Review of Hypertension in Pregnancy for Pharmacists
RELEASE DATE
February 1, 2021
EXPIRATION DATE
February 28, 2023
FACULTY
Amy Wu, PharmD
Freelance Writer
Sterling, Virginia
FACULTY DISCLOSURE STATEMENTS
Dr. Wu has no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint.
ACCREDITATION STATEMENT
 Pharmacy
Pharmacy
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-21-023-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER:
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients’ conditions and possible contraindications or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
GOAL
To review hypertension in pregnancy and treatment options for hypertensive disorders.
OBJECTIVES
After completing this activity, the participant should be able to:
- Review the different classifications of hypertensive disorders in pregnancy.
- Identify the risk factors for hypertensive disorders in pregnancy.
- Recognize the medication options used to treat hypertensive disorders in pregnancy.
- Describe the pharmacist’s role in patient counseling for hypertension in pregnancy.
ABSTRACT: Hypertension affects approximately 7% to 10% of all pregnancies. If left untreated, it can lead to significant maternal morbidity and mortality. Hypertension in pregnancy is defined as having a systolic blood pressure greater than 140 mm Hg and diastolic blood pressure greater than 90 mm Hg; it is classified into four types. Primary risk factors for hypertension in pregnancy include chronic hypertension, increased serum testosterone concentrations, collagen vascular disease, obesity, insulin resistance, gestational diabetes, diabetes mellitus, and thrombophilia. The mechanisms for pathogenesis are not well understood. Treatment depends on the level of blood pressure, the age of gestation, the occurrence of symptoms, and risk factors associated with them. Pharmacists provide counseling regarding drug-drug interactions, side effects, and adverse effects of antihypertensive medications.
Hypertension affects approximately 7% to 10% of all pregnancies in the United States.1 According to the World Health Organization, approximately 295,000 women and adolescent girls died due to pregnancy- and childbirth-related complications in 2015, and 99% of those occurred in low-resource settings. Hypertensive disorders, hemorrhage, and sepsis are responsible for more than half of all maternal deaths worldwide. 2 The pathogenesis of hypertension in pregnancy is not well understood. An imbalance in prostacyclin and thromboxane A2 metabolism is hypothesized to be involved in the pathogenesis of one type of hypertension in pregnancy, preeclampsia.3 Proper diagnosis, monitoring, and treatment are essential in protecting both mother and fetus if a hypertensive disorder occurs in pregnancy.
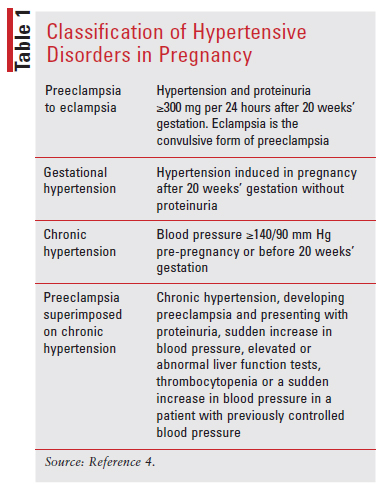
Classification of Hypertensive Disorders in Pregnancy
There are four hypertensive disorder classifications in pregnancy: preeclampsia/eclampsia, gestational hypertension, chronic hypertension, and preeclampsia superimposed on chronic hypertension.4
Preeclampsia to eclampsia is de novo hypertension after 20 weeks’ gestation accompanied by proteinuria, acute kidney injury, or liver injury, with or without upper-right quadrant or epigastric abdominal pain. Neurologic complications such as blindness, stroke, and severe headaches may occur. Hematologic complications include decreased platelet count, hemolysis, or disseminated intravascular coagulation. Uteroplacental dysfunction, such as fetal growth restriction, stillbirth, or abnormal umbilical artery Doppler waveform analysis, may also be present.5
Gestational hypertension is hypertension that appears de novo after 20 weeks’ gestation and then normalizes after delivery.5
Chronic or pre-existing hypertension is defined as having hypertension preconception or prior to 20 weeks’ gestation.5
Preeclampsia or eclampsia superimposed on chronic hypertension is pre-existing hypertension that then develops with signs and symptoms of preeclampsia or eclampsia after 20 weeks’ gestation.5 Refer to TABLE 1 for the classification of hypertensive pregnancy disorders.
