The Pharmacologic Management of Migraine
RELEASE DATE
January 1, 2022
EXPIRATION DATE
January 31, 2024
FACULTY
George A. DeMaagd, PharmD, BCPS
Professor of Pharmacy
Associate Dean of Academic Administration
Union University College of Pharmacy
Jackson, Tennessee
Kathryn Norville, PharmD, PGY1 Pharmacy Resident
Mayo Clinic, Department of Pharmacy
Phoenix, Arizona
Ashok Philip, PhD
Director of Assessment and Professor of Pharmaceutical Sciences
University of Arkansas for Medical Sciences, College of Pharmacy
Little Rock, Arkansas
FACULTY DISCLOSURE STATEMENTS
Drs. DeMaagd, Norville, and Philip have no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT
 Pharmacy
Pharmacy
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-22-003-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients’ conditions and possible contraindications or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
GOAL
To update participants on the epidemiology, pathophysiology, clinical presentation, diagnostic process, and treatment of migraine.
OBJECTIVES
After completing this activity, the participant should be able to:
- Describe the pathophysiological mechanisms and the clinical signs and symptoms of migraine.
- List some nonpharmacologic interventions for the management of migraine.
- Discuss the commonly used abortive (acute) and preventive (prophylactic) medications and their role in managing migraine.
- Understand the etiology and management of medication overuse headache.
ABSTRACT: Migraine headache has major effects on patients and their families, results in significant disability, and is a leading cause of outpatient and emergency-department visits. The pathophysiology of migraine has evolved over the years, and the most recent evidence points to the role of the trigeminovascular system. The clinical presentation of migraine is heterogeneous, and although it is described as having four phases, not all patients experience each phase. The management of migraine may include both abortive and preventive interventions, and although there is no cure for this disease, appropriate treatment can have an impact on quality of life. Pharmacists are often the front line for patients seeking advice on headaches, and an understanding of when to refer patients is important.
Migraine headache (HA) is a chronic neurologic disorder that has major effects on patients and their families, results in significant disability, and is a leading cause of outpatient and emergency-department (ED) visits. Migraine is the third most prevalent disease globally, affecting 14% of adults; it is three times more common in females, and 70% of patients have a family history. The common occurrence of migraine in young adults during their productive years impacts employment, relationships, parenting, and overall quality of life (QOL).1-8 Migraine results in billions of dollars in direct and indirect costs, and appropriate management has a significant effect on overall disease burden.9,10
PATHOPHYSIOLOGY
The pathophysiology of migraine has evolved over the years, and the most recent evidence points to the role of the trigeminovascular system (TGVS). Activation of the TGVS, with its peripheral components (meningeal vasculature) and central components (higher brain centers), involves the release of neuropeptides, e.g., calcitonin gene–related peptide (CGRP), neurotransmitters, and other proinflammatory substances, resulting in vasodilation and neurogenic inflammation. The result is a propagation of noceptive transmission throughout the TGVS that results in a hypersensitized pain environment, which now can be targeted by specific therapies that act on receptors within this system.11-15
DIAGNOSIS, PRESENTATION, AND CLASSIFICATION
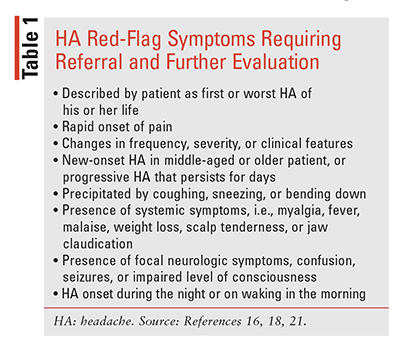
The clinical presentation of migraine is heterogeneous and the diagnostic process is challenging, often requiring a practitioner with experience in HA management. A comprehensive medical history should be conducted in order to rule out secondary HA causes, including potentially serious etiologies that require further evaluation (TABLE 1).16-20
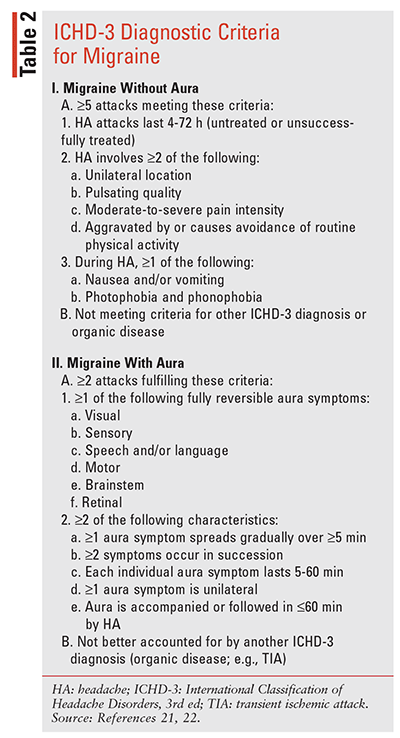
The Headache Classification Subcommittee of the International Headache Society has developed a comprehensive system for the classification of migraine (International Classification of Headache Disorders, 3rd edition; ICHD-3) that can assist in the diagnostic process (TABLE 2).21 ICHD-3 defines episodic migraine (EM) as fewer than 15 HA days/month and chronic migraine (CM) as 15 or more HA days/month for more than 3 months. EM and CM may be used to gauge treatment patterns and success, as patients may transition between these two HA phases.22,23 Because 60% of female migraine patients experience HAs associated with their menstrual cycle, the ICHD-3 also includes menstrual migraine types in its classification system.24,25
CLINICAL PRESENTATION
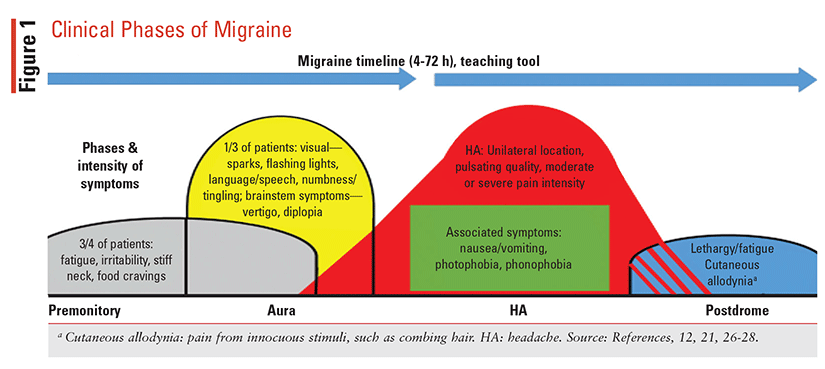
As noted, the clinical presentation of migraine is heterogeneous, and although it is described as having four phases, not all patients experience each phase. The phases—premonitory, aura, HA, and postdrome—and their features are described in FIGURE 1.12,21,26-28
TRIGGERS, RISK FACTORS, AND ASSOCIATED COMORBIDITIES
Migraine HAs are influenced by risk factors and triggers, and each patient’s individualized therapeutic plan should include a self-evaluation of potential triggers and exacerbating factors and their documentation in an HA diary. Triggers include stress, menses, weather changes, exposure to bright flashing lights, altered sleep patterns, allergens, and various odors. Food and chemical triggers include various substances found in foods (e.g., tyramine, caffeine, alcohol). Risk factors include altered sleep patterns, skipping meals, and dehydration.29-32 Migraine HAs are also associated with numerous comorbidities, including cerebrovascular disease (CBVD) and cardiovascular disease (CVD), psychiatric conditions (e.g., depression, anxiety), and others, including sleep disorders and autoimmune diseases. These comorbid conditions may complicate migraine presentation and affect its management.33-37
TREATMENT OVERVIEW
A comprehensive and individualized treatment approach should include patient and family education, risk factor evaluation, nonpharmacologic interventions, and pharmacologic therapies. Considerations should include frequency and severity of attacks, associated symptoms, level of disability, comorbidities, contraindications, concomitant medications, pregnancy/lactation status, prior treatment successes and failures, patient preference, costs, and expectations of the plan.38,39 Management may involve both acute (abortive) and preventive (prophylactic) interventions, and although there is no cure for migraine, appropriate treatment can have an impact on QOL.40-42
ABORTIVE (ACUTE) TREATMENT
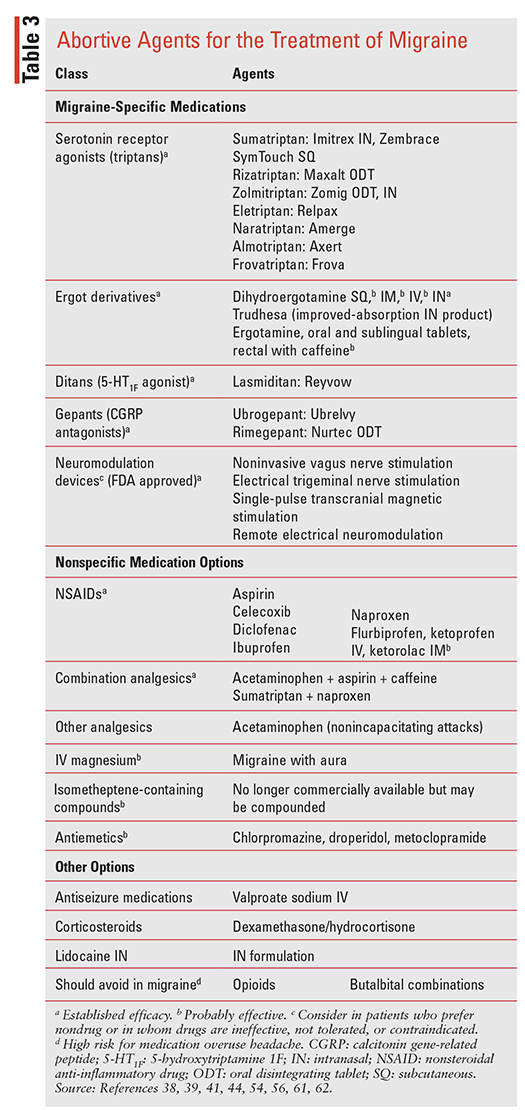
