The Pharmacist's Role in Managing Postpartum Depression
RELEASE DATE:
September 1, 2019
EXPIRATION DATE:
September 30, 2021
FACULTY:
Tammie Lee Demler, BS, PharmD, MBA, BCGP, BCPP
Clinical Associate Professor
State University of New York at Buffalo School of Medicine,
Department of Psychiatry
Director of Psychiatric Pharmacy Residency Programs
State University of New York at Buffalo School of Pharmacy and
Pharmaceutical Sciences
Buffalo, New York
FACULTY DISCLOSURE STATEMENTS
Dr. Demler has no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT:
Pharmacy
 Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-19-087-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE:
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER:
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients' conditions and possible contraindications or dangers in use, review of any applicable manufacturer's product information, and comparison with recommendations of other authorities.
GOAL:
To educate pharmacists about postpartum depression (PPD), including risk factors, symptoms, and treatment options.
OBJECTIVES:
After completing this activity, the participant should be able to:
- Describe PPD's prevalence, diagnostic criteria, and risk factors as well as the challenges associated with this mood disorder.
- List the medications currently available to treat PPD.
- Discuss the safety concerns for these medications during pregnancy and lactation.
- Identify opportunities to improve prescriber and patient education regarding emerging safety and labeling information.
ABSTRACT: Postpartum depression (PPD), a serious mental health condition, can have significant adverse effects on both mother and infant. This mood disorder often goes unrecognized, and even when it is diagnosed, PPD may be undertreated owing to prescriber and patient concerns about the safety risks associated with the agents used for treatment. In addition to keeping abreast of new medications approved for PPD treatment, it is imperative that pharmacists become fluent in interpreting the pregnancy and lactation risks of medications; additionally, they must offer patient education that supports shared decision making and emphasize the importance of adherence to the drug once a regimen has been initiated.
Postpartum depression (PPD) is a serious mental health condition that is estimated to occur in roughly 10% to 20% of mothers worldwide. This disorder has been consistently observed in both high- and low income countries, with higher rates in low- and middle-income countries.1,2
Characterized by clinically significant depressive symptoms that are often accompanied by anxiety, PPD is defined as a major depressive episode (MDE) resulting in significant impairment whose onset of symptoms occurs during pregnancy or the 4 weeks following delivery.3,4 However, researchers and clinicians who are engaged in daily clinical practice generally describe a broader range of presentation lasting through 4 weeks to 12 months after childbirth.5 A highly variable rate of depression has been reported during the perinatal period (i.e., from 22 completed weeks [154 days] of gestation through 7 days after birth).6 While prevalence rates of depression during pregnancy may be as low as 10%, rates as high as 29% have been reported during the postpartum period.7,8 Women with a previous episode of PPD are recognized to be at higher risk, with roughly 25% experiencing a subsequent episode.9 In some cases, PPD symptoms may not meet full criteria for an MDE; therefore, there may be hesitancy to treat. In fact, treatment rates are much lower in pregnant and postpartum women: roughly 14% compared with approximately 26% in their generalpopulation nonpregnant peers.10,11
Sleep disturbance is a common symptom of PPD. Although excessive daytime sleepiness could be interpreted as a consequence of stress and exhaustion associated with care of the newborn, sleep disruption, anxiety, and irritability are also symptoms of PPD. Because these symptoms have a significant negative impact on both mother and infant, intervention is necessary. In extreme cases of PPD, obsessive thoughts about and preoccupation with the baby's health, care, and feeding, along with suicidal ideation and worry about causing the baby harm, can occur.5,12 Although PPD is a maternal diagnosis, increased rates of depression have been found to occur in new fathers as well.5
Risk Factors
