Safe Use of Analgesics in Acute Pediatric Pain
|
FACULTY:
Evan R. Horton, PharmD
Associate Professor of Pharmacy Practice
MCPHS University
School of Pharmacy–Worcester/Manchester
Worcester, Massachusetts
Sarah L. Howard, PharmD
PGY-1 Pharmacy Resident
Baystate Medical Center
Pharmacy Department
Springfield, Massachusetts
FACULTY DISCLOSURE STATEMENTS:
Drs. Horton and Howard have no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT:
Pharmacy
 Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-16-021-H05-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
FEE INFORMATION:
Payment of $6.50 required for exam to be graded.
TARGET AUDIENCE:
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@jobson.com
DISCLAIMER:
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients' conditions and possible contraindications or dangers in use, review of any applicable manufacturer's product information, and comparison with recommendations of other authorities.
GOAL:
To provide pharmacists with information to ensure safe and effective analgesia in pediatric patients, including the impact of developmental pharmacology and proper dosing on the use of analgesics, as well as appropriate medication selection and updates on FDA analgesic alerts.
OBJECTIVES:
After completing this activity, the participant should be able to:
- Describe how human physiological development may impact appropriate medication selection for children of various ages.
- Recognize common dosing errors for pediatric patients.
- Evaluate the effectiveness and safety of commonly used analgesics for certain indications.
- Discuss recent FDA Drug Safety Communications regarding the risks of using codeine and tramadol in pediatric patients.
- Recommend adverse event monitoring for families with children using analgesics.
ABSTRACT: More than 25% of hospitalized pediatric patients will experience pain during their care. Because pediatric patients are three times more likely than their adult counterparts to experience a medication error, the safe and accurate dosing of analgesics is imperative due to the potential dangers posed by the overdose of these medications. Pharmacists are empowered to assist with recommending and monitoring these agents through proper knowledge of human physiological development and its impact on drug therapy; practicing medication safety as it relates to pediatric patients; and understanding common misconceptions about analgesic efficacy and safety. Recent FDA alerts have highlighted the dangers of using certain opioid medications in pediatric patients with genetic polymorphisms that affect their ability to properly metabolize the drugs. These alerts showcase the impact that medication missteps can have on pediatric patients compared to their adult counterparts. Pharmacists play an integral role in ensuring that analgesics are used properly and safely in the pediatric population.
The use of analgesic medications within the pediatric population is vast. Pharmacists play an important role in product selection, proper dosing, and monitoring. From simple scenarios such as OTC acetaminophen for flulike aches to complex inpatient opioid infusions for sedation or chronic pain syndromes, there is no age or setting within pediatric practice that does not require a firm understanding of analgesics. Historic misconceptions about pediatric pain perception have contributed to the undertreatment of pediatric patients with regard to analgesia.1 Lack of consistent, standardized pain measurement tools has been a contributing factor when pediatric patients have not received appropriate analgesia. Studies have confirmed that undermanaged pain in infants can lead to alterations in pain tolerance, maligned responses to pain as the individual ages, and a predisposition to chronic pain.2 It is estimated that over 25% of hospitalized children experience some type of pain during their stay.3
Background
Even as practitioners increase their awareness of acute pain syndromes in pediatrics, the lack of clinical evidence hinders their approach. When possible, nonpharmacologic methods of pain control should be considered. The American Academy of Pediatrics (AAP) recommends oral sucrose, pacifier use, and facilitated tucking, as well as kangaroo care (skin-to-skin contact) to help console neonates and infants and potentially reduce the need for analgesic pain medications.4 A 2013 Cochrane review supports the use of sucrose to reduce crying, grimacing, and motor hyperactivity. Studied concentrations varied from 12% to 50%, and doses ranged from 0.05 to 3 mL, although clinically, the most typical doses are 0.05 to 0.1 mL of 24% concentration no more than 10 times per day.5
Sucrose is believed to activate the endogenous opioid system and may have mediating action on cholinergic pathways.6 The use of vapocoolant sprays (ethyl chloride) have shown effectiveness in reducing pain associated with cannulation in children 6 years and older.7 In a study of 80 children aged 6 to 12 years, vapocoolant spray reduced pain scores on a 100 mm visual analog scale by 19 mm compared to placebo (95% CI, 6-32; P <0.01). A comparison of vapocoolant spray versus lidocaine injection in adults favored the spray; however, these results have not yet been replicated in pediatric patients.8
When considering pharmacologic options, practitioners must rely on three basic concepts to help select appropriate analgesics: 1) utilization of all available data, including expert consensus; 2) extrapolation from adult data; and 3) matching of medication mechanisms to patient’s pain pathophysiology.9 Addi-tionally, practitioners need to be aware of safety alerts regarding analgesics. Recent alerts from the FDA have warned of the potential dangers of the opioid medications codeine and tramadol.10-12 Pharmacists must be able to understand the positive and negative aspects of all analgesic drug classes and relay those concerns to prescribers as well as their patients.