Abortive (acute) treatment of migraine may include risk management, nonpharmacologic options, or the use of abortive pharmacotherapies (TABLE 3). Therapy should be initiated early (e.g., premonitory phase), with the goals being pain and symptom relief/freedom within 2 hours, limited repeat dosing, avoidance of side effects, reduced disability, and lessened use of resources (e.g., ED visits).38,39,42,43 Nonpharmacologic abortive HA management includes interventions and devices supported by the recent consensus statement from the American Headache Society (AHS). Education on lifestyle modifications (e.g., proper nutrition, trigger identification, psychosocial interventions, sleep and stress management, quiet dark rooms, reduced sensory input, aerobic exercise, yoga, cold packs, acupuncture) may also offer relief.38,45-53 Neuromodulating devices should also be considered, especially in patients who are unable to tolerate or have contraindications to drugs or who prefer nondrug options. Four such devices are FDA approved (TABLE 3); they work by using electrical current or magnetic fields to modulate pain transmission, and they may be used alone or in combination with pharmacotherapy.54,55
Options for pharmacologic management include ergot derivatives, 5-hydroxytriptamine (5-HT) receptor agonists (triptans), various analgesics, antiemetics, and the recently approved migraine mechanism–specific drugs—ditans and gepants, which further target the 5-HT and CGRP receptor systems, respectively.11-15,55-58
TABLE 3 lists the available agents and notes their established or probable efficacy as reported by the recent AHS consensus statement.38,42,43,56-58 Treatment usually involves a stratified approach, with analgesics used for mild attacks and additional options including triptans, dihydroergotamine (DHE), gepants, or ditans considered for moderate-to-severe attacks.38,39,59-62 In some cases, repeat dosing or combinations of these medications may be needed to relieve the migraine and accompanying symptoms.38,42,43,56-58 Other factors that require consideration in the abortive treatment plan include gastric stasis and nausea/vomiting, which may influence drug absorption and compliance.38,42,43 Management should also include education on the risk of medication overuse headache (MOH; discussed below).44
Analgesics
The analgesics, including aspirin (ASA; alone or in combination with caffeine), other nonsteroidal anti-inflammatory drugs (NSAIDs), and acetaminophen (APAP), may be useful for mild-to-moderate migraine attacks.63-78 APAP is less effective than NSAIDs, although it is beneficial as a component of combination analgesics (e.g., combined with ASA and caffeine), as it works synergistically.65,79-88 In doses of 900 mg to 1,000 mg, ASA may be beneficial and cost-effective, although tolerability may be problematic; this can be improved if it is combined with an antiemetic.66,89-96 Other NSAIDs are reported to be effective options for mild-to-severe migraine pain and associated symptoms, either as an alternative to or in combination with a triptan.67-78 Pharmacokinetic (PK) differences between NSAIDs provide options for faster onset (e.g., ibuprofen) or longer duration (e.g., naproxen).73,74 Recently, an oral solution of celecoxib with improved solubility and absorption was approved for migraine treatment. NSAIDs’ side effects may limit their role in some patients, although the addition of an antiemetic may improve tolerability.97 Other analgesics, including tramadol, which has limited data, and isometheptene, which is no longer available and therefore must be compounded, are not recommended.98-100
Although previously they were commonly prescribed for migraine, opiates or compounds containing a barbiturate (butalbital) should be avoided in migraine owing to lack of efficacy, abuse potential, additive disability, and strong association with MOH.44,101-111 MOH is a chronic, often unrecognized disorder that affects millions of people in the United States and has a worldwide prevalence of 1% to 2%.44,112-117 The ICHD-3 defines MOH as HAs occurring on ≥15 days per month for ≥3 months while the patient is using opioids, barbiturates, combination analgesics, triptans, or ergots ≥10 days per month or simple analgesics ≥15 days per month.21 The pathology of MOH includes a central hypersensitivity (opioids) and/or dysregulation of glutamatergic/serotonergic transmitter systems.118-120 Management includes treatment protocols that involve tapering off the offending agent, preventive therapy, and education to avoid patterns of overuse that result in this syndrome.121,122
Serotonergic Drugs
The serotonergic drugs utilized in the acute treatment of migraine include historical ergotamine derivatives, 5-HT receptor agonists (triptans), and the recently approved ditan (lasmiditan). Each of these drug classes is beneficial for migraine pain and related symptoms, with action at various 5-HT receptors and other neurotransmitter systems, e.g., ergots.123-128
Ergots or Ergot Alkaloids (EAs): Derived from a fungus, the EAs were first identified for HA efficacy in the 1800s, but their use is limited today because of the availability of better-tolerated alternatives.127-129 Their broad spectrum of 5-HT, adrenergic, and dopaminergic receptor activity provides efficacy in migraine similar to that of the triptans, although side effects and adverse reactions (ARs) are problematic. The two available EAs are ergotamine (rarely used in U.S.) and DHE; DHE is the preferred agent owing to fewer vasoconstrictive properties, improved efficacy, tolerability, and multiple administration options, including intravenous (IV), intramuscular (IM), subcutaneous (SQ), and intranasal (IN). DHE’s onset occurs within minutes, with the IN formulation offering the fastest response and the option of patient self-administration.127-130
EAs’ side effects and ARs are well documented, including nausea/vomiting, altered sense of taste, difficulty swallowing, dizziness, flushing, cramps, paresthesia, rhinitis, and fatigue. More serious ARs are associated with these agents’ vasoconstrictive properties, including ergotism, gangrene, cerebral ischemia and cardiac concerns (boxed warning), resulting in numerous contraindications in the vascular area, along with pregnancy, lactation, postmenopausal status, and basilar and hemiplegic migraine. Potential drug interactions include serotonergic drugs, some antidepressants, triptan use within 24 hours, and ischemic potentiation (boxed warning) from use with CYP3A4 pathway inhibitors (e.g., antifungals, others).131-138
The role of ergots in migraine is limited, although they may have utility in some triptan nonresponders, refractory patients, and patients with HA-recurrence issues. First-dose clinic administration is recommended based on risk assessment, along with concurrent use of antiemetics.139-142 A novel DHE IN delivery formulation released in 2021 uses a breath-triggered system to improve drug delivery.143 Education on the appropriate use of these products by providers and pharmacists is essential to ensure the best outcomes.
Selective 5-HT Receptor Agonists (Triptans): The triptans have had the greatest impact on acute migraine treatment over the past 30 years. Their 5-HT1B and 5-HT1D receptor action provides vasoconstriction, inhibits release of vasoactive peptides, and blocks nociceptive pain transmission, offering a more selective receptor profile versus the ergots.144-149
The approval of sumatriptan (SMT) in 1990 was followed by the arrival of six more triptans and an SMT combination with naproxen. All of the triptans have demonstrated efficacy in the abortive treatment of migraine, although PK differences may provide some differentiation within the class.150-157 Agents with the fastest onset of action include SQ, SMT, and IN SMT and zolmitriptan, with onset in 10 to 20 minutes.
