because the only contraception with evidence for potential decline in efficacy in obesity (patients >90 kg) is the patch, although BMI can be calculated for monitoring to assess potential weight gain.12,14
Contraceptives should not be used in specific chronic conditions and with certain medications; the United States Medical Eligibility Criteria for Contraceptive Use, 2016 (US MEC), should be reviewed to identify these.14 Most states that allow pharmacist prescribing of contraception will have guidelines regarding the chronic conditions and medications pharmacists can prescribe contraceptives with. Patients with a hormone-related cancer history, coagulation history, migraine with aura, and gastrointestinal absorption issues will need careful consideration and may need referral. Other disease states or conditions (e.g., dyslipidemias, migraines without aura) may not have any special considerations. The US MEC provides a ranked safety evaluation and suggestions for preferred contraception and contraception to be avoided in certain conditions. For example, smokers aged less than 35 years generally can use combination oral contraceptives (COCs). In patients aged above 35 years, safety is of concern, and patients who smoke more than 15 cigarettes a day should not use COCs owing to the risk of myocardial infarction (MI) and stroke. Patients who smoke less than 15 cigarettes a day should only use COCs when another contraceptive form is not acceptable to the patient or not available.14
Overview of Available Contraceptive Options
Contraceptive products that pharmacists can prescribe and administer will vary from state to state. Pharmacists should be well informed of all contraceptive options to ensure that they recommend the best option based on patient health, medications, preferences, and cost considerations. Long-acting reversible contraceptives (LARCs) are recommended first-line because their failure rate with typical use is 0.05% to 0.8% and they provide prolonged, effective contraception for 3 to 10 years.12,14-16 LARC methods have been found to have a higher continuation rate compared with short-acting contraceptives up to 2 years after initiation.15 LARC includes progestin-only implants and levonorgestrel-containing or copper-eluting intrauterine devices (IUDs). Although IUDs and implants must be inserted at a trained medical office, pharmacists need to understand why these are the best option for patients so they can adequately counsel them on this therapy and refer them to a clinician who can provide this service.11 Community pharmacists who are prescribing contraceptives should educate patients about the benefits of LARCs and facilitate referrals when patients are interested.15 Patients should be made aware of the full range of contraceptives they are eligible for and should be advised of the effectiveness, side effects, and unique benefits of each product.14
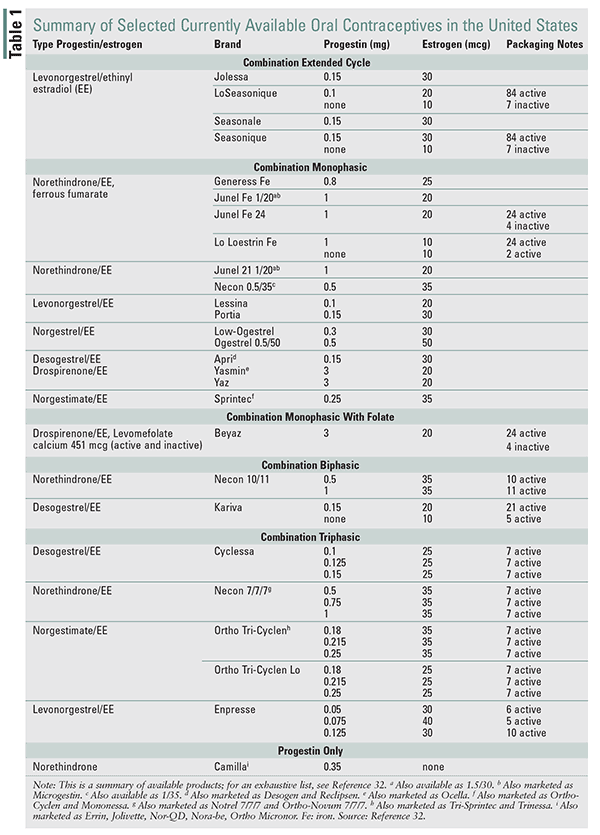