As cultural competence increases in the United States, it is important to keep in mind that ethnicity, race, and cultural factors may pose an increased risk of PPD. Some studies have concluded that refugee women are at higher risk because of local language barriers and short duration of residence in the destination country.13 Studies suggest that one in three migrant women may experience PPD, a much higher rate than that for their nonmigrant counterparts in the destination country. The combination of prior history of depression and inadequate social support is strongly associated with perinatal depression regardless of other factors.14 Although the rapid hormonal alterations that occur during and after pregnancy are thought to play a significant role in PPD, these changes are not solely responsible for its development.5,15 Because the strongest risk factor for PPD is a history of untreated depression, healthcare providers (HCPs) should be appropriately proactive when mood and anxiety disorders occur during pregnancy.12
Pregnancy can be a wellness motivator for women who may not otherwise seek preventive healthcare, and HCPs should take these opportunities to screen perinatal patients for depression. The role of universal PPD screening has been debated, but instruments have been used to assess patients for PPD for many years. Although the Edinburgh Postnatal Depression Scale has been a validated screening tool since its publication in 1987, alternative screening options that offer a more racially and demographically diverse assessment have been used more recently in primary care settings.12
Diagnosis and Clinical Course
The diagnostic criteria for PPD are the same as those for major depressive disorder (MDD), with symptoms that result in clinically significant distress or impaired functioning and are not attributable to another medical condition or a substance. Like MDD, PPD has a variable course, with some women reporting spontaneous remission within weeks of onset and others (approximately 20%) still exhibiting signs of depression a year or more after childbirth.5 Awareness that PPD increases the risk of numerous adverse outcomes in both mothers and newborns is increasing. In addition to altered physical and social development in the child, there is evidence that diminished cognition and reduced maternal attachment resulting in impaired emotional development are possible consequences.16
The monitoring of maternal mental health during pregnancy and afterward is a critical step in suicide prevention. A study conducted by Grigoriadis and colleagues found that roughly 4% of deaths occurring during pregnancy were attributed to suicide and that the prevalence of suicide increased postpartum; additionally, fewer than one-half of the deceased subjects had been receiving mental health services within the last month of life despite having regular contact with their obstetrician.17 The vulnerability of pregnant women who may be at risk for death by suicide is something that HCPs must consider at every stage of pregnancy and postpartum recovery.
A study by Khalifeh and colleagues evaluated data on 4,785 women aged 16 to 50 years who died by suicide in the UK.18 Subjects were categorized by age and according to perinatal or nonperinatal period. About 1 in 50 women aged 16 to 50 years and roughly 1 in 25 in the subgroup aged 20 to 35 years died by suicide during the perinatal period. It was concluded that the perinatal period is a high-risk time for women with depression and a risk of suicide and that such patients need careful monitoring and treatment.18
Pregnancy-Related Treatment Concerns
In general, it is optimal to reduce medication exposure during pregnancy in order to eliminate risks to the fetus. In addition to prescriber hesitancy to treat depressive symptoms during pregnancy, women who become pregnant while taking antidepressants may opt to discontinue them based on their own perception of risk or need.19 Although nonpharmacologic interventions offer an effective option for mild depression, drug therapy is indicated—and is considered the mainstay of treatment—for more severe depression, which harbors substantial risk for both mother and infant.
Adverse outcomes, including congenital malformations, are potentially associated with the use of antidepressants during pregnancy; however, outcomes such as premature delivery and spontaneous abortion have been linked to untreated or insufficiently treated maternal depression.20 Although most studies have focused on adverse outcomes, there is evidence that selective serotonin reuptake inhibitor (SSRI) use in pregnancy could have positive effects.21 Hunter and colleagues found that antidepressant treatment can reduce maternal anxiety and mitigate the adverse consequences of altered infant sensory gating, which increases the risk of impaired attentional function.21