The oral agents have an onset of action ranging from 30 to 120 minutes, with faster onset related to improved bioavailability and/or central nervous system (CNS) penetration. Orally disintegrating tablets (ODTs) are perceived to have a faster onset versus other forms. Frovatriptan (FVT) and naratriptan (NAT) have the longest half-life, supporting longer duration and potential for less HA recurrence. Metabolism is via the CYP450 and/or monoamine oxidase (MAO)-A system, supporting evaluation for significance in patients with hepatic disease or on concurrent drugs. NAT and almotriptan have a greater dependence on renal elimination, supporting dose adjustments in some patients.158-162 Owing to their greater receptor selectivity, the triptans are usually well tolerated; side effects include dizziness, flushing, myalgias, paresthesias, mild injection-site reactions (SQ), and nasal-cavity discomfort (IN). More severe ARs, although rare, include cardiac events; however, chest sensations may be due to effects on esophageal motor function.150-156,163-168
Contraindications to triptans include CVD and CBVD (or family history) and certain risk factors, e.g., postmenopausal status, age >40 years, smoking, obesity, diabetes, other ischemic disorders, and hemiplegic or basilar migraine. Considerations for use in high-risk patients include pretreatment screening and first-dose observation. Although triptans are not recommended in pregnant patients, there is lack of evidence supporting harmful outcomes.165-174 Drug interactions include multiple concurrent serotonergic agents, ergots within 24 hours, inhibitors of CYP450 3A4 and 2D6 systems (e.g., paroxetine, ketoconazole), and MAO inhibitors, with triptans metabolized by this pathway.175-182 Another potential interaction with questionable significance is propranolol’s inhibition of MAO-A metabolism in triptans metabolized by this pathway, e.g., rizatriptan.183
Clinical trials of the triptans for abortive treatment of migraine have reported efficacy in 50% to 90% of patients, with similar, improved, or faster onset and greater HA freedom reported in some trials versus comparator abortives.184-187 The combination of SMT and naproxen was found to have additive effects and good tolerability in the acute management of migraine.188 The triptans are considered first-line options in patients with moderate-to-severe attacks, or for mild migraine in patients intolerant or nonresponsive to analgesics. The choice of triptan may involve multiple factors. The use of SQ and IN may be good options if a faster onset is needed or if the patient is experiencing nausea/vomiting or responded poorly to oral agents. The use of FVT and NAT may be beneficial in patients with HA-recurrence issues or for short-term prophylaxis of menstrual migraine (5-6 days).24,25,189-196 Other considerations include cost, previous response and/or tolerability, and patient preference.197-211 Administration should occur at the first sign or symptom of migraine, ideally in the premonitory phase. Approximately 30% of patients have an unsatisfactory response to triptans; however, an adequate trial of at least two drugs in the class is recommended prior to considering other options.38,39,212,213 Although MOH is uncommon with triptans, it is recommended to limit triptan use to ≤10 days per month.214
When dispensing triptans, pharmacists should play a major role in monitoring and educating patients about their appropriate use, potential side effects, and expected efficacy.
Recently Approved Abortive Agents
Ditans: The oral ditan lasmiditan (LMT; Reyvow), which was approved in 2019, has selective properties targeting 5-HT1F receptors throughout the TGVS, and its lack of vasoconstrictive properties differentiates it from the triptans. LMT binds to the 5-HT1F receptor ≥400 times more versus the triptans, providing a potentially safer agent for CVD and CBVD.215-217
LMT’s approval was based on two randomized, placebo-controlled, double-blind trials in more than 3,000 patients with migraine, with results at all doses, that reported a reduction in pain and other migraine symptoms similar to triptan responses; no additional abortives were administered during the study, but 20% of patients were on concurrent preventives.218-222 LMT is rapidly absorbed within 2 hours without interference from food, and metabolism is primarily via non-CYP enzymes with no active metabolites and insignificant renal clearance. The drug was not studied in severe hepatic disease. Significant side effects with LMT are primarily CNS-related (25%-40%) and include lethargy, dizziness, and sedation, resulting in labeling advising patients not to drive or perform activities requiring mental alertness for 8 hours post dose; the drug is labeled FDA schedule V although there is no evidence of high-level abuse. Other significant side effects include gastrointestinal effects and paresthesias. Drug interactions include cautious use with alcohol/other CNS depressants and other serotonergic agents, including avoidance of ergots and triptans within 24 hours. Dosing is 50-mg to 200-mg tablets as one dose per 24 hours regardless of original dose, and the safety of treating four or more attacks over 30 days is unclear. Similar to the triptans, caution is advised in order to avoid MOH.215-217 The AHS recommends the use of LMT as an option in migraine patients who are older than 18 years and are intolerant or unresponsive to two or more tripans, as determined by a validated screening tool or clinician verification.38,39,223
Gepants: The gepants, the first of which became available in 2020, are a class of small-molecule, oral CGRP antagonists approved for the abortive treatment of migraine. Their small size, short half-lives, and penetration of the blood-brain barrier enable targeting of the CGRP receptor in the peripheral and central TGVS.38,39,224-228 In addition to abortive use, approval has been granted to some agents in the class for the prevention of migraine (discussed below).
Ubrogepant (UBP), the first approved agent in this class, peaks after oral absorption in 1.5 hours, with high-fat foods delaying peaks only. Metabolism is via the CYP3A4 pathway, with insignificant metabolites and 40% of the parent drug recovered unchanged in the feces or urine. Initial dosing is 50 mg to 100 mg, and the dose may be repeated in 2 hours (maximum 200 mg/24 hours); a reduction of 50% is recommended in severe renal or hepatic disease, and avoidance is recommended in the case of end-stage disease. Approval of UBP was based on data from two randomized, double-blind, placebo-controlled trials that found a significant reduction in migraine pain, associated symptoms, and functional disability within 2 hours after administration. Trial patients were on concurrent preventive therapies, including topiramate, botox, propranolol, and amitriptyline. UBP may be used to treat up to eight migraines in a 30-day period. Reported side effects include nausea, somnolence, dry mouth, and treatment-emergent respiratory infections. The liver-enzyme elevations reported in the trials were considered unlikely related to UBP, and the drug appeared safe and well tolerated at 1 year. Use in pregnancy and lactation is not recommended based on embryofetal effects and weight loss, respectively, in animal models. UBP is associated with significant drug interactions involving the CYP3A4 pathway. Recommendations include avoidance with strong inhibitors of the CYP3A4 pathway (e.g., clarithromycin), dose reductions with weak or moderate inhibitors (e.g., fluconazole), and avoidance with strong CYP3A4 inducers (e.g., phenytoin, rifampin). In addition, UBP dose reductions are recommended with concurrent use of breast cancer resistance protein (BCRP) or P-glycoprotein (P-gp) inhibitors.229-234
Rimegepant (RMP), which was approved soon after UBP, offers an ODT option for the abortive treatment of migraine. RMP’s PK profile is similar to that of UBP, and the drug is dosed at 75 mg daily with no additional dosing for 24 hours. Approval of RMP was based on results from a randomized, double-blind, placebo-controlled trial that demonstrated significant reductions in moderate-to-severe migraine and associated symptoms at 1 to 2 hours post dose. Rescue abortive therapy was allowed, but the majority of patients used only their RMP dose in 24 hours. As with the UBP studies, some patients were on other preventive therapies. RMP was well tolerated, with nausea and urinary tract infections found to be more common versus placebo. Contraindications include history of hypersensitivity and severe hepatic impairment.235-238 Human data in pregnancy are lacking, but animal studies report fetal variations and weight loss at higher doses. No information supports a role for this agent in renal and hepatic impairment. Similar to UBP, RMP is associated with significant drug interactions involving the CYP3A4 system, and recommendations include avoidance with strong inhibitors, avoidance of additional doses within 48 hours with moderate inhibitors, and avoidance with moderate and strong CYP3A4 inducers. In addition, this agent should be avoided with concurrent inhibitors of the P-gp and BCRP efflux systems.224,226-228 The AHS recommends gepants as an alternative option in migraine patients older than 18 years who are intolerant or unresponsive to two or more tripans, as determined by a validated screening tool or clinician verification.38,39,223