Most available oral contraceptives (OCs) are monophasic, providing active tablets of the same dose of estrogen and progestin for 21 days followed by placebo for 7 days, during which menses begins.17 Doses of estrogen have decreased from 50 mcg of ethinyl estradiol (EE) when oral contraceptives were first approved to 20 to 35 mcg. With the decrease in the EE dose, patients experience fewer adverse effects (e.g., nausea) and fewer complications (e.g., hormone-affected cancers, vascular disease, blood clots).17 TABLE 1 provides a summary of currently available OC products. Biphasic and triphasic products are also commonly available, and there is one quadriphasic product available. All were designed to more closely mimic hormonal fluctuations that occur during the menstrual cycle to decrease adverse effects, but no data show any differences in safety and efficacy. Monophasic contraceptives are easier to identify and make it easier to manage adverse effects that patients may experience.17
Extended-cycle or continued-cycle contraceptives are also available. Extended-cycle contraceptives provide 24 days of active tablets followed by 4 days of placebo, while continuous-cycle contraceptives provide 84 active days followed by 7 placebo days or continuous activity (without placebo). These were designed to decrease the frequency of menses (extended-cycle) and to shorten the duration and severity of menses (continuous-cycle). Extended-cycle contraceptives have not been found to have any differences in bleeding and spotting.17 All OCs are similarly effective, and product selection should be based on patient preference for dosage form and cycle type, previous experience, cost, and management of adverse effects and comorbidities.17 OCs containing levonorgestrel or norethindrone in combination with EE <35 mcg are considered first-line.18
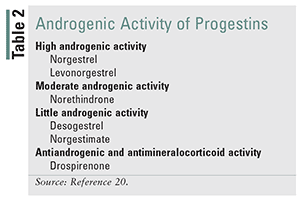
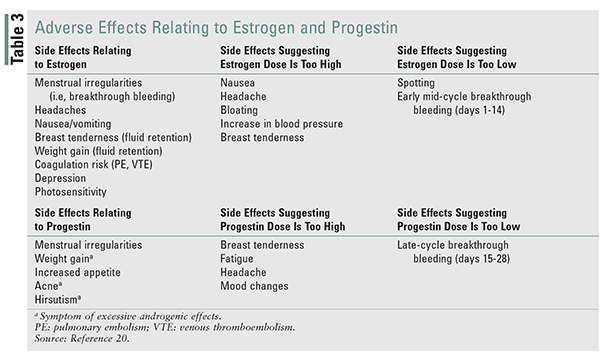
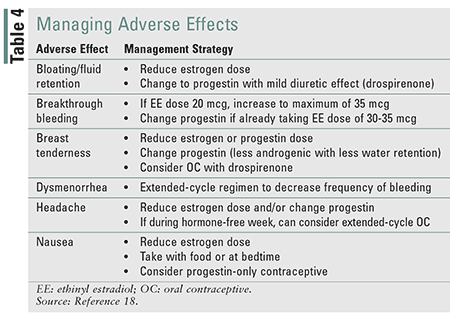
Common adverse effects of COCs include nausea/vomiting, breast tenderness, weight gain, acne/oily skin, breakthrough bleeding, and spotting.17 While not common, there is a two- to fourfold increase in venous thromboembolism (VTE) and an increased risk of MI and stroke with COCs.19 Adolescents, women weighing less than 50 kg (110 lb) or more than 35 years of age, and perimenopausal women are more likely to experience adverse effects; therefore, an EE dose of 20 to 25 mcg should be selected.17 EE doses of 20 mcg are likely as effective as those of 30 to 35 mcg, but may increase breakthrough bleeding.18 Nonadherence may be greater with EE doses above 35 mcg owing to increased frequency of adverse effects.17 Generally, these products have been removed from the market, but the remaining products should be used infrequently. Patients who experience acne, oily skin, or hirsutism should be prescribed an OC with an anti-androgenic progestin or a progestin with low androgen activity if cost is not prohibitive. TABLE 2 lists progestins from most to least androgenic. Extended-cycle and continuous-cycle OCs are ideal for patients for whom convenience is a priority, and these help to minimize adverse effects.17 Progestin-only pills (POPs) commonly cause irregular bleeding, but this appears to resolve by 1 year of therapy.20 Extended-cycle OCs will shorten or eliminate the hormone-free interval to manage common menstrual symptoms (e.g., excessive bleeding, menstrual pain, headaches, tiredness).21 TABLE 3 provides a comparison of adverse effects likely associated with estrogen and progestin, and ways to identify when doses are too high or low. TABLE 4 provides strategies for managing OC adverse effects.