Prevalence of Hypertensive Disorders in Pregnancy
Hypertensive disorders of pregnancy can complicate up to 10% of pregnancies and represent a significant cause of maternal and perinatal morbidity and mortality. Preeclampsia can occur in up to 35% of women with gestational hypertension and up to 25% of those with chronic hypertension. The prevalence of gestational hypertension in reproductive-aged women is approximately 7.7%.5
Pathogenesis
The mechanisms for the pathogenesis of hypertensive disorders in pregnancy are not well understood. Regarding preeclampsia, it is hypothesized that there is an imbalance in prostacyclin and thromboxane A2 metabolism involved in its pathogenesis; this has led to initial studies of aspirin for preeclampsia prophylaxis due to its inhibition of thromboxane A2 at lower doses.3 Hypertension associated with preeclampsia develops during pregnancy and remits after delivery, implicating the placenta as a central culprit of the disease. There may be a correlation due to a mechanism of reduced placental perfusion causing systemic vascular endothelial dysfunction. Studies suggest that abnormal cytotrophoblast invasion of spiral arterioles is an important factor that could cause reduced placental perfusion.
Eclampsia is one of the more serious complications of hypertension in pregnancy. Eclampsia is characterized by new-onset tonic-clonic, focal, or multi-focal seizures in the absence of other possible causes such as intracranial hemorrhage, epilepsy, cerebral arterial ischemia, and infarction, or drug use.1
In a low-resource setting, eclampsia is a substantial cause of maternal death. Seizures can progress to severe maternal hypoxia, aspiration pneumonia, and trauma. Some women may experience short-term and long-term changes such as impaired memory and cognitive function especially after recurrent seizures or uncorrected severe hypertension leading to cytotoxic edema or infection. Although permanent white matter loss has been found on magnetic resonance imaging due to eclampsia in up to one-fourth of women, this does not present as significant neurologic damage or deficit.6
Risk Factors for Hypertension in Pregnancy
A family or personal history of preeclampsia has been noted in mothers who have an above-average weight gain during pregnancy. Other risk factors include pregnancy assisted with reproductive technology, BMI greater than 30 prior to pregnancy, chronic hypertension, gestational diabetes, kidney disease, nulliparity, maternal age 30 years and older, multifetal gestations, obstructive sleep apnea, preeclampsia in a prior pregnancy, pregestational diabetes, systemic lupus erythematosus, and thrombophilia. Approximately one-third of mothers with antiphospholipid syndrome—an autoimmune condition with recurrent thrombosis—will develop preeclampsia.7
There are many risk factors for gestational hypertension, including chronic hypertension, increased serum testosterone concentrations, collagen vascular disease, black race, obesity, insulin resistance, gestational diabetes, diabetes mellitus, and thrombophilia. Epidemiological data showed that Asian women are at lower risk for hypertensive disorders in pregnancy, while African American and Hispanic women are at a higher risk. Arab women older than 30 years who have increased BMI are shown to have a higher risk of pregnancy-induced hypertension.7
It has been found that advanced maternal age is associated with an increased risk of chronic hypertension. This temporal relationship of maternal age and chronic hypertension could potentially be due to an increase in age-related vascular disease. Risk factors associated with chronic hypertension include pregestational diabetes mellitus, metabolic syndrome, obesity, smoking, and multifetal pregnancy.8
Diagnosis of Hypertensive Disorders in Pregnancy
Hypertension in pregnancy is classified as mild when systolic blood pressure is 140-149 mm Hg and diastolic blood pressure is 90-99 mm Hg; moderate when systolic blood pressure is 150-159 mm Hg and diastolic blood pressure is 100-109 mm Hg; and severe when systolic blood pressure is ≥160 mm Hg and diastolic blood pressure is ≥110 mm Hg.6
Gestational Hypertension: If systolic blood pressure is ≥140 mm Hg or diastolic blood pressure is ≥90 mm Hg, or both, and occurs on two occasions at least 4 hours apart after 20 weeks’ gestation in a previously normotensive woman, this is considered to be gestational hypertension.7 In situations where severe hypertension is a concern, the diagnosis may be within a shorter interval, such as minutes, instead of 4 hours, so that antihypertensive therapy can begin.6