Other Abortive Agents: Other nonmigraine mechanism– specific, non–FDA-approved abortive agents used for migraine include a variety of traditional therapies.239-243 Antiemetics, including the phenothiazine derivatives (chlorpromazine, prochlorperazine), metoclopramide, and the butyrophenones (droperidol), are useful for nausea associated with migraine but may benefit HA pain as well. Their action against pain may involve reduction of hypersensitivity to dopamine or suppression of the TGVS activation and/or nociception occurring in migraine.244 Small trials, case reports, and anecdotal evidence on various antiemetics of varying dosages and formulations reported efficacy versus placebo as well as similar, less, or greater efficacy versus comparators within 2 hours of administration.245-271
Although antiemetics are reported as safe, the butyrophenone droperidol has a boxed warning for proarrhythmic risk, and the entire class carries a dose-related risk of extrapyramidal side effects.272 Antiemetics may offer an alternative to other migraine abortives, including analgesics, triptans, ergots, and others, and may be a more appropriate option versus opioids in the ED setting.273-276 Low magnesium levels and their correlation with migraine attacks have suggested a role for magnesium sulfate in the treatment of migraine. Small trials have yielded inconsistent findings, although the AHS considers IV forms as probably effective in migraine with aura, offering a nonsedating alternative for selected patients.38,277-286 Small multicenter studies and cases series of valproate (VPA) IV have reported reductions in HA intensity and other migraine symptoms as well as similar or less efficacy versus comparators. VPA should be avoided in women of childbearing years but may be an alternative for some patients.287-292
Small trials of IN lidocaine have demonstrated inconsistent findings, although synergy has been reported to reduce migraine intensity when used in combination with antiemetics or NSAIDs.293-297 Other formulations of lidocaine have been used for migraine as a component of nerve blocks.298-300 The benefits and tolerability of glucocorticoids in acute migraine were reported in small trials and case reviews as dose-dependent responses, with one report noting less HA recurrence compared with opioids, suggesting an option in the ED setting.301-311 Other agents with limited data to support a role in aborting migraine include calcium channel blockers, granisetron, diphenhydramine, trimethobenazamide, and complementary agents such as feverfew, ginger, and peppermint oil.312-323
PREVENTIVE (PROPHYLACTIC) PHARMACOTHERAPY
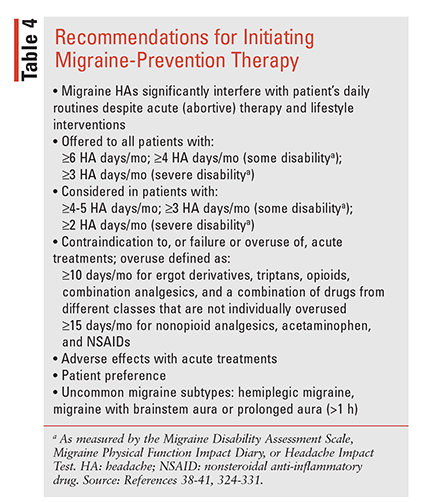
Prevention, the other component of migraine therapy, refers to the regular administration of medications for 6 to 12 months or longer while assessing response and tolerability. The overall goals of preventive (prophylactic) therapy include reducing the frequency, severity, and duration of HA attacks and reducing the need for and/ or improving responsiveness to abortive therapies and improving QOL. Criteria for qualifying a patient for preventive therapy (TABLE 4) are based on HA frequency, disability, and tolerability of acute therapies in addition to patient preference.38-41,324-331
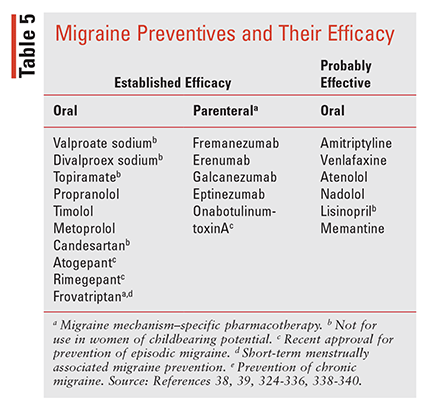
Evidence on the use of existing oral migraine preventive therapies indicates that approximately 3% to 13% of patients receive therapy, although up to 40% of EM and the majority of CM patients could benefit.38-41 Selection of the appropriate preventive therapy should be individualized using the frequency criteria in TABLE 4, along with considerations that include medication history (responses, tolerability), route, frequency of administration, pregnancy and breastfeeding status, comorbidities, potential drug interactions, and patient and provider preference. In addition, therapies should be coupled with patient and family education, lifestyle modifications, trigger tracking, and documentation in an HA diary.332-336 The AHS recently published an updated consensus statement on medications with “established efficacy”for migraine (supported by ≥2 class I trials) or “probably effective” (based on one or two class II trials), as defined by the American Academy of Neurology (AAN) evidence classification system.38-39,337 Preventive medications comprise nonmigraine mechanism–specific drug classes including beta-blockers, antiseizure agents, antidepressants (ATs), angiotensin receptor blockers (ARBs), and ACE inhibitors (TABLE 5) as well as migraine mechanism–specific agents including onabotulinumtoxinA (OBT), monoclonal antibodies (mAbs) targeting CGRP (TABLE 6), and recently approved gepants.338-340
Current first-line preventive options recommended by the the AHS are the nonmigraine mechanism–specific oral agents, including beta-blockers, antiepileptics, antidepressants, and the ARB candesartan, each of which has established efficacy in preventing migraine.38,39 The basic principles for starting these oral therapies are to initiate therapy with low doses and titrate upward every 2 weeks based on response and tolerability with trials of ≥2 to 3 months, although full benefits may take up to 6 months.
Success in migraine prevention includes reductions in attack frequency, defined as a 50% reduction in monthly migraine days (MMDs); decreases in attack severity and duration; improved response to acute treatments; reductions in disability and psychological distress; and improvements in QOL. If a patient experiences tolerability issues or lack of response after an adequate trial with one medication, alternative medications and/or combinations may be considered.324-339
Beta-Blockers
Beta-blockers, which have been used for preventive treatment of migraine since the mid-1900s, are FDA approved and are considered a treatment of choice in patients without contraindications. The in-class choices with trials supporting utility include propranolol, timolol, metoprolol, and nadolol; data are limited for atenolol, and avoidance of agents with intrinsic sympathomimetic activity is recommended. Migraine patients usually tolerate beta-blockers well, and trials have reported a 50% reduction in the frequency of MMDs.341 Beta-blockers’ mechanism of action (MOA) in migraine may involve modulating adrenergic and/or serotonergic neurotransmission in cortical or subcortical pathways.338 In addition to their co-benefits in patients with other comorbidities, the usual precautions and/or contraindications need to be considered, including asthma, bradycardia, and male sexual-function concerns.338,341
Antiseizure Medications
The antiseizure medications divalproex sodium and topiramate are FDA approved for migraine, with data reporting a ≥50% reduction in MMDs. Their proposed MOA includes enhancement of gamma-aminobutyric acid and/or modulation of neurotransmitter activity. The major limitation to their use is the risk of teratogenic effects, especially with valproic acid derivatives, and to a lesser extent with topiramate. Both should be avoided in pregnancy and in women of childbearing years. Their side-effect profile may also be problematic, especially their CNS effects (e.g., sedation, cognitive issues), which are more common with topiramate and may impact work and school performance. Divalproex sodium and topiramate should be dosed with slow titration to response and tolerability. Both agents may be options for patients with concurrent seizure disorders, and some patients may benefit from topiramate’s effect on weight loss. Contraindications and cautions include teratogenicity, hepatotoxicity (with divalproex sodium), and the risk of metabolic acidosis, myopia, and oligohydrosis (with topiramate).342-346 Other anticonvulsants that are utilized for aborting migraine include zonisamide, lamotrigine, and levetiracetam, although information supporting their role is limited.344
Antidepressants
ATs used in migraine prevention include the tricyclic antidepressants (TCAs) amitriptyline and nortriptyline and the serotonin-norepinephrine reuptake inhibitors (SNRIs) venlafaxine and duloxetine, with proposed MOAs involving modulation of neurotransmitters. The TCA class has the majority of data for use in migraine, especially amitriptyline, with a 50% response reported in trials. Nortriptyline may be a better-tolerated option; however, the sedating effects of both agents may benefit comorbid insomnia. Low initial dosing at bedtime is recommended with slow titration and monitoring, especially for anticholinergic side effects. Contraindications include a history of cardiac-rhythm disturbances. SNRIs have limited evidence supporting their role, but they may be an option in patients with comorbid depression and anxiety. Awareness of antidepressants’ boxed warning for suicidality risk is a relevant factor in young migraine patients.347,348
ARBs and ACE Inhibitors
The AHS lists the ARB candesartan as having established efficacy in migraine prevention based on clinical trials and a systematic review that reported a significant reduction in MMDs versus placebo and similar efficacy versus propranolol.349-352 Open-label and systematicanalysis data with ACE inhibitors in migraine prevention demonstrated a reduction in migraine frequency and severity with lisinopril/ramipril and enalapril, respectively.351,352 Although the MOAs of ARBs and ACE inhibitors are not entirely clear, serum levels of ACE are lower and angiotensin II and angiotensin levels are elevated in migraine patients, suggesting a relationship. In addition, the lipophilic agents in these drug classes appear to be more effective. The role of ARBs and ACE inhibitors in migraine will continue to evolve, and these agents are a preventive option, especially in patients with concurrent hypertension.349-352
