Respiratory Syncytial Virus Infection in Infants and Young Children
RELEASE DATE
August 1, 2023
EXPIRATION DATE
August 31, 2025
FACULTY
Brooke Barlow, PharmD
Neurocritical Care Clinical Pharmacy Specialist
Memorial Hermann The Woodlands Medical Center
The Woodlands, Texas
FACULTY DISCLOSURE STATEMENTS
Dr. Barlow has no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT
 Pharmacy
Pharmacy
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-23-079-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients’ conditions and possible contraindications or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
GOAL
To present the epidemiology, pathogenesis, diagnosis, prevention, and treatment of infants and children with respiratory syncytial virus (RSV) and define the pharmacist's role in patient care.
OBJECTIVES
After completing this activity, the participant should be able to:
- Discuss the epidemiology and pathogenesis of RSV in infants and young children.
- Describe the clinical presentation and diagnosis of RSV in infants and young children.
- Explain the use of palivizumab immunoprophylaxis for RSV in at-risk infants and young children.
- Identify the pharmacist's role in the care of infants and young children with RSV.
ABSTRACT: Respiratory syncytial virus (RSV) infects nearly all children by age 2 years and is the leading cause of hospitalization in infants and young children. Most children present with mild, cold-like disease, but susceptible individuals may develop severe disease. No vaccine is available for prevention of RSV in infants and children; however, in high-risk patients, immunoprophylaxis with the monoclonal antibody palivizumab may be considered during RSV season. RSV treatment remains largely supportive in nature, including fever control, rehydration, nutritional support, secretion clearance, and oxygen support, if needed. Antiviral therapy may be considered in patients with severe disease. Pharmacists have a key role in identifying candidates for palivizumab during RSV season and providing education on nonpharmacologic preventive measures that can be undertaken to lessen the spread of RSV.
Respiratory syncytial virus (RSV) is one of the chief causes of acute lower respiratory tract infection in children younger than age 5 years, with an estimated 33 million annual cases occurring globally.1,2 Although it is often a self-limiting illness, RSV can result in a high degree of morbidity and mortality, especially in patients with severe disease, and it is the leading cause of hospitalization in infants and young children. In the United States, RSV is estimated to account for approximately 60,000 to 80,000 hospitalizations and up to 300 deaths annually in children younger than age 5 years.3
To address the substantial worldwide disease burden, the World Health Organization developed the Battle against Respiratory Viruses (BRaVe) Initiative for the prioritization of research efforts focused on gaining a deeper understanding of the pathophysiology, epidemiology, prevention, and treatment of respiratory viruses, including RSV.4 Additionally, the CDC organized surveillance systems to monitor seasonal trends associated with RSV and research networks to assess the efficacy of preventive measures against RSV-associated hospitalizations.5,6 These efforts have greatly improved the overall knowledge of RSV, and notably its prevention, thereby enabling pharmacists to better ascertain that adequate preventive measures are put in place to improve patient outcomes.
Prior to the COVID-19 pandemic, RSV infections exhibited a distinct seasonality wherein the number of cases exhibited a rise (i.e., season onset) in late autumn, peaked in winter (mid-December to mid- February), and reached season offset (i.e., declined) in mid-April to mid-May (spring).5 The epidemiology, seasonality, and severity of RSV infections dramatically shifted when the COVID-19 pandemic began, however. Several countries experienced a reduced number of cases from 2019 to 2020, but they also had a spike in off-season outbreaks and increased disease severity.7 The initial reduction in cases was likely due to social distancing, masking, and other preventive measures undertaken to lessen the spread of COVID-19. When COVID-19 restrictions in the U.S. were lifted, an unusually early surge of RSV occurred in the autumn of 2022.8 It was unclear whether this was due to a more virulent strain of RSV; however, genomic analysis suggests that cases were consistent with previous viral lineages and that the surge was likely related to nonviral factors, including changes in immunity and lifting of COVID-19 infection-prevention measures. Continued surveillance and tracking of RSV infections will be critical to determining whether the case load, seasonality, and severity return to prepandemic levels.