Some of the general principles of prescribing during the perinatal period include using the fewest number of medications possible; using medications with the fewest metabolites and highest protein binding to lessen passage across the placenta; using the lowest effective dose; using medication with the lowest known risk to both mother and child; and educating the mother and family about the importance of taking the prescribed medication. Shared decision making is recommended to ensure the best chance of adherence. It is also important to predict and evaluate the pharmacokinetic changes that take place in pregnancy, with dose titrations planned in response to these alterations. It is important to present the absolute and relative risks in terms that the patient can understand, using common denominators (e.g., 10 in 100 or 25 in 100 rather than 1 in 10 or 1 in 4) and providing written material, when possible, to describe the risks.14 Absolute risk to the developing fetus is difficult to fully determine given the limited data available and the ethical barriers preventing randomized, placebo-controlled trials that would improve interpretation of these risks.14
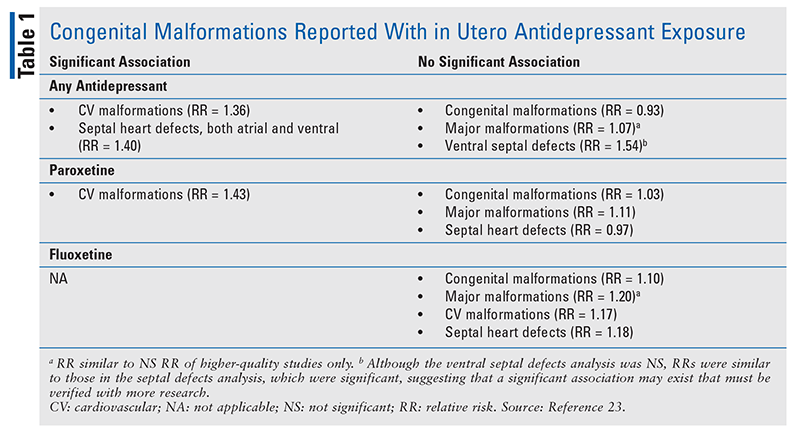
In addition to being small and of poor quality, the existing studies do not adequately evaluate confounding risks (e.g., smoking or substance use). The increasing use of pregnancy registries and databases, coupled with emerging safety information that establishes optimal use of antidepressants during pregnancy, has reduced the likelihood of adverse outcomes. A 2013 meta-analysis highlighted a potentially overrated risk of paroxetine exposure, with reports of only slightly increased risk of cardiac malformations (odds ratio [OR] 1.4) versus the greater OR of 1.72 (95% CI, 1.22-2.42) originally reported by Bar-Oz and colleagues.22 Previous studies had conflicting results and methodological limitations, frequently finding rates of major congenital malformation no higher than what is considered to be the established baseline of roughly 3% of births in North America.22,23 When Grigoriadis and colleagues examined congenital malformations potentially associated with antidepressant exposure during pregnancy, available data for fluoxetine and paroxetine permitted a more intensive evaluation (see TABLE 1 for a detailed summary).23
Assessing Pregnancy-Exposure Risk
According to the FDA, 6 million pregnancies occur in the U.S. each year, and 50% of pregnant women have reported taking at least one medication during their pregnancy. The rate of first-trimester prescription medication use has increased by more than 60%, and the use of four or more medications during the first trimester has risen from roughly 10% to almost 30%. Pregnant women have reported taking an average of 2.6 medications at any given time during their pregnancy.24
In 2014, the FDA published the Pregnancy and Lactation Labeling Rule (PLLR), which took effect on June 30, 2015.24 The PLLR requires all prescription drugs to remove the pregnancy letter category by June 2020, and all drugs approved on or after June 30, 2001, have additional content and formatting requirements. The PLLR also reorganizes labeling information to provide clearer descriptions of the relevant data in order to facilitate decisions about treatment in pregnant and nursing women. Included in the revised labeling is a subsection for pregnancy, labor, and delivery. To encourage registry participation, the PLLR requires the labeling to include a specific statement about the associated pregnancy registry (should one exist) that tracks exposures and provides follow-up contact information.24
The previously used pregnancy letter category system was criticized for being too simplistic and subject to misinterpretation, as the letters were mistakenly viewed as a grading system. The new system, however, has presented challenges to prescribers who are more familiar with the previous A, B, C, D, and X categories. TABLE 2 provides a crosswalk comparison of the two labeling systems.24,25 HCPs who frequently prescribe mental health medications to pregnant patients are more likely to be aware of which drugs pose greater fetal risk, so they may be more confident in deciding which medications should be used or avoided.24
Prepregnancy Management
The pharmacist can play a key role in helping women who are being treated for mental health conditions to avoid unplanned pregnancy by evaluating drug interactions that decrease the effectiveness of oral contraceptives; they can also monitor the use of high-risk medications and recommend the avoidance of contraindicated medications in women of childbearing age. Although antidepressants may be indicated for lifelong treatment in women with refractory PPD, consideration of proactive drug discontinuation prior to pregnancy may be a feasible option for patients who are in remission and have a low risk of relapse.14 Pharmacists can also inform patients that the use of medication for mental health conditions during pregnancy may be indicated and may, in fact, be appropriate and necessary.