MIGRAINE MECHANISM–SPECIFIC INJECTABLE PHARMACOTHERAPY
Currently, five injectable FDA-approved migraine mechanism–specific preventive therapies are available in the U.S.: OBT injection (approved in 2010) and four mAbs that target CGRP (approved between 2018 and 2020).38,39 In addition, two oral gepants, RMP and atogepant, were recently approved for the prevention of migraine.224,226,227,340
OBT (Botox)
The benefits of OBT in the prevention of migraine were discovered during the drug’s use for cosmetic purposes. It is proposed that OBT’s MOA in migraine is that it blocks the release of neuropeptides (e.g., CGRP, other proinflammatory substances, acetylcholine) and reduces the peripheral sensitization associated with migraine.353,354 Clinical trials and systematic reviews have reported an increase in HA-free days and a reduction in migraine frequency in 30% to 50% of patients, along with improved QOL and a favorable safety profile in CM and MOH patients.355-357 Another study found that 66% of patients who obtain a >50% response to OBT within their third course of treatment maintain this response over time.358
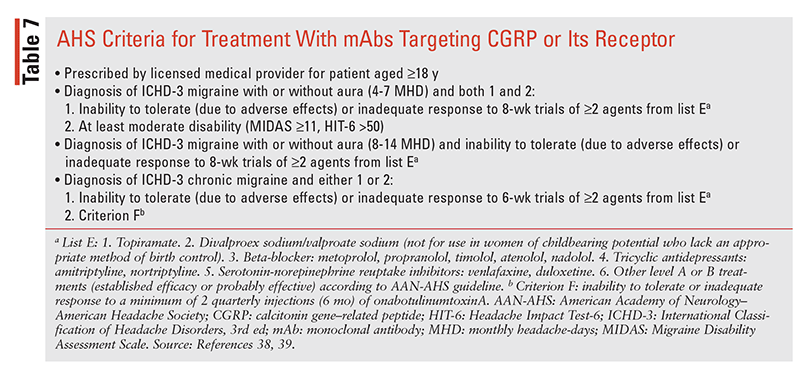
The AHS supports OBT use in patients with CM unless they cannot tolerate or have an inadequate response to a minimum of two quarterly injections (6 months).38,39 Nonserious ARs, reported in 60% of OBT patients versus 47% of placebo patients, included neck pain and weakness, spasms, stiffness, bronchitis, diplopia, ptosis, and facial paresis.355-358 OBT has a boxed warning for distant spread of toxin effect beyond injection areas in hours to weeks after treatment, and case reports describe dysphagia, breathing difficulties, and deaths, with the highest risk in children. Other serious events include hypersensitivity reactions and a rare risk of transferring (albumin content) the fatal viral disease/ variant Creutzfeldt-Jakob disease. In addition, caution is recommended for coadministration with drugs that interfere with neuromuscular transmission, e.g., aminoglycosides, and drugs with an additive anticholinergic side-effect profile. OBT is administered with follow-up and evaluation for response at dosing intervals. The recommended dosing protocol is injection of 155 units (optimal dose) at 31 to 39 sites every 12 weeks; higher doses have been administered, however, and a higher dose protocol is approved by the European Union.354,357
CGRP Antagonist mAbs
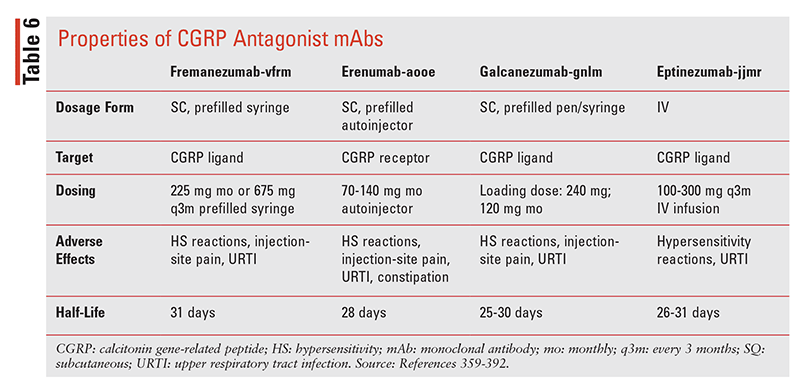
The mAbs targeting CGRP or its receptor have had a significant impact on the preventive management of migraine over the past 4 years. As discussed in the Pathophysiology section, CGRP and its association with TGVS are integral to migraine pain and related features.359 The four available agents (TABLES 5, 6) are erenumab (ENB; Aimovig), a CGRP receptor blocker, and galcanezumab (GZB; Emgality), fremanezumab (FZB; Ajovy), and eptinezumab (EZB; Vyepti), which act as ligand binders to CGRP. Each of these agents has reported efficacy in migraine prevention, may be administered without regard to dose titration, has an onset of action of days to weeks, and is effective and recommended in nonresponders or those intolerant to other preventives (TABLE 7).38,39,359-362 General properties of the four mAbs are listed in TABLE 6, including adverse drug reactions (ADRs) and basic kinetics, and other properties are discussed in the following sections.
ENB: ENB, the first mAb to be approved (2018), differs from the others in its class by acting as a CGRP receptor antagonist. It is administered as a monthly SQ dose, peaks in 6 days, reaches steady state in 3 months, and is metabolized by nonspecific proteolytic pathways. Clinical studies in both EM and CM noted reductions in MMDs, associated symptoms, and acute medication use, along with improved QOL and effectiveness in nonresponders to previous preventive therapies.361-367 No drug interactions were reported and the safety profile was similar to placebo. Reported ADRs are listed in TABLE 6, and the one side effect that appears unique to ENB versus other mAbs is constipation.361 In addition, the FDA recently added the potential for elevated blood pressure to ENB labeling based on reports of this finding during postmarketing surveillance, and monitoring is required.368 ENB should be used with caution in patients with underlying hypertension and avoided in patients with latex allergies and constipation issues.
GZB: GZB is one of the three mAbs that antagonize the activity of CGRP by binding to the ligand itself. Dosing is SC and involves a loading dose followed by monthly dosing. Its PKs are similar to other agents in the class, with peak levels in 5 days and metabolism via catabolic pathways similar to endogenous immunoglobulin G, and no drug interactions reported. Clinical studies in both EM and CM found reductions in MMDs, associated symptoms, and disability as well as effectiveness in nonresponders to previous preventive therapies.369-373 In addition, GZB was considered safe and well tolerated and had minimal side effects, with recent data compiled from controlled trials reporting no severe adverse effects.362,374,375
FZB: FZB’s MOA is similar to that of GZB. Dosing is SC, and administration may be monthly or quarterly. Its PKs are similar to other agents in the class, with peak levels in 5 to 7 days, steady state in 6 months, and metabolism by enzymatic proteolysis, and no drug interactions were reported. Clinical studies in EM and CM using both dosing regiments reported reductions in HA frequency, associated symptoms, and acute medication use as well as efficacy in refractory patients who had failed up to four previous preventives.360,376-379
EZB: Similar to FZB and GZB, EZB’s MOA is via binding to the CGRP ligand, but this agent is the first in its class to be administered quarterly by IV infusion. The IV formulation offers the fastest onset of action in the class although its PK and other properties are similar to the other agents.359 Clinical studies in EM and CM reported reductions in MMDs and associated symptoms as well as an early and sustained response; in addition, EZB was well tolerated and had an acceptable safety profile.380-383
Safety of CGRP Antagonist mAbs
The primary concern with any new class of medications is its safety profile over time, and data are accumulating in this area for the mAbs.384-392 mAbs and anti-CGRP agents have been 90% to 100% humanized but still have the potential to provoke an immune reaction; the prevalence is low, however, ranging from <1% to 18%, and ARs related to these reactions are rare.359-362,384 Additional concerns with the mAbs are their antagonism of CGRP and this neuropeptide’s baseline involvement in various physiological processes. These concerns are related to CGRP’s involvement in compensatory processes and its chronic inhibition and theoretical risks, e.g., response to ischemia in patients with comorbid brain pathology.385 Although these concerns need further evaluation, some study data indicated that plasma CGRP is detectable in patients treated chronically with anti-CGRP agents, suggesting that a level of this peptide and receptor activity are maintained.390
Safety data from open-label extension trials with ENB reported no increased risk of CVD, CBVD, or peripheral vascular disease in low-risk populations, e.g., no vascular events within 12 months.386,387 Regarding ENB’s influence on exercise time in stable angina patients, no significant differences were found in time to onset of ≥1 mm ST-segment depression, exercise-induced angina, and duration, and there were no reports of infarctions or failing compensatory mechanisms.388
Analysis of GZB trial data demonstrated no significant CVD adverse events related to blood pressure, pulse, or ECG parameters in the treatment groups.389 As noted above, based on recent labeling changes, patients placed on ENB, especially those with a history of hypertension, must be monitored for blood pressure elevation during the first few weeks of therapy.361,391 mAb use during pregnancy and lactation continues to be evaluated; data are limited, and no controlled studies are available. Recent reports in women who received mAbs prior to and during pregnancy and in lactating women suggested that mAbs may be safe. More research is needed to ensure the safety of these agents in pregnant and lactating women, and at the present time they are not recommended.392 Although initial data from clinical trials and follow-up analysis appear to indicate tolerability and safety, continued surveillance, monitoring, and reporting are necessary to ensure safety with long-term use.38,39,359-362
Gepants and Other Preventive Therapies
Two agents in the gepant class recently were approved for prevention of EM, adding to the body of oral preventive options. RMP, initially approved for abortive treatment of migraine, is now indicated for migraine prevention as well, and up to 18 doses per month may be used for both aspects of migraine care.340 Additionally, atogepant was recently approved for prevention of EM, with daily dosing.393