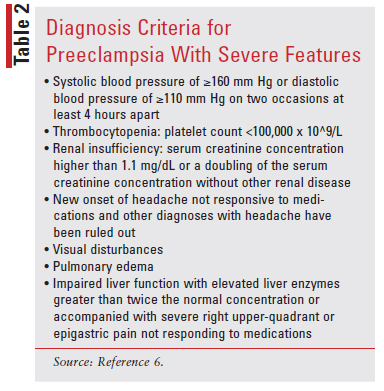
Preeclampsia: The diagnostic criteria for preeclampsia is hypertension that presents with one of the following: proteinuria of 300 mg or more per 24-hour urine collection or the same amount extrapolated from a timed collection; protein/creatinine ratio of 0.3 mg/dL or more, or dipstick reading of 2+. The dipstick reading should be used only if other quantitative methods are not available, because this method has high false-positive and false-negative results.9 Preeclampsia is also diagnosed if a new onset of hypertension is not accompanied by proteinuria but presents with one of the following: thrombocytopenia, renal insufficiency, new-onset headache unresponsive to medication, impaired liver function, or pulmonary edema.6
Ambulatory monitoring of blood pressure is valuable for the diagnosis and differential diagnosis of hypertension in pregnancy and follow-up. It allows for detection of cases of white-coat hypertension, prediction of preeclampsia, late-pregnancy prognosis, and adjustment of treatment. White-coat hypertension refers to measurements of elevated blood pressure that occur in a healthcare setting when measurements outside the healthcare setting are normal. It is important to distinguish white-coat hypertension from hypertension in pregnancy to avoid the potential adverse effects of unneeded antihypertensive therapy as well as unnecessary caesarean or preterm delivery.7
Preeclampsia With Severe Features: Patients with gestational hypertension with severe-range blood pressures (systolic blood pressure of ≥160 mm Hg or diastolic blood pressure of ≥110 mm Hg) are diagnosed with preeclampsia with severe features that can increase the risk of morbidity and mortality. TABLE 2 shows the criteria for diagnosing preeclampsia with severe features.
HELLP Syndrome: Another severe form of preeclampsia is referred to as HELLP syndrome. The acronym stands for hemolysis, elevated liver enzymes, and low platelet count. HELLP is associated with an increase in maternal morbidity and mortality; therefore, close monitoring is prudent. The parameters for making the diagnosis include elevation of lactate dehydrogenase to 600 IU/L or higher, elevated alanine aminotransferase and aspartate aminotransferase to more than twice the upper limit of normal, and decreased platelets to a count of less than 100,000 x 109/L. HELLP syndrome presents with nausea and vomiting in 50% of cases, right upper-quadrant pain, and generalized malaise in up to 90% of cases. Patients may have an atypical onset, with approximately 15% of patients lacking proteinuria or hypertension.6
Preeclampsia: Clinical Considerations, Prevention, and Management
During the initial evaluation for pregnancy, it is recommended that the following lab work is obtained: serum creatinine, a complete blood count, liver function tests, proteinuria, and comprehensive maternal and fetal clinical assessment. An initial ultrasonographic evaluation to estimate fetal weight, amniotic fluid amount, and fetal antepartum testing should be assessed. Fetal antepartum testing encompasses a nonstress test, fetal biophysical profile, fetal movement assessment, contraction stress test, umbilical artery Doppler velocimetry, and modified biophysical profile.10 Following the initial fetal evaluation, subsequent management will be based on the results of the initial tests and gestational age. Physicians will need to weigh the risks versus benefits of delivery.6
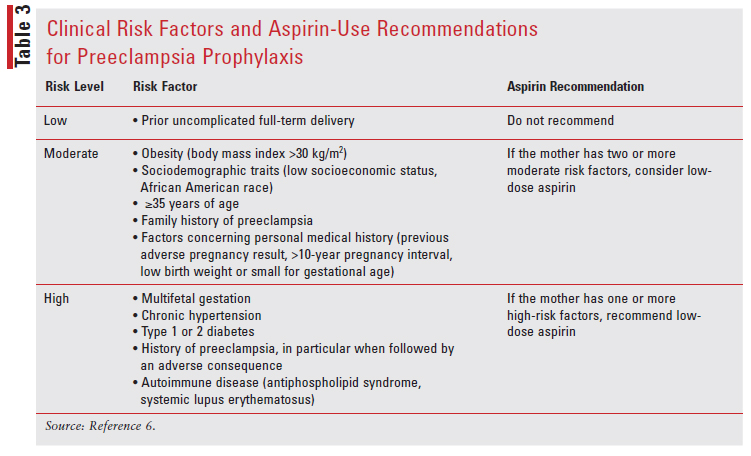
Women at high risk for preeclampsia are recommended to take aspirin and calcium supplements daily, especially when dietary calcium intake is low. There are not enough data to recommend taking vitamins C, E, or D for preeclampsia prevention.7
The American College of Obstetricians and Gynecologists recommends 81 mg of aspirin per day for preeclampsia prophylaxis in women with any of the high-risk factors for preeclampsia. It should be started between 12 and 28 weeks of gestation and continued until delivery.6 High-risk factors include autoimmune diseases, renal disease, multifetal gestation, previous pregnancy with preeclampsia, type 1 or type 2 diabetes mellitus, and chronic hypertension. Those with more than one of the moderate risk factors such as family history of preeclampsia, first pregnancy, a BMI greater than 30 kg/m2, age 35 years or older, sociodemographic factors, and personal-history factors are given aspirin between 12 weeks and 28 weeks of gestation, continuing until delivery.6,11 TABLE 3 summarizes the recommendation for aspirin use.