Generally, patients with significant comorbidities should be referred. Patients with risks of VTE, ischemic stroke, or MI who meet the appropriate criteria according to state protocol should be given a low-dose (20 mcg) estrogen contraceptive. Drospirenone has also been associated with a higher risk of VTE and should be avoided in patients with a history of VTE or high risk for VTE.19 Alternatively, a progestin-only contraceptive (i.e., POP) could be initiated. Progestin-only contraceptives should be used in patients with a history of blood clots or estrogen-dependent cancer.20 Levonorgestrel is associated with lower risks of VTE, stroke, and MI and is the recommended progestin in these cases.19 Low-dose EE does increase the likelihood of spotting, with spotting occurring during approximately two-thirds of menstrual cycles a year.19
Contraception Warnings, Contraindications, and Drug Interactions
While OCs are generally safe and well tolerated, there are some boxed warnings that pharmacists must be mindful of when prescribing contraception. Generally, prescribing algorithms will be designed to prevent certain patients from being prescribed an OC. Contraindications for COC use include history of breast cancer and other estrogen- or progestin-dependent cancers, high risk of arterial or venous thrombotic disease (e.g., diabetes with vascular disease, migraine with aura, history of deep VTE or pulmonary embolism).22 Estrogen-containing contraceptives should be avoided in women with a history of VTE, stroke, cardiovascular disease, or peripheral vascular disease.21 While OCs are generally considered to have greater benefits than risks for harm in obese patients, obesity itself is a risk factor for VTE, and ACOG suggests a progestin-only method may be safer in these patients.21
Breakthrough bleeding and unintended pregnancy appear to be increasing in women taking OCs, which may be due to decreasing doses of estrogen and progestins in OCs.23 Estrogen and progesterone are metabolized by the CYP450 3A4 isoenzyme.23,24 Medications that induce this enzyme may result in contraceptive failure, but it is not known if this interaction actually increases the risk of unintended pregnancy.24 Patients taking carbamazepine, ethosuximide, rifampin, phenytoin, phenobarbital, and primidone should use an OC with a higher dose of EE (50 mcg EE, American Academy of Neurology; 30 mcg, ACOG and US MEC), medroxyprogesterone, or a nonhormonal contraceptive.23,24 ACOG preferentially recommends IUDs or long-acting injectable progesterone in patients taking antiepileptic drugs (AEDs), but if a COC is preferred or must be used owing to a contraindication for IUD use, OCs should be used in conjunction with condoms.24 Unlike older AEDs, lamotrigine clearance can be induced by estrogen, and risk of seizures may increase in patients taking a COC with monotherapy lamotrigine.14,24 A combination AED of lamotrigine with a nonenzyme-inducing AED is likely safe.14 Additionally, many AEDs are potentially teratogenic, so special attention to adherence counseling and, possibly, a back-up contraceptive are important. Pharmacists must pay special attention to assessing for current medications to identify patients who are taking AEDs, because 74% of women on AEDs do not have documented contraception.24 If patients must take an AED with a COC, newer agents (e.g., gabapentin, valproate, topiramate) generally do not induce CYP3A4 and may be safe for combined use.24
Benzodiazepines metabolized by CYP3A4 (e.g., diazepam, triazolam, alprazolam, midazolam) must also be considered. These can either induce or compete with OCs. For short courses of benzodiazepine use, an alternative form of contraception (i.e., condoms) is recommended. For long courses, a higher dose of EE or a LARC is recommended.23 Another consideration is the use of OCs in patients with HIV who are taking antiretrovirals. The mechanism of the interaction again involves drug-drug interaction via the CYP450 enzyme and glucuronidation as well.25 Owing to new metabolic pathways, contraception options are increasing, but with the risks of contraceptive failure and antiretroviral failure, patients with HIV should be carefully managed in conjunction with the HIV prescriber. 25 Ketoconazole is a potent CYP3A4 inhibitor, but this would not result in a clinically significant drug interaction.26 St. John's wort should also be avoided because it may decrease the effectiveness of contraception.14