This article will review how pharmacists can navigate proper analgesia in pediatric patients, taking into account early human physiological development, proper medication dosing techniques, drugs to potentially avoid in certain situations, and how to help caregivers monitor children and adolescents requiring these medications. The use of neuropathic analgesics will not be covered due to lack of sound evidence in this population.
Pathophysiology of Pain and Developmental Pharmacology Issues
Until the latter part of the 20th century, there was a common misconception that pediatric patients, specifically neonates and infants, perceived pain differently from adult counterparts. It was initially thought that children may not possess the neurologic development to perceive pain.1 It is now known that humans develop the ascending pain pathway at 8 to 20 weeks’ gestation, with full communication to the thalamus (key to perceiving pain) by the end of the second trimester.6 The descending pain pathway is fully formed by 34 to 38 weeks’ gestation, highlighting that outside of the preterm period, neonates and infants have the physiological capacity to both sense and perceive pain. It has been established that the immature spinal cord possesses neurons with increased excitability, which may predispose neonates to hyperalgesia and allodynia.
In 2003, Kearns et al published a seminal article outlining how natural early human development impacts medication selection.13 It is now common knowledge among pediatric pharmacists that the roles absorption, distribution, metabolism, and excretion play is significant in how medications are initiated and monitored. Alterations in gastric pH and motility, as well as epidermal development, can affect the absorption capacity of certain medications. Topical analgesics such as lidocaine, as well as alcohol-based analgesics, should be avoided during infancy due to the underdevelopment of skin, which can lead to increased and unwanted absorption of substances, potentially leading to toxicity.
Pediatric patients possess higher amounts of total body water and reduced fat stores, altering the distribution of hydrophilic and lipophilic drugs, respectively. It has typically been understood that pediatric patients have a lower volume of distribution with respect to fat stores; however, with the rise in pediatric obesity, medications that are highly lipophilic, such as fentanyl, may require loading doses to achieve their desired effect.6
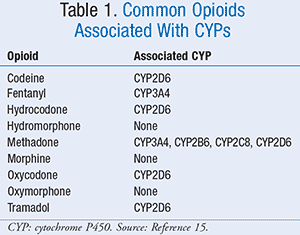
Currently, alterations in metabolism and excretion likely have the greatest known impact on analgesic selection. The developmental timeline of phase 1 enzymes such as cytochrome P450s (CYPs) is well established; however, the impact on drug metabolism and drug-drug interactions based on these concepts is not fully understood. Several opioid medications (e.g., oxycodone, hydrocodone, tramadol, codeine) are dependent upon CYPs for metabolism to active and inactive metabolites. The more prevalent issue in pediatrics is the potential for significant alterations in opioid metabolism due to CYP polymorphisms. Approximately 10% of codeine is metabolized to morphine via CYP2D6, which is necessary for analgesia, as codeine itself has extremely poor affinity for mu-opioid receptors.14 Identified polymorphisms of CYP2D6 can cause some patients (poor metabolizers) to receive no analgesia and others (ultrarapid metabolizers) to metabolize the drug to potentially deadly levels of morphine.10 Other opioids such as oxycodone and hydrocodone offer analgesia through both the parent compound and the active metabolites. In these cases, the effects of genetic polymorphisms are less pronounced but are not yet completely understood. Common opioids and their association with CYPs are described in TABLE 1.15
Phase II metabolism through glucuronidation and sulfation have a mixed impact with regard to pediatric analgesia. Glucuronosyltransferases (UGTs) that target acetaminophen are lacking through the first few years of life, as they are not in older children and adult counterparts.13 Acetaminophen metabolism is still realized through increased sulfation in infants. UGTs that target morphine are present as early as 24 weeks’ gestation and significantly increase between weeks 27 and 40. This rapid increase in metabolic capacity may necessitate increases in morphine dosing to achieve appropriate analgesia as the infant develops. UGT polymorphisms exist, but the data surrounding their impact on patient outcomes are inconsistent.