The priorities in managing mothers with preeclampsia during labor and delivery are prevention of seizures and control of hypertension.
Preeclampsia with severe features can lead to acute and long-term complications for the baby and mother. Complications for the mother may include myocardial infarction, stroke, acute respiratory distress syndrome, renal failure, pulmonary edema, retinal injury, or coagulopathy.6
Magnesium sulfate should be given to women with gestational hypertension or preeclampsia with severe features to help avoid and control seizures during stabilization and until 24 hours after delivery. There is, however, controversy regarding the use of magnesium sulfate in women without severe gestational hypertension or preeclampsia due to their lower risk of eclampsia.
Decisions on the use of magnesium sulfate in this patient group should be based on the preferences of doctors and patients as well as consideration of benefits and harms.2 According to the Magpie trial, which was a randomized, placebo-controlled trial with 10,110 participants, the seizure rate was lowered overall by more than 50% with magnesium sulfate treatment.12 It was shown that magnesium sulfate is more effective than phenytoin, diazepam, or nimodipine to reduce eclampsia. Magnesium sulfate is considered the drug of choice to prevent eclampsia during the intrapartum and postpartum periods.
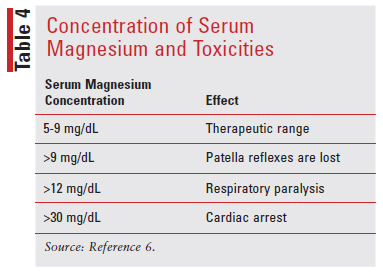
There is no consensus on the ideal dosage; even when magnesium is at a therapeutic level in the range of 4.8 to 9.6 mg/dL, seizures can still occur. Several studies have shown that with an infusion rate of 1 g per hour, which is associated with subtherapeutic magnesium levels, a reduction of eclampsia or recurrent convulsions is reported.13 It should also be noted that a higher BMI and larger volume of distribution can affect the dose and duration of magnesium sulfate required to obtain adequate magnesium levels. In mothers with a BMI greater than 35, the antepartum level of magnesium can be subtherapeutic for as long as 18 hours after infusion with an IV loading dose of 4.5 g followed by 1.8 g per hour. An increase in perinatal mortality has been associated with infusion rates greater than 2 g per hour. In the U.S. the preferred regimen is IV administration of a 4 g to 6 g loading dose over 20 to 30 minutes followed by 1 g to 2 g per hour as a maintenance dose.
If cesarean delivery is necessary, then the infusion of magnesium sulfate should begin before surgery and continue until 24 hours postsurgery. For mothers who are delivering vaginally, the infusion should also continue for 24 hours afterwards. If venous access cannot be obtained, then magnesium sulfate can be administered IM. The dose for IM injection is 10 g initially as the loading dose given as 5 g in each buttock, followed by 5 g every 4 hours. Magnesium sulfate can be mixed with 1 mL of xylocaine 2% solution because IM administration can be painful. Since magnesium sulfate acts as a smooth muscle relaxant, adverse effects such as respiratory depression and cardiac arrest can occur.6 TABLE 4 shows the toxicity of magnesium sulfate in correlation to the level of serum magnesium.