Antibiotic use in conjunction with OCs is controversial, with inconsistent evidence, but most broad-spectrum antibiotics do not affect efficacy.14,27 Twenty percent of women reporting to a family planning clinic with an unplanned pregnancy report concomitant OC and antibiotic use.27 A case cross-over study of more than 43,000 patients found 1,330 OC failures, but no association was found between use of OCs with antibiotics and risk of breakthrough pregnancy. However, owing to study limitations, elevated risk of pregnancy in patients taking OCs and antibiotics concomitantly cannot be ruled out.28 Generally, a significant impact on the pharmacokinetics of EE, levonorgestrel, and norethindrone has not been found. Yet individual patients have been found to have significant decreases in plasma concentrations of hormones, and these patients appeared to ovulate.27 The likely cause for decreased concentration is CYP3A4 induction affecting hydrolytic enzymes of intestinal bacteria that produce free, active hormone. Furthermore, use of antibiotics with OCs may interfere with enterohepatic cycling as well.26 The most likely affected antibiotics include penicillin derivatives and tetracyclines.27 Ampicillin, amoxicillin, metronidazole, and tetracyclines have the most documentation of contraceptive failure in case-report literature. Doxycycline has been found to decrease hormone levels, but there was no evidence of ovulation. While there is a definite interaction, it is unproven that these antibiotics increase the risk of unintended pregnancy.27 Best practice is to use a second form of contraception while taking these antibiotics. Cephalexin, clindamycin, and sulfamethoxazole-trimethoprim also have case reports documenting their association with contraception failure. These are most likely safe for use in patients taking OCs, but best practice would be use of a backup contraceptive method in these patients as well. These interactions appear to be the most significant in patients taking low doses of EE.26
Patient Counseling
When prescribing contraceptives, pharmacists must ensure that they provide patients all information regarding safe and effective contraceptive use, because patients will likely not receive education from another healthcare provider about this medication. Patients obtaining contraceptives from pharmacies compared with other healthcare facilities have been shown to receive less key information about their contraceptive, particularly with regard to side effects.29 Key details that need to be reviewed with a patient who is being dispensed or prescribed a contraceptive are listed in TABLE 5.
It is critical that patients be adequately trained in how to safely and appropriately use their contraception. While pharmacists are used to counseling about contraceptives, when there is no longer another healthcare provider involved in prescribing the medication, full assurance that the patient is adequately trained to use her contraceptive rests with the pharmacist. While OCs are 99% effective with "perfect use" (used correctly and consistently as directed), they are only 91% effective with "typical use" or "real use," wherein patients will have inconsistent use or incorrect use.16,30 Because of the consequences of unplanned pregnancy, pharmacists must make sure that patients understand the importance of proper use and adherence and should use the teach-back method to verify understanding. Pharmacists should also provide strategies to help patients remember to take their birth control prescription (e.g., using a phone reminder, timing with a consistent meal).
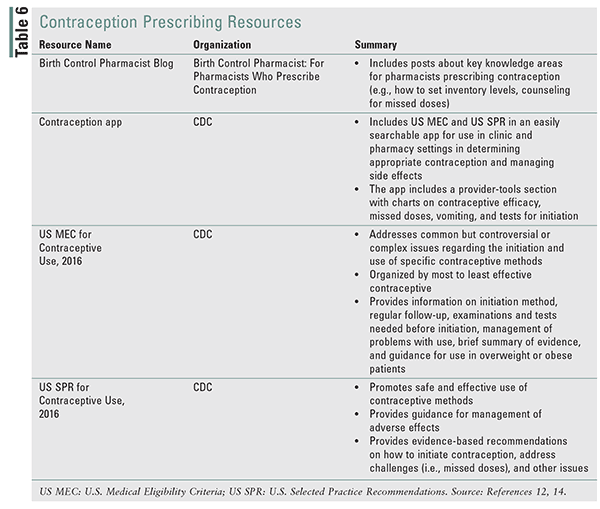
The Quick Start method of initiation should be reviewed with patients.1 Patients prescribed COC or POP can start contraception at any time using the Quick Start method, in which the patient takes the first dose upon receipt of the prescription.13,17 This method has been found to be associated with increased success in getting women to start OCs and continue through the third cycle of use.17 Two other common methods are Sunday start, when the patient starts a pill the Sunday after her menstrual cycle begins. This is the most common, because it allows for weekends free of menstrual cycles. Alternatively, patients can start a pill pack on the first day of menses.17 Backup contraception should be recommended if more than 5 days have elapsed since the last menses for COC and POP. Duration of backup contraception is 2 days for POP and 7 days for COC.13 Patients should be counseled regarding what to do when they miss a dose. This will vary based on the type of contraceptive product being used, so the package insert should be consulted. Generally, patients taking COCs should be counseled to take the missed dose as soon as possible if it has been 24 hours or more, but less than 48 hours, since the missed dose. The patient should continue her regular schedule, even if this means taking two doses in one day. No additional backup contraception is needed. If 48 hours or more have elapsed since the missed dose, the most recent missed dose should be taken as soon as possible, and other missed doses should be discarded. The remaining doses should be taken as scheduled and backup contraception (i.e., condoms) should be used for the next 7 days.13 If a patient misses a dose of a POP, she should take the missed dose as soon as possible. If it has been more than 3 hours since the missed dose, backup contraception should be used for the next 2 days.12 There is no evidence regarding potential loss of efficacy when patients experience nausea, vomiting, or diarrhea. TABLE 6 reviews key points that should be shared with patients.