Complete nephrogenesis occurs at 36 weeks’ gestation. As such, a potential 4-fold difference in the glomerular filtration rates (GFR) can be present between preterm and term neonates.14 Pediatric dosing guides such as NeoFax and Lexicomp’s Pediatric & Neonatal Dosage Handbook account for these differences and should be consulted for proper dose adjustments in preterm and term newborns.15,16 Within the first 2 weeks of life, neonates will experience a drastic increase in the GFR, followed by a subsequent decrease and then a gradual increase to near-adult rates within a few years.13 A rule of thumb for practitioners should be to carefully monitor any analgesics that are extensively excreted through the kidneys in neonates, infants, and children. Due to differences in renal function when compared to adults, children should be evaluated from a renal standpoint using the modified Schwartz equation (FIGURE 1) and/or dynamic direct shifts in serum creatinine (50% increase in 24 hours is considered significant).17
Medication Safety
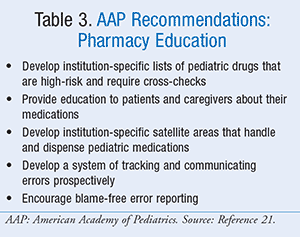
While it should be alarming that the majority of pediatric poisonings are now due to ingestion of medication versus household products such as bleach or disinfecting agents, it should be noted that more than 95% of these events involve accidental ingestion rather than a dosing error.18 One report found analgesic medications to be responsible for approximately 14% of all pediatric adverse drug events requiring hospitalization, second only to antibiotics.19 For nonaccidental ingestions, the AAP has established that pediatric patients are three times more likely than their adult counterparts to experience a medication error.20 Of these errors, incorrect dosing is by far the most common. The AAP has published recommendations for pharmacy actions (TABLE 2) and education (TABLE 3),20 which are primarily intended for inpatient practices but can be implemented in a variety of settings. While these recommendations are not specific to analgesics, the severity of overdoses of these drugs is all the more reason to be cognizant of the AAP’s guidance. Aside from these specific institutional recommendations, pharmacists can promote analgesic safety through common medication safety practices.
Because the majority of pediatric medications, including all analgesics, are dosed based on weight, pharmacists in all settings should first and foremost be double-checking patient weights prior to dispensing or recommending OTC analgesics. Adult doses should not be exceeded to achieve weight-based mg/kg accuracy. Weight-based dosing may also have an age-related component, which is often only highlighted in pediatric-specific dosing references.15,16 Pharmacists should be aware of medications that are dosed mg/kg/day versus mg/kg/dose, as misinterpreting these instructions could be a potential medication error. Pharmacists should read references carefully to interpret dosing recommendations. Dosing references are often subtle with regard to these instructions, and prescribers may overlook or confuse these concepts. Pharmacists should be wary of dosing that seems abnormally high or low, as it may be the result of misinterpretations of dosing recommendations. For this reason, the Pediatric Pharmacy Advocacy Group recommends that prescribers identify both the mg/kg and the full calculated dose when communicating prescription orders.21
Identification of trailing zeros (e.g., 1 vs. 1.0) and/ or lack of leading zeros (e.g., 0.1 vs. .1) can reduce the potential for adverse events of analgesics. Power-of-ten miscalculations can be extremely detrimental to pediatric patients. Even in a healthy adult, a 10-fold overdose of an opioid medication can lead to nausea, altered mental status, and potentially life-threatening events from respiratory depression. This same overdose in a pediatric patient is highly likely to lead to a life-threatening apneic event that will require medical interventions ranging from application of a reversal agent such as naloxone to prolonged treatment in an intensive care unit (ICU).