When Delivery Is Recommended
Due to the risk of harm to the child and mother, childbirth is advised in women who are diagnosed with preeclampsia or gestational hypertension with severe features at 34 weeks of gestation or beyond. However, the mother’s condition should first be stabilized. Delivery should also proceed if there is labor or prelabor rupture of membranes.6,14 If delivery is necessary before 34 weeks of gestation, then it is recommended to give corticosteroids to mature the fetal lungs. However, delivery should not be postponed in order to complete the administration of corticosteroids.15
In a patient who has serious preeclampsia prior to 34 weeks of gestation, expectant management should be considered on the basis of joint decision-making between the mother and physician. If there are reasons to believe the fetus may not survive, then expectant management is not advised and delivery should be performed. In addition, if the condition of the fetus and mother worsen, then delivery is also advised. Expedited delivery should occur regardless of gestational age after maternal stabilization if any of the following occurs: eclampsia, pulmonary edema, stroke, visual disturbances, persistent headaches unresponsive to treatment, suspected acute placental abruption or vaginal bleeding, uncontrolled severe range blood pressure with a systolic blood pressure of ≥160 mm Hg or diastolic blood pressure ≥110 mm Hg unresponsive to antihypertensive medication, or new or worsening renal dysfunction.6
Treatment of Hypertension in Pregnancy
Treatment of hypertension in pregnancy depends on the level of blood pressure, the age of gestation, the occurrence of symptoms, and risk factors associated with them. In general, nonpharmacologic therapy is recommended for pregnant women with mild-to-moderate hypertension in pregnancy who do not meet the pharmacologic therapy requirements. Lifestyle modification, such as salt restriction and strict bed rest, are not recommended in hypertension in pregnancy or preeclampsia, according to the World Health Organization. It is essential for women who want to become pregnant to adhere to recommendations to maintain or seek a healthy weight since obesity and gestational weight gain are both risk factors for hypertension in pregnancy, preeclampsia, or eclampsia.
When preeclampsia is diagnosed, the gestational age as well as the degree of increased blood pressure affects the use of antihypertensive treatment. Women with preeclampsia are likely to be delivered at term, and unless there is severe hypertension, treatment may be postponed, and postpartum blood pressure may be reassessed. If preeclampsia develops and the mother is not near term, expectant management is normally undertaken. Blood pressure can be safely reduced to 140/90 mm Hg with oral antihypertensives.7
It should be noted that there are no studies examining safe blood pressure treatment targets for pregnant women and that recommendations and reports usually prescribe treatment to achieve blood pressure levels that are likely to be protective against maternal acute cerebrovascular or cardiovascular adverse effects, typically between systolic levels of 140 and 155 mm Hg and diastolic levels of 90 and 105 mm Hg. Fetal monitoring is essential to help observe signs of fetal distress that could be due to reduced placental perfusion.16
Mild-to-Moderate Hypertension
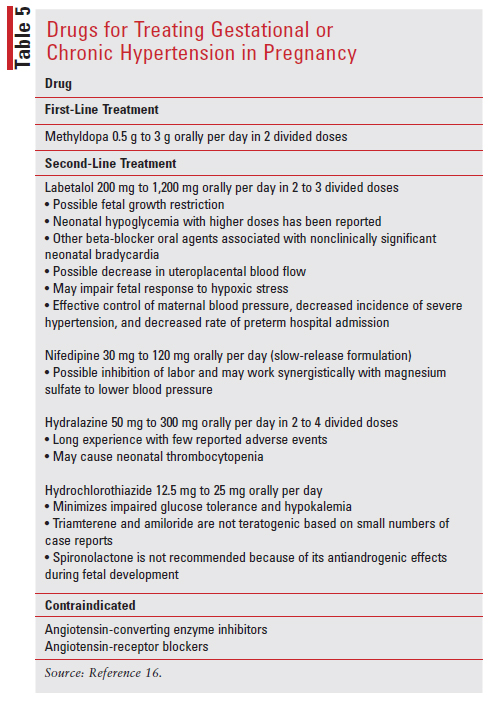
Data are inconclusive for the treatment of mild-to-moderate hypertension in pregnancy, so specific recommendations are lacking.7 There were contradictory findings in randomized, controlled trials comparing prescription drugs with placebo or no medication in moderate cases of hypertension in pregnancy. Studies have shown atenolol and labetalol prevented proteinuria in the treatment group compared with the control group. Methyldopa resulted in improved pregnancy outcome in mild pregnancy hypertension and mild preeclampsia with regard to mid-pregnancy abortions and progression to severe preeclampsia, respectively. Another trial of methyldopa in mild hypertension found that pregnancy was substantially extended by approximately 10.3 days, with no adverse effect on birth weight.17 Methyldopa is the drug of choice in hypertension in pregnancy because of its long record of safety and effectiveness; however, at the time of writing, there is a shortage of raw materials needed to manufacture methyldopa, and suppliers are unable to obtain this drug.18