Many other medications and complementary therapies have been utilized for prevention of migraine, with variable results. The presence of elevated glutamate levels in migraine led to assessment of the glutamate antagonist memantine, with a small trial and a systemic review suggesting benefits.394,395 The antiseizure drug lamotrigine has been reported to improve aura in an open-label study. Small clinical trials and case reports have evaluated numerous supplement therapies for migraine prevention, with mixed results; some of the supplements studied include oral magnesium, riboflavin, feverfew, vitamin B6/folic acid, melatonin, omega-3 fatty acids, vitamin D, ginger extract, and their combinations.396-399 Mindfulness-based stress-reduction programs, yoga, and tai chi may be adjunctive treatment options for some patients, as reduced HA frequency, pain intensity, and acute medication use, as well as improved QOL, have been reported. Manual therapies include osteopathic manipulation, massage and reflexology, acupuncture, and exercise programs. Small trials examining some of these therapies have reported reduced migraine days, frequency, duration, intensity, and disability compared with controls. The AAN is currently in the process of updating its guidance document for complementary therapies.45,57,317,318
Three neuromodulation devices—electrical trigeminal nerve stimulation, noninvasive vagus nerve stimulation, and single-pulse transcranial magnetic stimulation (TABLE 3)—are FDA approved for migraine prevention as monotherapy or used adjunctively, as described in the Abortive Treatment section. Although studies using these devices exhibit positive results, their use in clinical practice has been limited. A trial of one of these devices should be considered for migraine prevention in all patients as an adjunct to existing treatment. Patients with inadequate responses or poor tolerability to medications, those at risk for MOH or chronic HA, and those who prefer to avoid medications are particularly good candidates for these devices.38,39,46,54,55
Combination Preventive Therapy
Up to 60% of patients achieve some improvement with preventive monotherapy, but approximately 30% fail to respond or cannot tolerate the available medications, especially the nonmigraine mechanism– specific oral agents. Numerous combination preventives have been used, especially combinations of nonmigraine mechanism–specific agents. Some experts recommend monotherapy trials of at least two different agents for approximately 6 to 8 weeks prior to considering combination therapy.38,400 Medications studied and utilized in combination for migraine prevention include beta-blockers (propranolol, nadolol), amitriptyline, valproate, and topiramate. Additive reductions in MMDs have been reported for combinations of beta-blockers plus amitriptyline, divalproex, or topiramate. Considerations for these combination therapies include additive side-effect profiles and potential drug interactions, e.g., metabolic inhibition of amitriptyline by divalproex.401-405 Other reported combinations include riboflavin or magnesium and lamotrigine (when auras are bothersome) and atorvastatin and magnesium in combination with sodium valproate.406,407 The combination of amitriptyline and cognitive behavioral therapy was found to be effective in children and adolescents with migraine.408
The anti-CGRP mAbs have been investigated in patients on concurrent nonmigraine mechanism–specific therapies (e.g., beta-blockers, antiseizure drugs), and they are often initiated in patients on these existing preventive therapies. A recent retrospective chart review demonstrated reductions in MMDs, HA intensity, and disability and indicated tolerability in patients receiving mABs and OBT.409 Because mAbs are often initiated in patients on oral preventives (e.g., beta-blockers, antiseizure drugs), plans to reduce the dosage or taper off dual therapy should involve patient and practitioner working together based on response and tolerability.359-362
Selection and Management of Preventive Therapy
Selection of initial preventive therapy should take an individualized approach that considers HA severity, concurrent medications, contraindications, comorbidities, previous responses, pregnancy or childbearing age, patient preferences, and clinician experience. Partial responses or dose-limiting ARs may warrant alternative or—in some cases—combination therapies. Minimizing polypharmacy is important, but practitioners contemplating a single drug for multiple comorbidities should consider the risk of undertreating one condition. Drug therapy should be avoided in pregnant or lactating females and women trying to conceive; rather, nonpharmacologic interventions, plans, and education should be used.410 Neuromodulatory devices may also be effective in combination with other therapies and should be considered in all patients, especially if medications must be limited or avoided.
With the availability of the mAbs and OBT, the clinician has more options for preventive therapy and must balance efficacy, tolerability, and cost-effectiveness. The AHS recommends the use of evidence-based preventive treatments, when indicated, based on the options in TABLE 5, and the criteria in TABLE 7 should be followed regarding when to use the mAbs and OBT.38,39 Migraine is a disease that can fluctuate over time, with periods of remission and potentiation, and therapy should be evaluated on an ongoing basis. Discontinuation of therapy should involve joint decisions between patient and practitioner, as stopping and reinstituting therapy does not always restore HA control.411
Patient compliance with oral preventive treatment is poor, likely because of lack of effectiveness or tolerability issues. Compliance declines with time; only 17% to 20% of patients remain on oral preventives after 1 year, although recently approved monthly or quarterly injectables may result in improvement. Involving the patient in the therapeutic plan is important, as is clinician- and pharmacist-provided education on treatment expectations and goals, the importance of compliance, and the knowledge that overuse of the abortive agent can interfere with response to the preventive.412-415
THE PHARMACIST’S ROLE
Pharmacists are often the front line for patients seeking advice regarding HAs, and an understanding of when to refer patients is important. Pharmacists can triage patients based on red flags and can use available migraine screening tools, including the ID Migraine Screener and the Migraine Disability Assessment Questionaire (MIDAS).416 In addition, resources such as the National Headache Foundation and American Migraine Foundation websites provide essential information. Pharmacists can also assist with appropriate use of OTC medications and educate patients on the risks of MOH. Pharmacists should be involved in the interprofessional team approach to migraine care, ensure appropriate education, assist in the triage of HAs, perform therapeutic drug monitoring, and interact with third-party payers to help ensure optimal outcomes and cost-effective migraine care.19,47,95,417
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
- Lipton RB, Silberstein S. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention. Headache. 2015;55(suppl 2):103-112.
- Migraine Research Foundation. Migraine facts. https://migraineresearchfoundation.org/about- migraine/migraine-facts/. Accessed August 12, 2021.
- Burch RC, Loder S, Loder E, Smitherman TA. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55:21-34.
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211-1259.
- Vetvik KG, MacGregor EA. Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. Lancet Neurol. 2017;16:76-87.
- Hareendran A, Mannix S, Skalicky A, et al. Development and exploration of the content validity of a patient-reported outcome measure to evaluate the impact of migraine—the migraine physical function impact diary (MPFID). Health Qual Life Outcomes. 2017;15(1):224.
- Buse DC, Scher AI, Dodick DW, et al. Impact of migraine on the family: perspectives of people with migraine and their spouse/domestic partner in the CaMEO study. Mayo Clin Proc. 2016;25;S0025-6196(16)00126-9.
- Swanson SA, Zeng Y, Weeks M, Colman I. The contribution of stress to the comorbidity of migraine and major depression: results from a prospective cohort study. BMJ Open. 2013;3(3):e002057.
- Bonafede M, Sapra S, Shah N, et al. Direct and indirect healthcare resource utilization and costs among migraine patients in the United States. Headache. 2018;58:700-714.
- Messali A, Sanderson JC, Blumenfeld AM, et al. Direct and indirect costs of chronic and episodic migraine in the United States: a web-based survey. Headache. 2016;56(2):306-322.
- Noseda R, Burstein R. Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, cortical spreading depression, sensitization and modulation of pain. Pain. 2013;154(suppl 1):S44-S53.
- 12. Dodick DW. Migraine. Lancet. 2018;391(10127):1315-1330.
- Charles A. The pathophysiology of migraine: implications for clinical management. Lancet Neurol. 2018;17(2):174-182.
- Russo AF. Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu Rev Pharmacol Toxicol. 2015;55:533-552.
- Hansen JM, Hauge AW, Olesen J, Ashina M. Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia. 2010;30(10):1179-1186.
- Lipton RB, Bigal ME, Amatniek JC, Stewart WF. Tools for diagnosing migraine and measuring its severity. Headache. 2004;44:387-398.
- Tabatabai RR, Swadron SP. Headache in the emergency department: avoiding misdiagnosis of dangerous secondary causes. Emerg Med Clin North Am. 2016;34(4):695-716.
- Zodda D, Procopio G, Gupta A. Evaluation and management of life-threatening headaches in the emergency department. Emerg Med Pract. 2019;21(2):1-20.