The use of individualized, precisely measured doses of analgesics is an excellent strategy to avoid medication errors in pediatric patients. When possible, caregivers should be provided with individual, premade doses that reflect proper weight-based dosing. If this is not possible, caregivers should at least be offered an appropriate oral dosing syringe with clearly marked measurements and instructions. To aid in accurate pediatric dose delivery at home, prescribers should be encouraged to use doses rounded to the nearest teaspoon (5 mL) or half-teaspoon (2.5 mL) whenever possible and clinically appropriate. Providing multiple doses in a stock bottle without a proper measured dosing device is a risk factor for dosing errors at home. The use of nonstandardized oral dosing devices such as kitchen utensils is not recommended.
Caregivers should be cautious regarding the mixing of medications in food to increase palatability of liquid medications. This method, while often successful in masking the taste of poorly palatable medications, may lead to inaccurate dosing if the child does not consume all of the food in which the medication was mixed. In the case of analgesics, this could lead to reduced efficacy of doses and the need for repeat doses.
Specific Selection of Analgesic Agents Based on Evidence
Acetaminophen vs. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs): Two of the most commonly used analgesics in pediatric patients, both inpatient and outpatient, are acetaminophen and the NSAID ibuprofen. A 2010 meta-analysis reviewed 18 pediatric studies comparing these two medications for analgesia.22 Six trials concluded that ibuprofen was superior to acetaminophen, with a significant lower pain score than acetaminophen’s at 2 hours following medication administration. One study found ibuprofen to be more efficacious on the day of surgery, but not beyond that point, and the other 11 trials found no difference in efficacy between the two medications. When reviewing the safety of these medications, the authors reviewed 19 studies in children and found no significant difference in the incidence of one or more adverse events (OR 0.82; 95% CI, 0.60-1.12).
Opioid-Sparing Strategies: A 2006 study of the opioid-sparing effects of acetaminophen versus the NSAID ketoprofen showed no difference between the two individual patient groups, but it did show a positive effect when the drugs were combined (4.1 morphine doses [acetaminophen + ketoprofen] vs. 5.9 morphine doses [acetaminophen alone]; P <0.05).23 These results suggest that the combination of acetaminophen and NSAIDs may help to limit the use of opioid medications, potentially limiting adverse events. More recently, the development of IV acetaminophen, as well as the continued use of IV ketorolac, specifically following surgical procedures, has helped to reduce the overall use of opioids in both the short- and long-term.24 Reduction in the use of opioids leads to reductions in the overall incidence of postoperative nausea and vomiting.
There is pushback against use of IV ketorolac due to concerns of postsurgical bleeding and against IV acetaminophen due to increased formulary costs. IV acetaminophen is typically used for 48 hours post surgery at recommended doses of 7.5 mg/kg/dose every 6 hours.6 Ketorolac is dosed at 0.5 mg/kg/dose every 6 hours and is restricted to 5 days of use, just as it is in adults. Both IV acetaminophen and ketorolac are effective and appropriate for use in pediatric patients provided that dosing restrictions and proper monitoring are followed.
NSAIDs vs. Opioids: For decades, post-tonsillectomy/ adenoidectomy care often included an outpatient prescription for a codeine-containing medication, typically in combination with acetaminophen. Following 13 case reports of fatal or life-threatening events related to appropriate, standard doses of codeine, the FDA issued a safety update regarding the use of codeine in this setting.11 Many pediatric hospitals responded by severely restricting codeine use in pediatric patients or removing the medication from their formulary altogether. The most recent guidelines from the American Academy of Otolaryngology—Head and Neck Surgery (AAO-HNS) regarding tonsillectomy in children acknowledge the potential dangers of medications such as codeine and support limiting their use.25 With codeine use now considered to be contraindicated, practitioners must look to alternative analgesics.