Pathogenesis
RSV is a nonsegmented, negative-strand, enveloped RNA virus that belongs to the Pneumoviridae family.9,10 RSV exists as two major antigenic subgroups: type A and type B. Although both subtypes often cocirculate during RSV season, one subtype tends to predominate.11 Humans are the only known carriers of RSV, and transmission occurs via direct exposure to droplets from an infected individual or by self-inoculation after a surface contaminated with secretions is touched. Aerosolized particles from an infected person’s cough or sneeze can transmit the virus to individuals within a 3-foot radius.12 RSV can remain viable for several hours on surfaces and up to an hour on the hands.10,13 The mucosae of the nose and eyes are the primary portals of entry. Once inside the host, RSV migrates to the respiratory tract and replicates within the respiratory epithelium, targeting ciliated epithelium and type I and type II pneumocytes.14 The targeted actions on the epithelial lining result in impaired ciliary action, mucus clearance, and shedding of infected epithelial cells.
The innate immune system remains the first barrier of defense against RSV. Mononuclear cell infiltration within the respiratory tract to prevent viral invasion initiates a potent inflammatory response involving the release of cytokines and chemokines. This inflammatory cascade can induce edema in surrounding alveolar tissue, small-airway obstruction, and—in severe cases—bronchial necrosis.15 Young infants are at increased risk for developing atelectasis secondary to hyperinflation and airflow impedance during inspiration and expiration.10 The incubation period for RSV ranges from 2 to 8 days, and symptom onset of 4 to 6 days after exposure is most common.9 Viral shedding typically lasts 7 to 10 days; however, in some cases it may be detected for up to 30 days, and even longer in immunocompromised populations.9,14
Risk Factors
RSV infection is most common in infants and young children. Older adults, especially those with underlying medical conditions, are also at risk, but the prevalence is lower than in infants and young children, and other age groups are less likely to acquire RSV infection. This highly contagious virus infects nearly all children by age 2 years, and many people will be infected with it more than once in their lifetime. Some children may be asymptomatic or have only mild disease, whereas others develop severe disease and sequelae. Conditions that confer a high risk of severe RSV include premature birth, congenital heart disease, chronic lung disease, congenital malformations, neuromuscular disorders, and immunodeficiency. Premature infants and those with underlying chronic medical conditions not only have an increased risk of severe disease but also are at greater risk for hospitalization, need for ICU admission, and increased mortality.2
Other host-related factors that have been linked to severe RSV include birth during peak RSV season, lack of breastfeeding, male sex, Down syndrome, maternal smoking, malnutrition, failure to thrive, family history of atopy or asthma, and low cord serum RSV antibody titers.16 Environmental risk factors for RSV development include environmental pollution, crowded living conditions, increased altitude, low socioeconomic status, childcare attendance, and proximity to hospital care. Up to 85% of children with RSV who are admitted to the hospital, however, lack any of the known risk factors for severe disease, highlighting an important area needing additional research.17 Given that RSV is airborne and can also be acquired through direct contact, the risk of acquisition is greater in children and infants who attend daycare or have siblings in daycare.
Clinical Presentation and Diagnosis
Infants and children infected with RSV predominantly experience cold-like signs such as cough, rhinorrhea, sneezing, fever, and wheezing. Infants also may exhibit poor feeding, irritability, and fatigue.9,13 It is important to note that in some cases, apnea may be the only sign present in an infant.17 As the disease progresses to the lower respiratory tract, the cough may become more pronounced and productive and may be accompanied by dyspnea, tachypnea, and increased work of breathing. Bronchiolitis and pneumonia are the most common reasons for hospitalization due to impaired oxygenation, often warranting supplemental oxygen or ventilatory support.