- Wenzel RG, Sarvis CA, Krause ML. Over-the-counter drugs for acute migraine attacks: literature review and recommendations. Pharmacotherapy. 2003;23(4):494-505.
- National Headache Foundation. Headache tests. https://headaches.org/resources/headache- tests/. Accessed February 11, 2021.
- Headache Classification Committee of the International Headache Society (IHS) the International Classification of headache dirsorders, 3rd edition. Cephalalgia. 2018;38(1):1-211.
- Katsarava Z, Buse DC, Manack AN, Lipton RB. Defining the differences between episodic migraine and chronic migraine. Curr Pain Headache Rep. 2012;16(1):86-92.
- Bigal ME, Serrano D, Buse D, et al. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache. 2008;48:1157-1168.
- Cupini LM, Corbelli I, Sarchelli P. Menstrual migraine: what it is and does it matter? J Neurol. 2021;268(7):2355-2363.
- Maasumi K, Tepper SJ, Kriegler JS. Menstrual migraine and treatment options: review. Headache. 2017;57(2):194-208.
- Laurell K, Artto V, Bendtsen L, et al. Premonitory symptoms in migraine: a cross-sectional study in 2714 persons. Cephalalgia. 2016;36:951-959.
- Hansen JM, Charles A. Differences in treatment response between migraine with aura and migraine without aura: lessons from clinical practice and RCTs. J Headache Pain. 2019;20(1):96.
- Lipton RB, Bigal ME, Ashina S, et al. Cutaneous allodynia in the migraine population. Ann Neurol. 2008;63:148-158.
- Levy D, Strassman AM, Burstein R. A critical view on the role of migraine triggers in the genesis of migraine pain. Headache. 2009;49(6):953-957.
- Zaeem Z, Zhou L, Dilli E. Headaches: a review of the role of dietary factors. Curr Neurol Neurosci Rep. 2016;16(11):101.
- Borkum JM. Migraine triggers and oxidative stress: a narrative review and synthesis. Headache. 2016;56(1):12-35.
- National Headache Foundation. Resources. https://headaches.org/resources/#headache-tools. Accessed June 2020.
- Scher AI, Bigal ME, Lipton RB. Comorbidity of migraine. Curr Opin Neurol. 2005;18(3):305-310.
- Burch RC, Buse DC, Lipton RB. Migraine: epidemiology, burden, and comorbidity. Neurol Clin. 2019;37(4):631-649.
- Petrovics-Balog A, Majlath Z, Melinda L, et al. The effect of psychiatric comorbidities and stress-coping strategies on perceived quality of life in migraine. Ideggyogy Sz. 2019;72(11- 12):397-404.
- Elgendy IY, Nadeau SE, Bairey Merz CN, Pepine CJ. Migraine headache: an under- appreciated risk factor for cardiovascular disease in women. J Am Heart Assoc. 2019;8(22):e014546.
- Rambarat CA, Elgendy IY, Johnson BD, et al. Migraine headache and long-term cardiovascular outcomes: an extended follow-up of the Women’s Ischemia Syndrome Evaluation. Am J Med. 2017;130(6):738-743.
- Ailani J, Burch RC, Robbins MS; Board of Directors of the American Headache Society. The American Headache Society Consensus Statement: update on integrating new migraine treatments into clinical practice. Headache. 2021;61(7):1021-1039.
- American Headache Society. The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59(1):1-18.
- Bigal ME, Lipton RB. The preventive treatment of migraine. Neurologist. 2006;12:204-213.
- Loder E, Rizzoli P. Pharmacologic prevention of migraine: a narrative review of the state of the art in 2018. Headache. 2018;58(suppl 3):218-229.
- Tepper SJ. Acute treatment of migraine. Neurol Clin. 2019;37(4):727-742.
- Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55(1):3-20.
- Loder E, Weizenbaum E, Frishberg B; American Headache Society Choosing Wisely Task Force. Choosing wisely in headache medicine: the American Headache Society’s list of five things physicians and patients should question. Headache. 2013;53(10):1651-1659.
- Mauskop A. Nonmedication, alternative, and complementary treatments for migraine. Continuum (Minneap Minn). 2012;18(4):796-806.
- Puledda F, Shields K. Non-pharmacological approaches for migraine. Neurotherapeutics. 2018;15(2):336-345.
- Diamond ML, Wenzel RG, Nissan GR. Optimizing migraine therapy: evidence-based and patient-centered care. Expert Rev Neurother. 2006;6(6):911-919.
- Earles J, Folen RA, James LC. Biofeedback using telemedicine: clinical applications and case illustrations. Behav Med. 2001;27:77-82.
- Pistoia F, Sacco S, Carolei A. Behavioral therapy for chronic migraine. Curr Pain Headache Rep. 2013;17(1):304.
- Linde K, Allais G, Brinkhaus B, et al. Acupuncture for the prevention of episodic migraine. Cochrane Database Syst Rev. 2016;2016(6):CD001218.
- Noton D. Migraine and photic stimulation: report on a survey of migraineurs using flickering light therapy. Complement Ther Nurs Midwifery. 2000;6:138-142.
- Koseoglu E, Yetkin MF, Ugur F, Bilgen M. The role of exercise in migraine treatment. J Sports Med Phys Fitness. 2015;55(9):1029-1036.
- Finkel AG, Yerry JA, Mann JD. Dietary considerations in migraine management: does a consistent diet improve migraine? Curr Pain Headache Rep. 2013;17(11):373.
- Tepper SJ. Treating migraine with neuromodulation devices. https://americanmigrainefoundation.org/resource-library/spotlight-neuromodulation-devices- headache. Accessed July 7, 2020.
- Chou DE, Gross GJ, Casadei CH, Yugrakh MS. External trigeminal nerve stimulation for the acute treatment of migraine: open-label trial on safety and efficacy. Neuromodulation. 2017;20(7):678-683.
- Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78(17):1337-1345.
- Holland S, Silberstein SD, Freitag F, et al. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78(17):1346-1353.
- Moreno-Ajona D, Chan C, Villar-Martínez MD, Goadsby PJ. Targeting CGRP and 5-HT1F receptors for the acute therapy of migraine: a literature review. Headache. 2019;59(suppl 2):3- 19.
- Lipton RB, Stewart WF, Stone AM, et al. Stratified care vs step care strategies for migraine: the Disability in Strategies of Care (DISC) Study: a randomized trial. JAMA. 2000;284(20):2599- 2605.
- The Migraine Disability Assessment test. https://headaches.org/wp- content/uploads/2018/02/MIDAS.pdf. Accessed July 19, 2020.
- Ong JJ, De Felice M. Migraine treatment: current acute medications and their potential mechanisms of action. Neurotherapeutics. 2018;15(2):274-290.
- Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754-762.
- Lipton RB, Baggish JS, Stewart WF, et al. Efficacy and safety of acetaminophen in the treatment of migraine: results of a randomized, double-blind, placebo-controlled, population- based study. Arch Intern Med. 2000;160(22):3486-3492.
- Krinsky DL, Ferreri SP, Hemstreet BA, et al, eds. Handbook of Nonprescription Drugs: An Interactive Approach To Self-Care. 20th ed. Washington, DC: American Pharmacists Association; 2020.
- Derry S, Moore RA, McQuay HJ. Paracetamol (acetaminophen) with or without an antiemetic for acute migraine headaches in adults. Cochrane Database Syst Rev. 2010;(11):CD008040.
- Diener HC, Eikermann A, Gessner U, et al. Efficacy of 1000 mg effervescent acetylsalicylic acid and sumatriptan in treating associated migraine symptoms. Eur Neurol. 2004;52:50-56.
- Acute treatment of migraine attacks: efficacy and safety of a nonsteroidal anti-inflammatory drug, diclofenac-potassium, in comparison with oral sumatriptan and placebo. Diclofenac-K and the Sumatriptan Migraine Study Group. Cephalalgia. 1999;19:232-240.
- Smith TR, Sunshine A, Stark SR, et al. Sumatriptan and naproxen for the acute treatment of migraine. Headache. 2005;45(8):983-991.
- Stronks DL, Tulen JH, Bussmann HB, et al. Effects of naratriptan versus naproxen on daily functioning in the acute treatment of migraine: a randomized, double-blind, double-dummy, crossover study. Headache. 2003;43(8):845-852.
- Evers S, Rahmann A, Kraemer C, et al. Treatment of childhood migraine attacks with oral zolmitriptan and ibuprofen. Neurology. 2006;67:497-499.
- Kellstein DE, Lipton RB, Geetha R, et al. Evaluation of a novel solubilized formulation of ibuprofen in the treatment of migraine headache: a randomized, double blind, placebo-controlled, dose-ranging study. Cephalalgia. 2000;20:233-243.