Otolaryngologists have been reluctant to use NSAIDs for fear of secondary bleeding following tonsillectomy/adenoidectomy due to the potential antiplatelet effects of these medications. Numerous systematic reviews have now confirmed that NSAIDs do not increase the risk of hemorrhage in post-tonsillectomy patients, although researchers do caution against the use of these medications based on statistical interpretations of these reviews.26-28 Riggin et al concluded that although no statistically significant increase was seen in postoperative bleeding, wide confidence interval distribution should caution practitioners (RR 1.69; 95% CI, 0.71-4.01).27 AAO-HNS guidelines acknowledge the lack of data to support restricting NSAID use following this surgery.25 They also advise against the use of ketorolac, citing evidence that it may increase the risk of bleeding over other NSAIDs. In addition, orthopedic surgeons often avoid NSAIDs due to concerns of delayed bone growth following open and/or closed reductions of fractures.29
While practitioners may be softening to the idea that NSAIDs are not being associated with increased bleeding, the question of efficacy still remains. A 2015 study by Kelly et al compared standard-dose morphine versus ibuprofen (both in combination with acetaminophen) in 91 children (aged 1-10 years) following tonsillectomy.30 Results showed no statistically significant difference in the Faces Pain Scale or the Objective Pain Scale. Adverse events, including bleeding events, were the same between groups with the exception of oxygen desaturations per hour, which favored ibuprofen (↓ 1.79 ± 7.57 vs. ↑11.17 ± 15.02; P <0.01). These results are similar to a 1997 trial that examined ibuprofen versus acetaminophen plus codeine, where no statistically significant differences in pain perception or reporting were noted.31
The study by Kelly and colleagues highlights that although the response to morphine may be more predictable compared to that for drugs such as codeine or tramadol because of lesser variations in genetic polymorphisms, doses required to achieve appropriate analgesia may still pose a danger to patients who have a history of apnea and are exposed to anesthesia.30 Additional studies have shown similar efficacy outcomes between ibuprofen and codeine-based therapies used in fractures and musculoskeletal injuries.32,33
Recent FDA Warnings Regarding Analgesic Use in Pediatric Patients
Codeine: The FDA released a Drug Safety Communication in August of 2012 reporting the potential risk of adverse events or death with codeine use after tonsillectomy and/or adenoidectomy for obstructive sleep apnea in pediatric patients.10 A safety review of codeine was launched following case reports of four children, aged 2 to 5 years, who accidentally overdosed due to CYP polymorphism.10 All cases led to severe respiratory depression, and three of them were fatal. As previously mentioned, codeine requires conversion to morphine via CYP2D6 for adequate analgesia. Patients with CYP2D6 polymorphism for ultrarapid metabolism of codeine receive higher opioid exposure due to increased morphine concentrations in the blood, which can lead to an increased risk of adverse events.
During the safety review, the FDA found additional cases in which pediatric patients experienced severe respiratory depression or death after receiving codeine post tonsillectomy/adenoidectomy.11 These patients were found to be ultrarapid metabolizers via CYP2D6 and had dangerous concentrations of morphine in their blood. The combination of sleep apnea and reduced respiratory drive due to increased mu opioid-receptor agonism is the most likely explanation for the increased adverse effects in these specific patients. The codeine drug monograph has since been updated to include a new black box warning and contraindications for use in pediatric post-tonsillectomy/adenoidectomy pain management.
In July 2015, in response to a warning issued by the European Medicine Agency with regard to codeine use in asthmatic patients, the FDA released an additional Drug Safety Communication warning against the use of codeine-containing products for cough and cold in patients <18 years.34 In this announcement, the FDA recommended against the use of codeine in children <18 years and announced plans to institute an advisory committee to provide guidance on this safety issue.