Severe disease is most likely to occur within the first year of life, with 40% to 90% of infants requiring hospitalization.10 Although most signs and symptoms resolve within the 7- to 10-day time frame of viral shedding, the duration can vary based on immune status, comorbidities, disease severity, and course of illness. Furthermore, while the acute illness may resolve, some children continue to have respiratory signs, mainly cough, for a month or longer.10
In the community setting, RSV is often a clinical diagnosis, especially if it presents during the RSV season. The diagnosis may be confirmed using molecular diagnostic tests, such as reverse transcriptase-polymerase chain reaction (RT-PCR), that have been shown to have greater sensitivity and specificity than rapid antigen diagnostic assays.18 Samples should be obtained via nasopharyngeal swab or tracheal secretion. Several of the molecular diagnostic tests, such as RT-PCR, include multiplex assays to detect other viruses that often coexist with RSV.9 Cell culture is no longer preferred given the prolonged time to test result.18 A chest radiograph, which may be obtained in the hospital setting, will often demonstrate lower lobe consolidations, hyperinflation consistent with bronchiolitis, or diffuse consolidations consistent with pneumonitis.19
Prevention
Recent advances in the prevention of RSV have centered largely on adults, with two RSV vaccines approved by the FDA for use in vulnerable adults aged 60 years and older. To date, however, no vaccine is available for the prevention of RSV infections in infants and young children. Passive immunity against RSV may be derived from maternal antibodies, but this usually declines to an undetectable level by about age 6 months. Furthermore, preterm infants, infants born during peak RSV season, and those born to a multiparous mother are known to have lower antibody titers.20 Primary preventive measures, therefore, include good hygiene, routine hand washing, avoiding close contact with sick persons, refraining from touching face with unwashed hands, limiting time in childcare centers during peak RSV season, and ensuring that surfaces remain clean. Parents should be encouraged to keep the environment free of smoke. Some patients at high risk for severe disease may be candidates for immunoprophylaxis with palivizumab.
Palivizumab (Synagis) is a humanized monoclonal antibody that is directed against an antigenic site of the RSV fusion protein (F), which prevents the conformational change necessary for fusion with the plasma membrane of the respiratory epithelial cell.21 By blocking fusion with the respiratory epithelium, palivizumab prevents RSV from entering the cell, thereby halting replication.17 Palivizumab was FDA approved in 1998 for the prevention of serious lower respiratory tract infections caused by RSV in children at risk for severe disease.
Palivizumab’s efficacy for RSV prevention in patients at high risk for severe disease has been investigated in multiple randomized, controlled trials (RCTs), which demonstrated a 34% to 82% reduced rate of hospitalization compared with placebo.22 In a systematic review and meta-analysis of five RCTs, palivizumab reduced the rate of hospitalization for RSV (risk ratio [RR] 0.44, CI 0.30-0.64); however, no difference in mortality was observed at 2-year follow-up (RR 0.69, CI 0.42-1.15).23 Children at high risk for severe disease, including those with chronic lung disease or congenital heart disease, were the primary cohorts in these studies.
It is important to keep in mind that the package insert’s indication for palivizumab reflects the initial clinical-trial data for drug licensure and that the FDA does not provide guidance or recommendations on palivizumab use. Because the FDA approval did not specify a defined high-risk population, the American Academy of Pediatrics (AAP) developed a guidance statement (routinely updated based on ongoing research) to provide more precise recommendations on the optimal candidates for palivizumab therapy.24
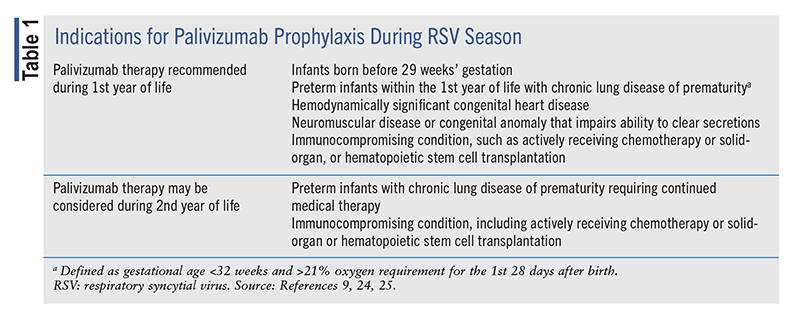
The 2023 AAP guidance recommends palivizumab prophylaxis during RSV season for the following patients (TABLE 1)9,24:
- Infants aged <12 months who were born before 29 weeks’ gestation
- Preterm infants within the first year of life who have chronic lung disease of prematurity (defined as gestational age <32 weeks and >21% oxygen requirement for the first 28 days after birth); prophylaxis may also be considered in the second year of life if continued medical support for chronic lung disease is still required
- Infants aged <12 months who have hemodynamically significant congenital heart disease (e.g., acyanotic heart disease, moderate-to-severe pulmonary hypertension)
- Children aged <2 years who undergo cardiac transplantation
- Infants aged <12 months who have neuromuscular disease or a congenital anomaly that impairs the ability to clear secretions
- Children aged <2 years who have an immunocompromising condition, including but not limited to those actively receiving chemotherapy or with solid organ or hematopoietic stem cell transplantation.