After Delivery and During Lactation
Recommendations for PPD treatment include continued use of the medication that successfully treated the symptoms during pregnancy and, in order to prevent relapse, avoidance of medication switching or discontinuation.14 Given the increased popularity of breastfeeding and societal and medical encouragement to do so, it is important for HCPs and patients to weigh the risks and benefits of breastfeeding against the potential risks of drug exposure to the infant.8 The health benefits of breastfeeding have been well established, and the World Health Organization states that exclusive breastfeeding is the best method during the first 6 months, followed by continued breastfeeding plus complementary food for up to 2 years or longer.26 Because treatment of maternal mental illness is crucial, finding appropriate treatment options is key to optimal management.
Although drug exposure and risks to the infant are lower during breastfeeding than during pregnancy, the mother may have concerns, so it is important for the pharmacist to provide reassurance. SSRIs are considered a first-line medication option for the treatment of depression in the perinatal period, and the amount of drug passing into breast milk is estimated to be up to 10 times lower than that estimated for in utero exposure. To establish the estimated dose of medication the nursing infant is receiving, evaluation is based on the milk-drug concentration and the average milk intake per day of 150 mL per kg of the infant's body weight per day.9 A relative infant dose of <10% of the maternal dose is considered negligible exposure and is associated with low risk in most healthy full-term infants.5,9 Øystein Berle and Spigset reviewed antidepressants with reported low infant doses, including fluvoxamine, paroxetine, sertraline, duloxetine, reboxetine, bupropion, and mirtazapine.9 With the exception of paroxetine, SSRIs are considered compatible with pregnancy should the mother become pregnant during the time of planned breastfeeding.
Despite the relatively safe profile of SSRIs, prescribers must be vigilant in identifying any potential adverse effects from the medications used, and higher-risk infants (i.e., those born prematurely or with compromised organ function) should be even more closely monitored.14 Importantly, in addition to any impaired metabolic capacity (including, but not limited to, preterm infancy), a predicted exaggerated drug effect should be anticipated whenever possible in newborns with a genetically predetermined impairment, such as that seen with CYP2D6 and CYP2C19 polymorphisms.9,14 Conversely, the pharmacodynamic effect of SSRIs on serotonin receptors does not seem to be affected; biological evidence has demonstrated that the reduced platelet effects and increased bleeding occurring in adults were not observed in infants.14,27
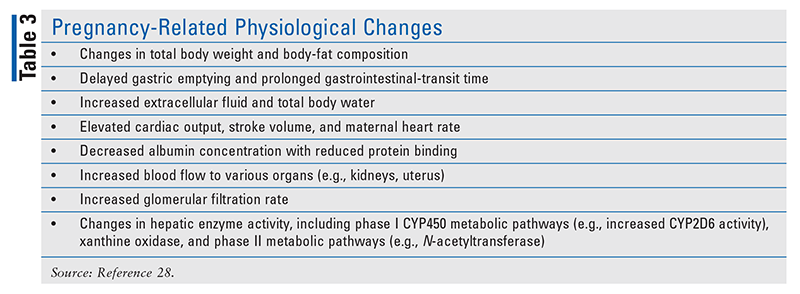
Pregnancy-Related Physiological Changes
The physiological alterations that occur during pregnancy vary with the progression of trimesters and return to baseline at various, uniquely individualized rates during the postpartum period. All of these changes can influence both the efficacy of medications and their potential for adverse effects. See TABLE 3 for a summary.28
Treatment Strategies
Nonpharmacologic interventions that are considered safe and appropriate during pregnancy include, but are not limited to, psychotherapy and electroconvulsive treatment. Pharmacologic treatment has generally included standard medication regimens established by consensus guidelines while recognizing that a pregnant patient is subject to different pharmacokinetic and pharmacodynamic consequences of the medications.28
Previous Treatment
The presumed cause of depression during the perinatal and postpartum periods is the theorized influence of hormonal changes associated with pregnancy, delivery, and lactation. The current standard of care for PPD includes the use of antidepressants in patients who have a history of severe depression or are experiencing moderate-to-severe symptoms, some of which are poor self-care, decreased appetite, and suicidal ideation. With the exception of paroxetine, SSRIs and selective serotonin-norepinephrine reuptake inhibitors are generally considered first-line treatment in women of childbearing age. Alternative medication options are usually based on previous history of efficacy and emerging safety information. It should be noted, however, that the current standard of care does not specifically address the currently recognized potential neurohormonal link to, and contributing cause of, PPD. In addition to a mechanism of action that is not directly linked to the current hormonal hypothesis, antidepressants have a low overall rate of remission and the onset of established efficacy is delayed weeks or months, which is suboptimal given that a rapid onset of action is necessary to prevent adverse outcomes in both newborn and mother.29