Tramadol: In September 2015, the FDA issued a Drug Safety Communication regarding the risks of using tramadol in children <17 years of age.12 Tramadol is a weak opioid agonist that has been used in pediatric patients for moderate-to-severe pain.35,36 The FDA is currently reviewing tramadol’s risks in the pediatric population because of the apparent increased risk of respiratory depression, particularly after tonsillectomy or adenoidectomy.12
Tramadol requires hepatic metabolism via CYP2D6 to the active metabolite O-desmethyltramadol, which acts on mu opiate receptors in the central nervous system.35-37 This active metabolite is further hepatically metabolized by CYP3A4 and CYP2D6 to undergo demethylation, glucuronidation, and sulfation to be excreted in the urine.12,35-37 The extensive hepatic metabolism required for tramadol’s conversion to an active metabolite can result in dangerous adverse events in the case of CYP polymorphisms, particularly for patients who are CYP2D6 ultrarapid metabolizers.35,36 The FDA issued this warning after a pediatric case of severe respiratory depression requiring pediatric intensive care after a single dose of outpatient oral tramadol post tonsillectomy.12,38 The patient was later determined to be an ultrarapid CYP2D6 metabolizer and had been exposed to increased concentrations of tramadol and its metabolites.
Until further review of the risks associated with tramadol use in pediatric patients, the FDA recommends careful monitoring or avoidance of the use of codeine and tramadol in pediatric patients.10-12
Monitoring and Counseling for Caregivers
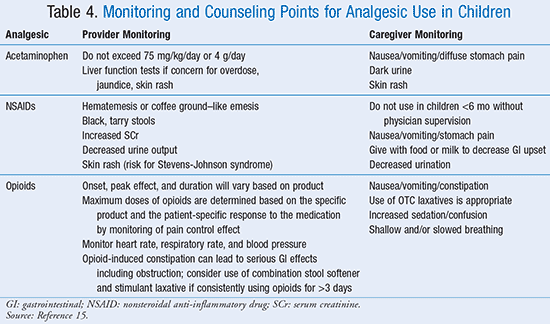
Inpatient and outpatient pharmacists alike are in a unique position to provide support to parents and caregivers of patients requiring analgesic use at home. For all analgesics, awareness of concentrations contained in a particular dosage form is imperative for safe administration at home, whether prescription or OTC. For liquid dosage forms, an appropriate dosing device, such as a syringe or dose cup, should be used for measuring doses, as flatware or kitchen spoons will not provide accurate dosing. Suspensions should be shaken well before dose preparation. Acetaminophen or ibuprofen may be contained in many OTC products, and caregivers must be aware in order to avoid unintentional duplication of therapy or overdose. Parents and caregivers are advised to avoid the use of aspirin in children <17 years because of the risk of Reye syndrome. Specific monitoring and counseling points for commonly used analgesics can be found in TABLE 4.15
Role of the Pharmacist
Pharmacists collaborating in the care of neonates and infants can reduce pain first and foremost by reducing unnecessary procedures. Coordinating blood draws so that necessary tests such as liver function and renal function, as well as therapeutic drug monitoring for medications such as vancomycin or aminoglycosides, can be accomplished with one vial will significantly reduce the number of needle sticks. It is estimated that the average neonatal ICU patient will experience approximately 10 painful procedures per day.39
Pharmacists participating in the care of pediatric patients should have a basic understanding of developmental pharmacology and how differences in human development, specifically during the neonatal and infantile periods, impact drug therapy. This understanding, coupled with knowledge of appropriate medication dosing references (e.g., Lexicomp’s Pediatric & Neonatal Dosage Handbook, NeoFax), will assist in the selection of the appropriate medication as well as proper dosing recommendations.15,16 Pharmacists who may not have direct access to the most appropriate dosing resources should at the very least understand the potential hazards and appropriate ways to navigate information that may be lacking, as previously discussed.
Pharmacists should have knowledge of which analgesics to avoid because of known adverse drug events and should stay current with FDA alerts regarding dangerous trends with specific medications. Understanding the evidence surrounding common analgesics such as acetaminophen and NSAIDs will assist in reducing the overuse of opioid analgesics. When stronger analgesics are warranted, pharmacists should be able to assist prescribers as well as caregivers with proper monitoring to help identify and avoid common yet dangerous medication adverse events.