Prematurity is one of the most important risk factors for severe RSV disease, and children born before 29 weeks' gestation have the highest risk of morbidity and mortality from RSV infection. Although palivizumab prophylaxis can reduce the risk of severe disease in this cohort, the risk remains high, emphasizing the critical need for improved preventive measures. The risk of RSV in infants with neuromuscular disease is ill defined; however, the risk appears to rise with increasing age because of the progressive nature of neuromuscular conditions. Palivizumab prophylaxis should therefore be considered in patients with anatomical abnormalities that impair secretion clearance, including those with neuromuscular disorders, those with congenital diaphragmatic hernia, and those requiring tracheostomy or ventilator support who are at increased risk for respiratory infections.24
Despite evidence suggesting that children with Down syndrome and cystic fibrosis are at high risk for RSV, data do not currently support routine prophylaxis in these patients. However, immunoprophylaxis may be considered in children with cystic fibrosis and evidence of nutritional deficiencies, chronic lung disease, or history of pulmonary exacerbations requiring hospitalization.24
Further research is necessary to establish the role of palivizumab in preventing severe RSV disease in children who live in low- and middle-income countries or in tropical regions, are malnourished, are exposed to smoke, or are living in an overcrowded environment, given the high risk of RSV acquisition and the potential for poor outcomes.24
Palivizumab is given as an intramuscular injection at a dosage of 15 mg/kg administered monthly for a maximum of 5 months during RSV season (TABLE 2).21 The first dose should be administered at the onset of RSV season, which can vary from early September to December. To ascertain the optimal timing of initiation, the onset of the current season can be determined by identifying the first week in which the local RSV RT-PCR positivity rate was 3% or higher.9 The AAP’s guidance, which is general, is to administer the first dose in early November and continue monthly for 5 consecutive months, which should provide adequate coverage through April for most infants and children in the U.S.25 However, seasonality may differ in some regions, so an assessment of local positivity rates is suggested.
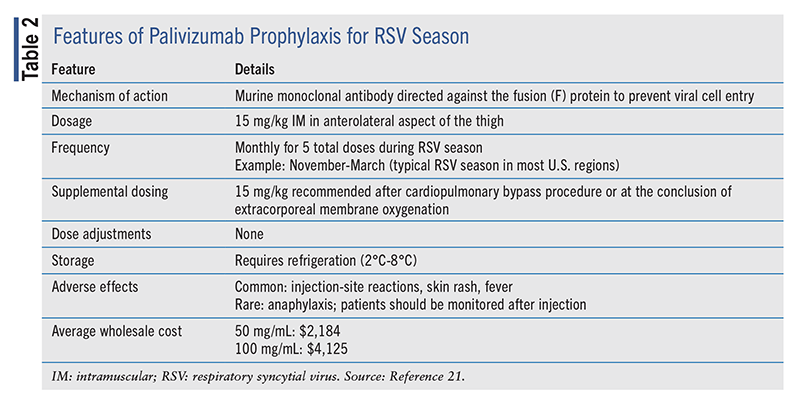
Five doses of palivizumab are estimated to achieve the high plasma levels necessary to provide more than 6 months of adequate protection; therefore, no more than five doses are recommended.25,26 Importantly, in children with congenital heart disease requiring surgical procedures such as cardiopulmonary bypass, a supplemental postoperative palivizumab dose of 15 mg/kg should be administered because a 58% decline in serum concentrations has been observed after surgical procedures. Supplemental dosing is also recommended after extracorporeal membrane oxygenation because of the potential for suboptimal serum concentrations.9,21 Palivizumab does not interfere with the immune response to live or attenuated vaccines; therefore, immunization schedules should be followed regardless of palivizumab administration.