Birth weights were lower than those of the untreated/placebo category for newborns of mothers who took antihypertensive treatment during pregnancy. A decrease of 10 mm Hg in mean arterial pressure was associated with a decrease of 145 g in mean birth weight, as observed in the post hoc study.19 In late pregnancy, atenolol and metoprolol appear to be safe.7 While the initial acute control of blood pressure is treatment with parenteral antihypertensive therapy, oral medications can be used as expectant management is continued. It is common to use labetalol and calcium channel blockers. Short-acting oral nifedipine can be added gradually if the maximum dose of labetalol is insufficient in achieving the desired blood pressure goal or if the dosage is limited by adverse effects.6
The classes of antihypertensives that are contraindicated in pregnancy are angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs). ACEIs and ARBs are contraindicated in pregnancy due to their increased risk of fetopathy, a disease or disorder in the fetus. If a pregnancy is confirmed, women who are treated with an ACEI or ARB before conception should be advised to switch to a different antihypertensive. Studies in both pregnant women and animals have shown decreased glomerular perfusion pressure, reduced urine output by the fetus, increased risk of fetal hypotension, impaired renal tubular development, complete anuria or oligohydramnios, pulmonary hypoplasia, limb contractures, cranio-facial deformities and impaired ossification, decreased placental and umbilical perfusion, and intrauterine growth restriction.7 TABLE 5 summarizes treatment options for gestational or chronic hypertension in pregnancy.
Severe Hypertension
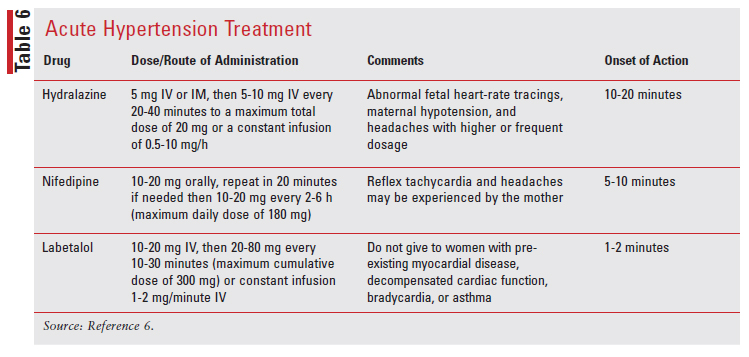
If a woman has an acute onset of severe hypertension (systolic blood pressure of ≥160 mm Hg or diastolic blood pressure of ≥110 mm Hg) lasting at least 15 minutes or longer, treatment with antihypertensive medications should be administered, usually with oral nifedipine or IV hydralazine, or labetalol. Treatment should be initiated as soon as possible and within 30 to 60 minutes of presentation in order to prevent adverse effects such as renal failure, stroke, congestive heart failure, or myocardial ischemia.14 TABLE 6 summarizes the antihypertensive medications used for urgent blood pressure control in pregnancy.
Clinical Follow-Up
Depending on the severity of hypertension, the type and frequency of clinical follow-up can vary. Clinical follow-up includes blood pressure measurements using a mercury sphygmomanometer with the patient in the sitting position and examination of any signs or symptoms indicative of clinical deterioration. Biochemical and urinary tests include urine dipstick, proteinuria urinalysis, urine dipstick >1+, complete blood counts (hematocrit, hemoglobin, platelets), liver enzymes, serum creatinine, and electrolytes. Fetal monitoring also depends on the level of hypertensive disorder. Chronic hypertensive pregnancies require fetal ultrasound, umbilical Doppler velocimetry, and evaluation of the amniotic fluid volume between gestational weeks 28 to 30 and weeks 32 to 34. If fetal activity is irregular, cardiotocography is recommended. For cases of mild-to-severe gestational hypertension, fetal follow-up is also recommended. For cases of extreme gestational hypertension or preeclampsia, it is recommended that fetal ultrasound, amniotic fluid volume assessment, and umbilical Doppler velocimetry be performed no more regularly than every 2 weeks. If irregular fetal activity, menstrual bleeding, or worsening of a pre-existing condition is detected, cardiotocography is advised.7
Pharmacist’s Role
For both the mother and fetus, pregnancy-induced hypertension is a common health condition with adverse effects. The pathogenesis of hypertension in pregnancy is not yet completely understood. Pharmacists in the community can help counsel patients about drug-drug interactions, side effects, and adverse effects of antihypertensive medications. Patients can also be counseled on the importance of following up with providers for blood pressure monitoring, lab work, and fetal monitoring.