Summary
Recent FDA alerts regarding life-threatening events related to opioid use in pediatric patients have aimed a new-found national spotlight on the need for safe and rational use of analgesics in this population. Pharmacists with a keen understanding of basic developmental pharmacology, medication safety processes, the evidence surrounding the use of common analgesics in the pediatric population, and proper monitoring for providers and caregivers will be well situated to advocate for patients.
REFERENCES
- Swafford LI, Allen D. Pain relief in the pediatric patient. Med Clin North Am. 1968;52:131-136.
- Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618-1625.
- Kozlowski LJ, Kost-Byerly S, Colantuoni E, et al. Pain prevalence, intensity, assessment, and management in a hospitalized pediatric population. Pain Manag Nurs. 2014;15(1): 22-35.
- American Academy of Pediatrics Committee on Fetus and Newborn, American Academy of Pediatrics Section on Surgery, Canadian Paediatric Society Fetus and Newborn Com-mittee, et al. Prevention and management of pain in the neonate: an update. Pediatrics. 2006;118(5):2231-2241.
- Stevens B, Yamada J, Lee GY, et al. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database Syst Rev. 2013;(1):CD001069.
- Johnson PN. Pain and sedation. In: Eiland L, Todd T, eds. Advanced Pediatric Therapeutics. Memphis, TN: Pediatric Pharmacy Advocacy Group; 2015:202-214.
- Farion KJ, Splinter KL, Newhook K, et al. The effect of vapocoolant spray on pain due to intravenous cannulation in children: a randomized controlled trial. CMAJ. 2008;179(1):31-36.
- Page DE, Taylor D. Vapocoolant spray vs subcutaneous lidocaine injection for reducing pain of intravenous cannulation: a randomized, controlled clinical trial. B J Anaesth. 2010;105(4):519-525.
- Mathew E, Kim E, Goldschneider KR. Pharmacological treatment of chronic non-cancer pain in pediatric patients. Pediatr Drugs. 2014;16:457-471.
- FDA Drug Safety Communication: codeine use in certain children after tonsillectomy and/or adenoidectomy may lead to rare, but life-threatening adverse events or death. August 15, 2012. www.fda.gov/Drugs/DrugSafety/ucm313631.htm. Accessed January 25, 2016.
- FDA Drug Safety Communication: safety review update of codeine use in children; new boxed warning and contraindication on use after tonsillectomy and/or adenoidectomy. February 20, 2013. www.fda.gov/Drugs/DrugSafety/ucm339112.htm. Accessed January 25, 2016.
- Tramadol: Drug Safety Communication—FDA evaluating risks of using in children aged 17 and younger. September 21, 2015. www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm463499.htm. Accessed January 25, 2016.
- Kearns GL, Abdel-Rahman SM, Alander SW, et al. Drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349:1157-1167.
- Madidi P, Koren G. Pharmacogenetic insights into codeine analgesia: implications to pediatric codeine use. Pharmacogenomics. 2008;9(9):1267-1284.
- Taketomo CK, Hodding JH, Kraus DM. Pediatric & Neonatal Dosage Handbook: A Comprehensive Resource for All Clinicians Treating Pediatric and Neonatal Patients. 21st ed. Hudson, OH: Lexicomp; 2014.
- NeoFax. New York, NY: Truven Health Analytics; 2016. http://micromedex.com/neofax-pediatric. Accessed January 25, 2016.
- GFR calculators for children. National Institute of Diabetes and Digestive and Kidney Disease. Updated March 1, 2012. www.niddk.nih.gov/health-information/health-communication-programs/nkdep/lab-evaluation/gfr-calculators/children-conventional-unit/Pages/default.aspx. Accessed February 1, 2016.
- Schillie SF, Shehab N, Thomas KE, Budnitz DS. Medication overdoses leading to emergency department visits among children. Am J Prev Med. 2009;37(3):181-187.