Early termination of palivizumab therapy is recommended in children who experience breakthrough RSV infections requiring hospitalization, given the less than 0.5% likelihood of a second RSV hospitalization during the same season. RSV resistance to palivizumab has been detected in up to 5% of children admitted with breakthrough infections while receiving prophylaxis. Resistance is thought to be conferred through amino-acid substitutions in the F protein that prevent palivizumab binding. Continuation of palivizumab in these cases in not recommended, and treatment should focus on supportive measures. Furthermore, palivizumab is not recommended for the treatment of active RSV infection or for the prevention of healthcare-associated RSV. If an outbreak occurs in a high-risk hospital unit during RSV season, strict infection-prevention measures, including adequate hand and cough hygiene, should be emphasized.21
Palivizumab is well tolerated; the most common adverse effects are injection-site pain, fever, and skin rash. Anaphylaxis is rare but may occur after a single or subsequent dose exposure. Patients should be monitored closely, and permanent discontinuation is recommended if a hypersensitivity reaction occurs. Palivizumab should be used cautiously in patients with bleeding disorders or thrombocytopenia, as bleeding can occur after intramuscular injection.21
Concerns have been raised regarding the cost benefit of palivizumab, given its high cost (ranging from $3,221 to $12,568 for a season).27 In the most recent cost-efficacy analysis conducted by the AAP, palivizumab prophylaxis was associated with an incremental cost-efficacy ratio of $5,188 to $791,295 per quality-adjusted life-years in preterm infants, with similar cost benefits demonstrated in infants with chronic lung disease supported by current guidance.28 The AAP's recent guidance suggests that the high cost of palivizumab is associated with minimal health benefit given the high cost of the intervention; therefore, the intervention is not considered to have a cost benefit in any cohort.24 Judicious prescribing of palivizumab for those patients most likely to obtain benefit is important for minimizing the financial burden associated with this therapy.
Treatment
No therapies are available that hasten recovery from RSV infection, so treatment remains largely supportive. Supportive measures to provide comfort include fever control, rehydration, nutritional support, secretion clearance, and oxygen support, if required. Supplemental oxygen may be recommended if oxygen saturation falls below 90%. The addition of bronchodilators, corticosteroids, nebulized epinephrine, hypertonic saline, or leukotriene inhibitors is not recommended in patients with bronchiolitis given the lack of evidence to support a benefit with their use.29-31 Antibiotic therapy is frequently employed in infants hospitalized for RSV, in part due to the presence of fever or evidence of infiltrates on the chest radiograph raising concern about concurrent bacterial infection. However, bacterial coinfection is an unusual complication, as it occurs in fewer than 2% of patients with RSV infection.32 Antibiotic therapy is therefore unnecessary in most patients; it should be reserved for those with evidence of secondary bacterial infection. IV fluid therapy should be used for resuscitation and to maintain euvolemia given the degree of insensible losses in children with fevers and increased respirations. Adequate nutrition should be administered to support the high metabolic requirements of children and infants during acute infections. Appropriate precautions should be taken in infants and children hospitalized with RSV in order to mitigate the risk of nosocomial transmission.
Ribavirin for inhalation solution (Virazole) is the only antiviral to be approved by the FDA for the treatment of hospitalized infants and children with severe lower respiratory tract infection due to RSV.33 Ribavirin, a synthetic nucleoside analogue, acts by inhibiting viral replication. It is supplied as a powder to be prepared as a solution for nebulization, and it may be administered via continuous nebulization in mechanically ventilated patients or via intermittent nebulization in patients who are not mechanically ventilated.
Despite the FDA approval, ribavirin for inhalation solution is not currently recommended for use in infants or children with RSV because some trials found only a modest clinical benefit and improvement in oxygenation but no improvement in duration of hospitalization, mortality, or respiratory failure.9,34,35 Additionally, ribavirin has a high acquisition cost, with a single day of treatment estimated at $29,000.36 Being a known teratogen, ribavirin is contraindicated in pregnancy (Category X).37 Healthcare workers who are or may be pregnant should not administer this medication, as harm can occur even when precautions are taken. Adverse effects of ribavirin include nausea and potentially severe bronchospasms leading to sudden deterioration of respiratory function. Close monitoring of respiratory function is required, and coadministration with bronchodilators may be considered to minimize the risk of bronchospasms.37 Given the lack of clear benefit and the potential for adverse effects, ribavirin for inhalation solution is not recommended for routine use in hospitalized infants and children with RSV infection.9
The Pharmacist’s Role
RSV, one of the most significant and common pathogenic infections of childhood, is associated with significant morbidity and mortality and a high financial burden. Pharmacists can play a key role in educating parents, family members, and patients about important infection-prevention measures for lowering the risk of RSV acquisition or transmission. Encouraging parents to undertake smoking cessation and minimize children and infants’ exposure to secondhand smoke is important for preventing worsening pulmonary complications of RSV. Parents should maintain regular contact with their child’s daycare center for information regarding RSV spread among the children to know when to take appropriate precautions. Employing adequate hand hygiene (i.e., washing with soap and water) and cough hygiene, minimizing the touching of eyes or mucous membranes with unwashed hands, and maintaining a sanitary environment are other critical preventive measures for lessening transmission. For any child, the importance of breastfeeding, adequate nutrition, avoidance of crowds, prevention of smoke exposure, and all members of the household keeping up-to-date on immunizations should be emphasized.