Brexanolone
Although progesterone is well known for its role in pregnancy, its function in stress adaptation, with increased production a likely response to neural-cell injury, is poorly understood.30 One of the newest medications for PPD, brexanolone, was developed as an analogue of the progesterone metabolite allopregnanolone, which increases during pregnancy and declines abruptly after birth. The decline of allopregnanolone is thought to cause symptoms of depression and anxiety in postpartum women. This neuroactive steroid has potent positive allosteric-modulator activity at both synaptic and extrasynaptic gamma-aminobutyric acid (GABA) receptors.31
The role of allopregnanolone in the overall regulation of emotion, depression, and anxiety is thought to include regulatory effects on hypothalamic-pituitary-adrenal axis function in addition to GABAergic mechanisms. Previous consideration of this steroidal hormone as a novel therapeutic target had been limited by pharmacokinetic challenges, including low bioavailability and extensive oxidation to the ketone.32
In March 2019, the FDA approved brexanolone (Zulresso) for the treatment of PPD in adults; however, the product launch was delayed until June 2019, when the Drug Enforcement Administration (DEA) finalized its ruling on the drug's assigned designation as a Schedule IV controlled substance.33,34 According to the manufacturer, the FDA previously granted brexanolone injection a Priority Review and Breakthrough Therapy designation.31,34 In the several years prior to approval of brexanolone, the downregulation of neurosteroidal biosynthesis as a likely contributor to the development of depression and anxiety disorders was vigorously debated. Reduced levels of allopregnanolone—such as that seen postpartum—in peripheral blood or cerebrospinal fluid have been associated with major depression, anxiety disorders, premenstrual dysphoric disorder, negative schizophrenia symptoms, and aggression.
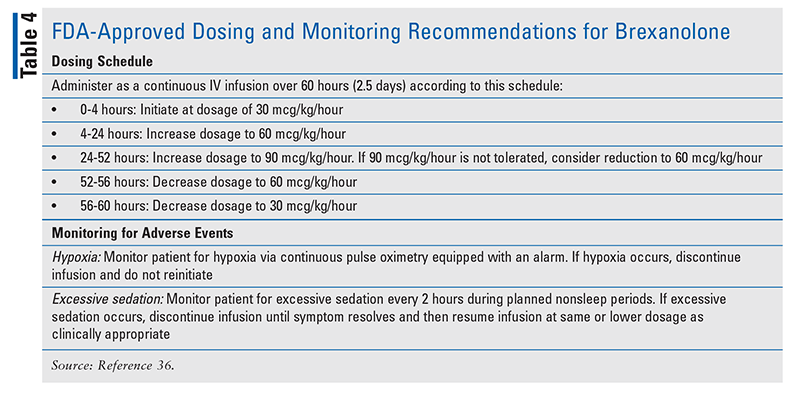
The efficacy of brexanolone was demonstrated in two randomized, double-blind, placebo-controlled, multicenter studies of women aged 18 to 45 years with onset of an MDE in the third trimester within 4 weeks of delivery.35 Study 1 patients were diagnosed with severe PPD based on Hamilton Depression Rating Scale (HAM-D) scores of 26 or higher, and study 2 patients had HAM-D scores of 20 to 25, indicating moderate PPD. Patients in both studies received a 60-hour continuous infusion of either brexanolone or placebo and were followed for 4 weeks. All study 2 brexanolone patients received the recommended target dosage of 90 mcg/kg/hour; in study 1 brexanolone patients, a subset received 60 mcg/kg/hour and the rest were given 90 mcg/kg/hour. The primary endpoint was the mean change in HAM-D total score (i.e., symptoms) from baseline to hour 60; an additional endpoint was mean change in HAM-D total score at day 30. For study 1 and study 2 patients, the 90 mcg/kg/ hour target dosage was significantly superior to placebo in improving depressive symptoms over baseline (–17.7 points and –14.6 points on HAM-D vs. –14 points for placebo; P = .0252 and P = .0013, respectively); in study 2, the 60 mcg/kg/hour dosage also was superior to placebo (–19.5 points vs. –14 points, respectively; P = .016). The improvement in depression symptoms was superior to that for placebo at the end of day 30. The authors did not report a significant comparative difference between the 60 mcg/kg/hour and 90 mcg/kg/hour dosages.35 See TABLE 4 for the full dosing recommendations.36