Adverse effects can have a significant impact on patient adherence and should be discussed with the patient before contraceptive selection. An agreeable adverse-effect profile for the patient should be selected. Excessive or deficient amounts of estrogen or progestin are the most common causes of adverse effects and should be monitored in follow-up.17 TABLE 4 provides strategies to manage common adverse effects associated with OCs. Patients should also be advised of noncontraceptive benefits of COCs, including regulated menses, diminished premenstrual dysphoric disorder, and decreased dysmenorrhea.21
Other Considerations
Pharmacists interested in prescribing contraception must ensure that they have an adequate workflow design to create a seamless patient experience. Furthermore, they must ensure that they have adequate training to perform appropriate assessment, prescribe the best selection, identify patients who need referral, and do proper follow-up with patients. This is critical in meeting patient expectations for adequate take-up of the service and to ensure that quality care is delivered that improves patient outcomes. In 2016, ACOG publicly opposed pharmacist prescribing of OCs, viewing this approach to increasing contraceptive access as replacing the barrier of a physician prescription with the barrier of a pharmacist prescription.31 Each state will enact pharmacist prescribing of contraception somewhat differently.11 Pharmacists who intend to prescribe will need to review their Board of Pharmacy website to ensure they have completed the necessary training and certification, have registered if required, and are using any required documentation processes or prescribing algorithms.11 Pharmacists must also be cognizant of the cost of contraceptives, as cost may negatively impact patient adherence and maintenance of OCs.
Conclusion
Pharmacist prescribing provides an opportunity for increased access to contraception for patients and may allow for better management of adverse effects associated with contraception owing to the pharmacist's medication expertise and accessibility. Pharmacists must ensure that they adequately screen patients prior to initiation of contraception and should provide thorough education to prevent the risk of unplanned pregnancy resulting from poor administration technique or lack of acceptance of the contraceptive because of issues such as dosage form or adverse effects.
REFERENCES
- Rodriguez MI, Anderson L, Edelman AB. Pharmacists begin prescribing hormonal contraception in Oregon: implementation of House Bill 2879. Obstet Gynecol. 2016;128(1):168-170.
- Landau SC, Tapias MP, McGhee BT. Birth control within reach: a national survey on women's attitudes towards and interest in pharmacy access to hormonal contraception. Contraception. 2006;74(6):463-470.
- American College of Obstetricians and Gynecologists. Committee on Gynecologic Practice. Committee Opinion No. 544: over-the-counter access to oral contraceptives. Obstet Gynecol. 2012;120(6):1527-1531.
- Mollison C. Pharmacists' role in managing contraceptive care contin-ues to evolve. Pharmacy Times. www.pharmacytimes.com/conferences/apha-2018/pharmacists-role-in-managing-contraceptive-care-continues-to-evolve. Accessed June 21, 2018.
- National Alliance of State Pharmacy Associations. Pharmacists authorized to prescribe birth control in more states. https://naspa. us/2017/05/pharmacists-authorized-prescribe-birth-control-states/. Accessed June 21, 2018.
- Rodriguez MI, McConnell KJ, Swartz J, Edelman AB. Pharmacist prescription of hormonal contraception in Oregon: baseline knowledge and interest in provision. J Am Pharm Assoc. 2016;56:521-526.
- Hawryluk M. Rollout of Oregon contraceptive-access program has been bumpy. www.bendbulletin.com/localstate/6263821-151/rollout-of-oregon-contraceptive-access-program-has-been-bumpy. Accessed June 21, 2018.
- Gardner JS, Miller L, Downing DF, et al. Pharmacist prescribing of hormonal contraceptives: results of the Direct Access study. J Am Pharm Assoc. 2008;48:212-221.