Pharmacists are also essential in identifying potential candidates for palivizumab therapy. Evaluating patients for the aforementioned risk factors that warrant palivizumab prophylaxis can help reduce the rates of hospitalization for RSV infection in susceptible patients. Parents should be counseled about adverse effects of palivizumab treatment and about monitoring for injection-site reactions. Developing a patient-specific calendar listing dates for receiving the palivizumab therapy can help families stay on track with immunoprophylaxis injections and minimize the risk of missed doses. Parents should be informed that palivizumab does not interfere with the child's response to immunizations and that it is critical for infants and children to stay on schedule for routine childhood vaccinations.
Finally, pharmacists can serve as stewards for patients who develop RSV infections to prevent unnecessary antibiotic and antiviral use in children and infants who are hospitalized for RSV. If the decision is made to initiate ribavirin in a specific patient, pharmacists must educate other healthcare workers about this drug’s adverse effects and teratogenicity and employ measures to prevent exposure in those who could experience harm from it. Pharmacists should also keep abreast of the current literature surrounding prevention and treatment of RSV infections, including any potential vaccines and novel therapeutics in the pharmaceutical pipeline.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
- Li Y, Wang X, Blau DM, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022;399(10340):2047-2064.
- Duan Y, Jiang M, Huang Q, et al. Incidence, hospitalization, and mortality in children aged 5 years and younger with respiratory syncytial virus-related diseases: a systematic review and meta-analysis. Influenza Other Respir Viruses. 2023;17(5):e13145.
- Hansen CL, Chaves SS, Demont C, Viboud C. Mortality associated with influenza and respiratory syncytial virus in the US, 1999- 2018. JAMA Netw Open. 2022;5(2):e220527.
- Legand A, Briand S, Shindo N, et al. Addressing the public health burden of respiratory viruses: the Battle against Respiratory Viruses (BRaVe) Initiative. Future Virol. 2013;8(10):953-968.
- Rose EB, Wheatley A, Langley G, et al. Respiratory syncytial virus seasonality—United States, 2014-2017. MMWR Morb Mortal Wkly Rep. 2018;67(2):71-76.
- CDC. RSV-NET overview and methods. www.cdc.gov/rsv/research/rsv-net/overview-methods.html. Accessed June 8, 2023.
- Bozzola E, Barni S, Villani A. Respiratory syncytial virus pediatric hospitalization in the COVID-19 era. Int J Environ Res Public Health. 2022;19(23):15455.
- Adams G, Moreno GK, Petros BA, et al. Viral lineages in the 2022 RSV surge in the United States. N Engl J Med. 2023;88(14): 1335-1337.
- Committee on Infectious Diseases, American Academy of Pediatrics; Kimberlin DW, Barnett ED, Lynfield R, Sawyer MH. Red Book: 2021-2024 Report of the Committee on Infectious Diseases (32nd edition). https://publications.aap.org/redbook/book/347/Red-Book-2021-2024-Report-of-the-Committee-on. Accessed July 7, 2023.
- Walsh EE, Hall CB. Respiratory syncytial virus (RSV). In: Bennett JE, Blaser MJ, Dolin R, eds. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. Elsevier; 2015:1948-1960.e3.
- Gilca R, De Serres G, Tremblay M, et al. Distribution and clinical impact of human respiratory syncytial virus genotypes in hospitalized children over 2 winter seasons. J Infect Dis. 2006;193(1):54-58.
- Hall CB, Douglas RG Jr. Modes of transmission of respiratory syncytial virus. J Pediatr. 1981;99(1):100-103.
- Walsh EE. Respiratory syncytial virus infection: an illness for all ages. Clin Chest Med. 2017;38(1):29-36.
- van Drunen Littel-van den Hurk S, Watkiss ER. Pathogenesis of respiratory syncytial virus. Curr Opin Virol. 2012;2(3):300-305.