Monitoring During Administration: Brexanolone has a black box warning requiring the monitoring of patients for excessive sedation or sudden loss of consciousness during administration.36 During clinical trials, 4% of patients experienced loss of consciousness or altered consciousness, and 5% experienced sedation and somnolence necessitating dose reduction or interruption. The infusion should be stopped immediately if signs or symptoms of excessive sedation occur or if pulse oximetry reveals hypoxia or hypoxemia. Once symptoms of excessive sedation have resolved, infusion may be resumed, either at a lower dose or cautiously at the same dose; however, administration should not be restarted following an episode of hypoxia. Although there was no apparent association between timing of the dose and alteration or loss of consciousness, time to full recovery was between 15 and 60 minutes after dose interruption. As with any central nervous system (CNS) depressant, the patient should avoid consuming alcohol or other CNS-depressing substances, performing activities requiring coordination and concentration or alertness, such as driving or operating machinery, until any CNS-depressant effects have dissipated. Patients should be instructed and reminded not to bring children who need care to the scheduled infusion or to drive following treatment. Brexanolone should be avoided in patients with end-stage renal disease with an estimated glomerular filtration rate of <15 mL/ minute/1.73 m2 because of the risk of adverse effects associated with potential accumulation of the solubilizing agent in the formulation.36
Increased Risk of Suicide With Antidepressants: All antidepressants carry a black box warning for increased risk of suicide. However, brexanolone is categorized as a neuroactive GABA type A receptor– positive modulator indicated for treatment of PPD, and it does not carry the same black box warning as the standard antidepressant medications. Because the risk of suicidal ideation with brexanolone is unknown, the suicide warning in the package insert is a general recommendation to closely monitor any at-risk patients, with discontinuation suggested in the event of worsening depression or emergence of suicidality.36 The patient should be counseled to report any episodes of suicidal thinking or behavior.
Safety in Pregnancy and Breastfeeding: Women who may be candidates for PPD treatment and who may also become pregnant during this time should be counseled that currently no information is available to ascertain any drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes with brexanolone use during pregnancy.36 Some animal reproductive studies that used brexanolone doses exceeding the maximum recommended human dose showed fetal harm including, but not limited to, fetal developmental toxicity, maternal toxicity, and neurobehavioral deficits. In animal studies assessing other drugs that enhance GABAergic transmission, drug administration in rats caused neurodegeneration in the developing brain during the window of vulnerability that corresponds to the human brain development that takes place during the third trimester.36
According to the American Academy of Pediatrics, women with PPD can seek mental health intervention while still meeting their own personal breastfeeding goals, and treatment should be built around preserving breastfeeding plans. Although breastfeeding is encouraged, if efforts to nurse contribute to worsening maternal symptoms, alternative forms of feeding should be explored.37 Data from a small lactation study examining the extent and effects of brexanolone in 12 nursing women demonstrated that the relative infant dose is low, with exposures of up to 2% of maternal weight-adjusted dose found in breast milk. Extrapolated data showing low adult oral bioavailability further suggest that the actual infant exposure is likely less; however, specific data on brexanolone's effects on nursing infants are not currently available.36
REMS Program: Because of the risk of excessive sedation or sudden loss of consciousness discussed earlier, the patient, treatment facility, wholesaler, distributor, and dispensing pharmacy must be enrolled or certified, as applicable, in the Zulresso Risk Evaluation Management Strategy (REMS) program. In order to meet REMS requirements, an HCP must be on-site for the entire 60-hour treatment to monitor the patient (and intervene, if necessary). Monitoring must include pulse oximetry and evaluation for excessive sedation every 2 hours during planned nonsleep periods.36 The DEA has established a Schedule IV controlled-substance status for this medication; therefore, safeguarding against unintended access and misuse, as per regulation, is required.