REFERENCES
1. Granger J, Alexander BT, Bennett WA, Khalil RA. Pathophysiology of pregnancy-induced hypertension. Am J Hypertens. 2001;14(6 Pt2):178S-185S.
2. WHO recommendations on drug treatment for non-severe hypertension in pregnancy. Geneva: World Health Organization; August 11, 2020. www.who.int/publications/i/item/9789240008793. Accessed January 26, 2021.
3. Schiff E, Peled E, Goldenberg M, et al. The use of aspirin to prevent pregnancy-induced hypertension and lower the ratio of thromboxane A2 to prostacyclin in relatively high risk pregnancies. N Engl J Med. 1989;10;321(6):351-356.
4. Kattah A, Garovic V. The management of hypertension in pregnancy. Adv Chronic Kidney Dis. 2013;20(3):229-239.
5. Braunthal S, Brateanu A. Hypertension in pregnancy: pathophysiology and treatment. SAGE Open Med. 2019;7:2050312119843700.
6. American College of Obstetricians and Gynocologists Committee on Practice Bulletins-Obstetrics. Gestational Hypertension and Preeclampsia. ACOG: Practice Bulletin, Number 222. Obstet Gynecol. 2020;135(6):e237-e260.
7. Kintiraki E, Papakatsika S, Kotronis G. et al. Pregnancy-induced hypertension. Hormones. 2015;14:211-223.
8. Ananth CV, Duzyj CM, Yadava S, Mangas G. Changes in the prevalence of chronic hypertension in pregnancy, United States, 1970 to 2010. Hypertension. 2019;74(5):1089-1095.
9. Phelan LK, Brown MA, Davis GK, et al. A prospective study of the impact of automated dipstick urinalysis on the diagnosis of preeclampsia. Hypertens Pregnancy. 2004;23:135-142.
10. Preboth M. ACOG Guidelines on antepartum fetal surveillance. Am Fam Physician. 2000;62(5):1184-1188.
11. Atallah A, Lecarpentier E, Goffinet F, et al. Aspirin for prevention of preeclampsia. Drugs. 2017;77(17):1819-1831.
12. Altman D, Carroli G, Duley L, et al. Do women with preeclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Magpie Trial Collaboration Group. Lancet. 2002;359:1877-1890.
13. Dayicioglu V, Sahinoglu Z, Kol E, Kucubas I. The use of standard dose of magnesium sulphate in prophylaxis of eclamptic seizures: do body mass index alterations have any effect on success? Hypertens Pregnancy. 2003;22:257-265.
14. Croke L. Gestational hypertension and preeclampsia: a practice bulletin from ACOG. Am Fam Physician. 2019;100(10):649-650.
15. Balogun OA, Sibai BM. Counseling, management, and outcome in women with severe preeclampsia at 23 to 28 weeks’ gestation. Clin Obstet Gynecol. 2017;60:183-189.
16. Podymow T, August P. Update on the use of antihypertensive drugs in pregnancy. Hypertension, 2008;51:960-969.
17. Weitz C, Khouzami V, Maxwell K, Johnson JW. Treatment of hypertension in pregnancy with methyldopa: a randomized double blind study. Int J Gynaecol Obstet. 1987;25:35-40.
18. Wheeler M. Methyldopa tablets. Updated December 8, 2020. ASHP drug shotages database. www.ashp.org/drug-shortages/current-shortages/ drug-shortage-detail.aspx?id=462&loginreturnUrl=SSOCheckOnly#:~:te xt=Reason%20for%20the%20Shortage.%20Accord%20has%20methyldopa%20on,count%20bottles.%20Teva%20discontinued%20methyldopa%20tablets%20in%202018. Accessed January 14, 2020.
19. Von Dadelszen P, Ornstein MP, Bull SB, et al. Fall in mean arterial pressure and fetal growth restriction in pregnancy hypertension: a meta-analysis. Lancet. 2000; 355(9198):87-92.