- Johnson JE, Gonzales RA, Olson SJ, et al. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod Pathol. 2007;20(1):108-119.
- Sommer C, Resch B, Simões EAF. Risk factors for severe respiratory syncytial virus lower respiratory tract infection. Open Microbiol J. 2011;5:144-154.
- Barr R, Green CA, Sande CJ, Drysdale SB. Respiratory syncytial virus: diagnosis, prevention and management. Ther Adv Infect Dis. 2019;6:2049936119865798.
- Henrickson KJ, Hall CB. Diagnostic assays for respiratory syncytial virus disease. Pediatr Infect Dis J. 2007;26(Suppl 11):s36-s40.
- Gonçalves A, Rocha G, Guimarães H, et al. Value of chest radiographic pattern in RSV disease of the newborn: a multicenter retrospective cohort study. Crit Care Res Pract. 2012;2012:861867.
- Receveur M, Ottmann M, Reynes JM, et al. Level of maternal antibodies against respiratory syncytial virus (RSV) nucleoprotein at birth and risk of RSV very severe lower respiratory tract infection. Influenza Other Respir Viruses. 2023;17(1):e13025.
- Synagis (palivizumab) product information. Waltham, MA: Sobi Inc; November 2020.
- Committee on Infectious Diseases. From the American Academy of Pediatrics: policy statements—modified recommendations for use of palivizumab for prevention of respiratory syncytial virus infections. Pediatrics. 2009;124(6):1694-1701.
- Garegnani L, Styrmisdóttir L, Roson Rodriguez P, et al. Palivizumab for preventing severe respiratory syncytial virus (RSV) infection in children. Cochrane Database Syst Rev. 2021;11(11):CD013757.
- Caserta MT, O'Leary ST, Munoz FM, Ralston SL; Committee on Infectious Diseases. Palivizumab prophylaxis in infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2023;152(1):e2023061803.
- American Academy of Pediatrics Committee on Infectious Diseases; American Academy of Pediatrics Bronchiolitis Guidelines Committee. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134(2):415- 420.
- Borchers AT, Chang C, Gershwin ME, Gershwin LJ. Respiratory syncytial virus—a comprehensive review. Clin Rev Allergy Immunol. 2013;45(3):331-379.
- Shahabi A, Peneva D, Incerti D, et al. Assessing variation in the cost of palivizumab for respiratory syncytial virus prevention in preterm infants. Pharmacoecon Open. 2018;2(1):53-61.
- Mac S, Sumner A, Duchesne-Belanger S, et al. Cost-effectiveness of palivizumab for respiratory syncytial virus: a systematic review. Pediatrics. 2019;143(5):e20184064.
- Liu F, Ouyang J, Sharma AN, et al. Leukotriene inhibitors for bronchiolitis in infants and young children. Cochrane Database Syst Rev. 2015;(3):CD010636.
- Gadomski AM, Scribani MB. Bronchodilators for bronchiolitis. Cochrane Database Syst Rev. 2014(6):CD001266.
- Fernandes RM, Bialy LM, Vandermeer B, et al. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst Rev. 2013(6):CD004878.
- Purcell K, Fergie J. Concurrent serious bacterial infections in 2396 infants and children hospitalized with respiratory syncytial virus lower respiratory tract infections. Arch Pediatr Adolesc Med. 2002;156(4):322-324.
- Virazole (ribavirin for inhalation solution) product information. Bridgewater, NJ: Bausch Health US, LLC; May 2019.
- Guerguerian AM, Gauthier M, Lebel MH, et al. Ribavirin in ventilated respiratory syncytial virus bronchiolitis. A randomized, placebo-controlled trial. Am J Respir Crit Care Med. 1999;160(3):829-834.
- Law BJ, Wang EE, MacDonald N, et al. Does ribavirin impact on the hospital course of children with respiratory syncytial virus (RSV) infection? An analysis using the Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) RSV database. Pediatrics. 1997;99(3):e7.
- Chemaly RF, Aitken SL, Wolfe CR, et al. Aerosolized ribavirin: the most expensive drug for pneumonia. Transpl Infect Dis. 2016;18(4):634-636.
- Krilov LR. Safety issues related to the administration of ribavirin. Pediatr Infect Dis J. 2002;21(5):479-481.