- Wu JP, Gundersen DA, Pickle S. Are the contraceptive recommendations of family medicine educators evidence-based? A CERA survey. Fam Med. 2016;48(5):345-352.
- Jebara T, Cunningham S, MacLure K, et al. Stakeholders' views and experiences of pharmacist prescribing: a systematic review. Br J Clin Pharmacol. June 5, 2018; Epub ahead of print.
- Anderson L, Borgelt LM, Clark P, Moore G. Encouraging compre-hensive and consistent pharmacist education for provision of contraception. J Am Pharm Assoc. 2018;58:113-116.
- Curtis KM, Jatiaoui TC, Tepper NK, et al. U.S. selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65(4):1-66.
- CDC. When to start using specific contraceptive methods. www. cdc.gov/reproductivehealth/contraception/pdf/When-To-Start_508Tagged.pdf. Accessed June 21, 2018.
- Curtis KM, Tepper NK, Jatiaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65(3):1-103.
- Rafie S, McIntosh J, Shealy KM, et al. Roles of the pharmacist in the use of safe and highly effective long-acting reversible contraception: an opinion of the women's health practice and research network of the American College of Clinical Pharmacy. Pharmacotherapy. 2014;34(9):991-999.
- CDC. Reproductive health: contraception. www.cdc.gov/reproductivehealth/contraception/index.htm. Accessed June 21, 2018.
- Shrader SP, Ragucci KR. Contraception. In: Dipiro JT, Talbert RL, Yee GC, et al., eds. Pharmacotherapy: A Pathophysiologic Approach. 10th ed. New York, NY: McGraw-Hill; 2017.
- Stewart M, Black K. Choosing a combined oral contraceptive pill. Aust Prescr. 2015;38(1):6-11.
- Lam Y, Coe C, Mounsey A. Which combined OC to prescribe with CV safety in mind? J Fam Pract. 2017;66(7):454-456.
- Mospan CM. Contraception. In: Munyer T, Hunter M, Hoover R, et al., eds. Pharmacotherapy First: A Multimedia Learning Resource. Washington, DC: American Pharmacists Association; 2018.
- Bonnema RA, McNamara MC, Spencer AL. Contraception choices in women with underlying medical conditions. Am Fam Physician. 2010;82(6):621-628.
- Lexi-Drugs Online. Hudson, OH: Lexi-Comp Inc. http://online.lexi. com. Accessed June 21, 2018.
- Michalets EL. Update: clinically significant cytochrome P-450 drug interactions. Pharmacotherapy. 1998;18(1):84-112.
- Onysko M, Anderson HM, Cai S, Winslow BT. 5 drug interactions you don't want to miss. J Fam Pract. 2017;66(11):680-686.
- Tittle V, Bull L, Boffito M, Nwokolo N. Pharmacokinetic and pharmacodynamic drug interactions between antiretrovirals and oral contraceptives. Clin Pharmacokinet. 2015;54(1):23-34.
- Zhanel GG, Siemens S, Slayter K, Mandell L. Antibiotic and oral contraceptive drug interactions: Is there need for concern? Can J Infect Dis. 1999;10(6):429-433.
- Dickinson BD, Altman RD, Nielsen NH, Sterling ML. Council on Scientific Affairs, American Medical Association. Drug interactions between oral contraceptives and antibiotics. Obstet Gynecol. 2001;98(5 pt 1):853-860.
- Toh S, Mitchell AA, Anderka M, et al. National Birth Defects Pre-vention Study. Contraception. 2011;83(5):418-425.
- Amin ME, Chewning B. Pharmacists' counseling on oral contraceptives: a theory informed analysis. Res Soc Admin Pharm. 2016;12:669-681.
- Planned Parenthood. How effective is the birth control pill? www. plannedparenthood.org/learn/birth-control/birth-control-pill/how-effective-is-the-birth-control-pill. Accessed June 21, 2018.
- American College of Obstetricians and Gynecologists. ACOG statement on pharmacist prescribing laws. www.acog.org/About-ACOG/News-Room/Statements/2016/ACOG-Statement-on-Pharmacist-Prescribing-Laws. Accessed June 21, 2018.
- Monthly Prescribing Reference (MPR). Oral contraceptives. www.empr.com/clinical-charts/oral-contraceptives/article/123837. Accessed June 21, 2018.