- Cohen AL, Budnitz DS, Weidenback KN, et al. National surveillance of emergency department visits for outpatient adverse events in children and adolescents. J Pediatrics. 2008;152(3):416-421.
- American Academy of Pediatrics Committee on Drugs and Committee on Hospital Care. Prevention of medication errors in the pediatric inpatient setting. Pediatrics. 2003;112(2):431-436.
- Levine SR, Cohen MR, Blanchard NR. Guidelines to preventing medication errors in pediatrics. J Pediatr Pharmacol Ther. 2001;6:427-443.
- Pierce CA, Voss B. Efficacy and safety of ibuprofen and acetaminophen in children and adults: a meta-analysis and qualitative review. Ann Pharmacother. 2010;44(3):489-506.
- Hiller A, Meretoja OA, Korpela R, et al. The analgesic efficacy of acetaminophen, ketoprofen, or their combination for pediatric surgical patients having soft tissue or orthopedic procedures. Anesth Analg. 2006;102(5):1365-1371.
- Baley K, Michalov K, Kossick M, McDowell M. Intravenous acetaminophen and intravenous ketorolac for management of pediatric surgical pain: a literature review. AANA J. 2014;82(1):53-64.
- Baugh RF, Archer SM, Mitchel RB, et al. Clinical practice guideline: tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;144:S1.
- Chan DK, Parikh SR. Perioperative ketorolac increases post-tonsillectomy hemorrhage in adults but not children. Laryngoscope. 2014;124(8):1789-1793.
- Riggin L, Ramakrishna J, Sommer DD, Koren G. A 2013 updated systematic review and meta-analysis of 36 randomized controlled trials; no apparent effects of non-steroidal anti-inflammatory agents on the risk of bleeding after tonsillectomy. Clin Otolaryngol. 2013;38(2):115-129.
- Lewis SR, Nicholson A, Cardwell ME, et al. Nonsteroidal anti-inflammatory drugs and perioperative bleeding in paediatric tonsillectomy. Cochrane Database Syst Rev. 2013;7: CD003591
- Garcia-Martinez O, De Luna-Bertos E, Ramos-Torrecillas J, et al. Repercussions of NSAIDS drugs on bone tissue: the osteoblast. Life Sciences. 2015;123:72-77.
- Kelly LE, Sommer DD, Ramakrishna J, et al. Morphine or ibuprofen for post-tonsillectomy analgesia: a randomized trial. Pediatrics. 2015;135(2):307-313.
- St Charles CS, Matt BH, Hamilton MM, Katz BP. A comparison of ibuprofen versus acetaminophen with codeine in the young tonsillectomy patient. Otolaryngol Head Neck Surg. 1997;117(1):76-82.
- Clark E, Plint AC, Correll R, et al. A randomized controlled trial of acetaminophen, ibuprofen, and codeine for acute pain relief in children with musculoskeletal trauma. Pediatrics. 2007;119(3):460-467.
- Drendel AL, Gorelick MH, Weisman SJ, et al. A randomized clinical trial of ibuprofen versus acetaminophen with codeine for acute pediatric arm fracture pain. Ann Emerg Med. 2009;54(4):553-560.
- FDA Drug Safety Communication: FDA evaluating the potential risks of using codeine cough-and-cold medicines in children. July 1, 2015. www.fda.gov/Drugs/DrugSafety/ucm453125.htm. Accessed January 25, 2016.
- Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43(13):879-923.
- Paar WD, Frankus P, Dengler HJ. The metabolism of tramadol by human liver microsomes. Clin Investig. 1992;70(8):708-710.
- Ultram ER (tramadol) prescribing information. Titusville, NJ: Janssen Pharmaceuticals Inc; July 2014.
- Orliaguet G, Hamza J, Couloigner V, et al. A case of respiratory depression in a child with ultrarapid CYP2D6 metabolism after tramadol. J Pediatr. 2015;135(3):753-755.
- Carbajal R, Rousset A, Danan C, et al. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA. 2008;300(1):60-70.