Conclusion
The pharmacist's role in mental health care has traditionally been recognized to be primarily that of gatekeeper of safe medication use. Predicting and communicating drug interactions and offering therapeutic recommendations while enabling patients' access to essential medication by facilitating prior authorizations continue to be mainstays of practice. However, it is imperative that all additional avenues for pharmacist intervention be explored to ensure optimized medication management in mental health. Among these interventions is the notable need for education that surpasses the basic medication-counseling encounter. The pharmacist also must be vigilant in advocating for his or her patient, making swift recommendations for mental health referral if symptoms such as depression and anxiety are suspected.
Some key principles pharmacists must regularly communicate to their patients are that PPD is common and that it is potentially disabling if not treated. Patients need reassurance that PPD is treatable and should be counseled that once treatment is initiated, adherence is necessary for recovery. Because patients may be hesitant to seek treatment during pregnancy and breastfeeding for fear of potential harm to the fetus or nursing infant, pharmacists can tell patients that not all PPD treatment requires pharmacotherapy. Mild cases of depression may be addressed with psychosocial strategies, such as peer support and counseling. In moderate-to-severe depression, pharmacotherapy with first-line SSRIs is often indicated, along with psychological therapy; however, more information is now available to ensure robust risk-versus-benefit assessment. In refractory depression or suboptimal response to treatment, pharmacists can facilitate shared decision making by offering additional information in accordance with patient preference. All HCPs, including pharmacists, should proactively assist patients who are motivated to seek preventive healthcare during the perinatal period. They should also observe these patients for signs of evident change in mood and encourage them to report depressive symptoms.
REFERENCES
- Fisher J, Cabral de Mello M, Patel V, et al. Prevalence and determinants of common perinatal mental disorders in women in lowand lower-middle-income countries: a systematic review. Bull World Health Organ. 2012;90:139G-149G.
- Parsons CE, Young KS, Rochat TJ, et al. Postnatal depression and its effects on child development: a review of evidence from low- and middle-income countries. Br Med Bull. 2012;101:57-79.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Arlington, VA: American Psychiatric Publishing; 2013:186.
- Kanes S, Colquhoun H, Gunduz-Bruce H, et al. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet. 2017;390:480-489.
- Stewart DE, Vigod S. Postpartum depression. N Engl J Med. 2016;375:2177-2186.
- World Health Organization. Maternal and perinatal health. www. who.int/maternal_child_adolescent/topics/maternal/maternal_perinatal/en. Accessed June 24, 2019.
- Shakeel N, Eberhard-Gran M, Sletner L, et al. A prospective cohort study of depression in pregnancy, prevalence and risk factors in a multi-ethnic population. BMC Pregnancy Childbirth. 2015;15:5.
- den Besten-Bertholee D, van der Meer DH, ter Horst PGJ. Quality of lactation studies investigating antidepressants. Breastfeeding Med. 2019;14:359-365.
- Øystein Berle J, Spigset O. Antidepressant use during breastfeeding. Curr Womens Health Rev. 2011;7:28-34.
- Bennett IM, Marcus SC, Palmer SC, Coyne JC. Pregnancyrelated discontinuation of antidepressants and depression care visits among Medicaid recipients. Psychiatr Serv. 2010;61:386-391.
- Vesga-López O, Blanco C, Keyes K, et al. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry. 2008;65:805-815.
- Wisner KL, Sit DK, McShea MC, et al. Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. JAMA Psychiatry. 2013;70:490-498.
- Fellmeth G, Fazel M, Plugge E. Migration and perinatal mental health in women from low- and middle-income countries: a systematic review and meta-analysis. BJOG. 2017;124:742-752.
- Howard LM, Molyneaux E, Dennis CL, et al. Non-psychotic mental disorders in the perinatal period. Lancet. 2014;384:17751788.
- Mehta D, Newport DJ, Frishman G, et al. Early predictive biomarkers for postpartum depression point to a role for estrogen receptor signaling. Psychol Med. 2014;44:2309-2322.
- Stein A, Pearson RM, Goodman SH, et al. Effects of perinatal mental disorders on the fetus and child. Lancet. 2014;384:18001819.
- Grigoriadis S, Wilton AS, Kurdyak PA, et al. Perinatal suicide in Ontario, Canada: a 15-year population-based study. CMAJ. 2017;189:E1085-E1092.
- Khalifeh H, Hunt IM, Appleby L, Howard LM. Suicide in perinatal and non-perinatal women in contact with psychiatric services: 15 year findings from a UK national inquiry. Lancet Psychiatry. 2016;3:233-242.
- Petersen I, Gilbert RE, Evans SJ, et al. Pregnancy as a major determinant for discontinuation of antidepressants: an analysis of data from the Health Improvement Network. J Clin Psychiatry. 2011;72:979-985.
- Ross LE, Grigoriadis S, Mamisashvili L, et al. Selected pregnancy and delivery outcomes after exposure to antidepressant medication: a systematic review and meta-analysis. JAMA Psychiatry. 2013;70:436-443.
- Hunter SK, Mendoza JH, D'Anna K, et al. Antidepressants may mitigate the effects of prenatal maternal anxiety on infant auditory sensory gating. Am J Psychiatry. 2012;169:616-624.
- Bar-Oz B, Einarson T, Einarson A, et al. Paroxetine and congenital malformations: meta-analysis and consideration of potential confounding factors. Clin Ther. 2007;29:918-926.
- Grigoriadis S, VonderPorten EH, Mamisashvili L, et al. Antidepressant exposure during pregnancy and congenital malformations: is there an association? A systematic review and meta-analysis of the best evidence. J Clin Psychiatry. 2013;74:e293-e308.
- Dinatale M. The pregnancy and lactation labeling rule (PLLR). www.fda.gov/media/100406/download. Accessed June 29, 2019.
- Paxil (paroxetine) package insert. Weston, FL: Apotex; January 2017.
- World Health Organization. Breastfeeding. www.who.int/nutrition/topics/exclusive_breastfeeding/en. Accessed June 24, 2019.
- Epperson N, Czarkowski KA, Ward-O'Brien D, et al. Maternal sertraline treatment and serotonin transport in breast-feeding mother-infant pairs. Am J Psychiatry. 2001;158:1631-1637.
- FDA Center for Drug Evaluation and Research. Guidance for industry: pharmacokinetics in pregnancy—study design, data analysis, and impact on dosing and labeling. www.fda.gov/media/71353/ download. Accessed June 30, 2019.
- Sage Therapeutics. Sage Therapeutics announces FDA approval of ZULRESSO™ (brexanolone) injection, the first and only treatment specifically indicated for postpartum depression. https://investor.sagerx.com/news-releases/news-release-details/sage-therapeuticsannounces-fda-approval-zulressotm-brexanolone. Accessed June 20, 2019.
- Schumacher M, Mattern C, Ghoumari A, et al. Revisiting the roles of progesterone and allopregnanolone in the nervous system: resurgence of the progesterone receptors. Prog Neurobiol. 2014;113:6-39.
- Kanes S, Colquhoun H, Gunduz-Bruce H, et al. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet. 2017;390:480-489.
- Schüle C, Nothdurfter C, Rupprecht R. The role of allopregnanolone in depression and anxiety. Prog Neurobiol. 2014;113:79-87.
- FDA. FDA approves first treatment for post-partum depression. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ ucm633919.htm. Accessed June 20, 2019.
- Business Wire. Sage Therapeutics announces U.S. Drug Enforcement Administration scheduling of ZULRESSO™ (brexanolone) injection. www.businesswire.com/news/home/20190614005243/en. Accessed June 24, 2019.
- Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, doubleblind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392:1058-1070.
- Zulresso (brexanolone) package insert. Cambridge, MA: Sage Therapeutics, Inc; March 2019.
- American Academy of Pediatrics. Postpartum depression & breastfeeding. www.healthychildren.org/English/ages-stages/baby/ breastfeeding/Pages/Postpartum-Depression-Breastfeeding.aspx. Accessed June 29, 2019.