- Codispoti JR, Prior MJ, Fu M, et al. Efficacy of nonprescription doses of ibuprofen for treating migraine headache. A randomized controlled trial. Headache. 2001;41:665-679.
- Kloster R, Nestvold K, Vilming ST. A double-blind study of ibuprofen versus placebo in the treatment of acute migraine attacks. Cephalalgia. 1992;12(3):169-171.
- Andersson PG, Hinge HH, Johansen O, et al. Double-blind study of naproxen vs. placebo in the treatment of acute migraine attacks. Cephalalgia. 1989;9(1):29-32.
- Dahlof C, Bjorkman R. Diclofenac-K (50 and 100 mg) and placebo in the acute treatment of migraine. Cephalalgia. 1993;13:117-123.
- Dib M, Massiou H, Weber M, et al. Efficacy of oral ketoprofen in acute migraine: a double- blind randomized clinical trial. Neurology. 2002;58:1660-1665.
- Harden RN, Carter TD, Gilman CS, et al. Ketorolac in acute headache management. Headache. 1991;31:463-464.
- Nebe J, Heier M, Diener HC. Low-dose ibuprofen in self-medication of mild to moderate headache: a comparison with acetylsalicylic acid and placebo. Cephalalgia. 1995;15:31-35.
- Hamalainen ML, Hoppu K, Valkeila E, Santavuori P. Ibuprofen or acetaminophen for the acute treatment of migraine in children: a double-blind, randomized, placebo-controlled, crossover study. Neurology. 1997;48(1):103-107.
- Loder E. Fixed drug combinations for the acute treatment of migraine: place in therapy. CNS Drugs. 2005;19(9):769-784.
- Lipton RB, Stewart WF, Ryan RE Jr, et al. Efficacy and safety of acetaminophen, aspirin, and caffeine in alleviating migraine headache pain: three double-blind, randomized, placebo- controlled trials. Arch Neurol. 1998;55:210-217.
- Goldstein J, Hoffman HD, Armellino JJ, et al. Treatment of severe, disabling migraine in an over- the-counter population of migraine sufferers: results from three randomized, placebo-controlled studies of the combination of acetaminophen, aspirin, and caffeine. Cephalalgia. 1999;19:684- 691.
- Silberstein SD, Armellino JJ, Hoffman HD, et al. Treatment of menstruation-associated migraine with the non-prescription combination of acetaminophen, aspirin, and caffeine: results from three randomized, placebo-controlled studies. Clin Ther. 1999;21(3):475-491.
- Goldstein J, Silberstein SD, Saper JR, et al. Acetaminophen, aspirin and caffeine in combination versus ibuprofen for acute migraine: results from a multicenter, double-blind, randomized, parallel-group, single-dose, placebo-controlled study. Headache. 2006;6(3):444-453.
- Goldstein J, Silberstein SD, Saper JR, et al. Acetaminophen, aspirin, and caffeine versus sumatriptan succinate in the early treatment of migraine: results from the ASSET trial. Headache. 2005;45:973-982.
- DeMaagd GA. Migraine headache: the pharmacist and the role of OTC medications. Pharmacy Times. 2007;73(3):106-117.
- Lipton RB, Diener HC, Robbins MS, et al. Caffeine in the management of patients with headache. J Headache Pain. 2017;18(1):107.
- Peroutka S, Lyon JA, Swarbrick J, et al. Efficacy of diclofenac sodium softgel 100 mg with or without caffeine 100 mg in migraine without aura: a randomized, double-blind, crossover study. Headache. 2004;44:136-141.
- Lipton RB, Goldstein J, Baggish JS, et al. Aspirin is efficacious for the treatment of acute migraine. Headache. 2005;45(4):283-292.
- Diener HC, Lampl C, Reimnitz P, Voelker M. Aspirin in the treatment of acute migraine attacks. Exp Rev Neurother. 2006;6(4):563-573.
- MacGregor EA, Dowson A, Davies PT. Mouth-dispersible aspirin in the treatment of migraine. Headache. 2002;42(4):249-255.
- Lange R, Schwarz JA, Hohn M. Acetylsalicylic acid effervescent 1000 mg (aspirin) in acute migraine attacks; a multicentre, randomized, double-blind, single-dose, placebo-controlled parallel group study. Cephalalgia. 2002;20:663-667.
- Lampl C, Voelker M, Diener HC. Efficacy and safety of 1,000 mg effervescent aspirin: individual patient data meta-analysis of three trials in migraine headache and migraine accompanying symptoms. J Neurol. 2007;254(6):705-712.
- Diener HC, Bussone G, de Liano H, et al. Placebo-controlled comparison of effervescent acetylsalicylic acid, sumatriptan, and ibuprofen in the treatment of migraine attacks. Cephalalgia. 2004;24(11):947-954.
- Biglione B, Gitin A, Gorelick PB, Hennekens C. Aspirin in the treatment and prevention of migraine headaches:possible additional clinical options for primary healthcare providers. Am J Med. 2020;133(4):412-416.
- Kirthi V, Derry S, Moore RA, McQuay HJ. Aspirin with or without an antiemetic for acute migraine headaches in adults. Cochrane Database Syst Rev. 2010;(4):CD008041.
- Elyxyb (celecoxib) package insert. Hyderabad, Telangana (India): Dr. Reddy’s Laboratories Limited; May 2020.
- Silberstein SD, Freitag FG, Rozen TD, et al. Tramadol/acetaminophen for the treatment of acute migraine pain: findings of a randomized, placebo-controlled trial. Headache. 2005;45(10):1317-1327.
- Winner P, Cady RK, Ruoff GE, et al. Twelve-month tolerability and safety of sumatriptan- naproxen sodium for the treatment of acute migraine. Mayo Clin Proc. 2007;82(1):61-68.
- Diamond S, Medina JL. Isometheptene—a non-ergot drug in the treatment of migraine. Headache. 1975;15(3):211-213.
- Tepper S. Opioids should not be used in migraine. Headache. 2012;52(suppl 1):30-34.
- Buse DC, Pearlman SH, Reed ML, et al. Opioid use and dependence among persons with migraine: results of the AMPP study. Headache. 2012;52(1):18-36.
- Lipton RB, Buse DC, Friedman BW, et al. Characterizing opioid use in a US population with migraine: results from the CAMeo study. Neurology. 2020;95:e457-e458.
- Fisher MA, Glass S. Butorphanol (Stadol): a study in problems of current drug information and control. Headache. 1997;48:1156-1160.
- Biondi DM. Opioid resistance in chronic daily headache: a synthesis of ideas from the bench and bedside. Curr Pain Headache Rep. 2003;7(1):67-75.
- Mathew NT. Transformed migraine, analgesic rebound, and other chronic daily headaches. Neurol Clin. 1997;15(1):167-186.
- Panconesi A, Anselmi B, Franchi G. Increased adverse effects of opiates in migraine patients. Cephalalgia. 1995;15(2):159-160.
- Yaksh T. Opioids, analgesia, and pain management. In: Brunton LL, Hilal-Dandan R, Knollmann BC, eds. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 13th ed. New York, NY: McGraw-Hill Education; 2018.
- Mihic S. Hypnotics and sedatives. In: Brunton LL, Hilal-Dandan R, Knollmann BC, eds. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 13th ed. New York, NY: McGraw-Hill Education; 2018.
- Diener HC, Katasarva Z. Analgesic/abortive overuse and misuse in chronic daily headache. Curr Pain Headache Rep. 2001;5:545-550.
- Mathew NT, Kurman R, Perez F. Drug-induced refractory headache—clinical features and management. Headache. 1990;13:634-638.
- Wilson MC, Jimenez-Sanders R. Medication overuse headache. https://americanmigrainefoundation.org/resource-library/medication-overuse-headache-2/. Accessed August 13, 2020.
- Saper JR, Da Silva AN. Medication overuse headache: history, features, prevention and management strategies. CNS Drugs. 2013;27(11):867-877.
- Ferrari A, Savino G, Gallesi D, et al. Effect of overuse of the anti-migraine combination of indomethacin, prochlorperazine and caffeine (IPC) on the disposition of its components in chronic headache patients. Pharmacol Res. 2006;54:142-149.
- Zed PJ, Loewen PS, Robinson G. Medication-induced headache: overview and systematic review of therapeutic approaches. Ann Pharmacother. 1999;33:61-72.
- Tepper SJ. Medication-overuse headache. Continuum (Minneap Minn). 2012;18(4):807- 822.
- Wakerley BR. Medication-overuse headache. Pract Neurol. 2019;19(5):399-403.
- Diener HC, Holle D, Solbach K, Gaul C. Medication-overuse headache: risk factors, pathophysiology and management. Nat Rev Neurol. 2016;12(10):575-583.
- Bigal ME, Rapoport AM, Sheftell FD, et al. Transformed migraine and medication overuse in a tertiary headache centre—clinical characteristics and treatment outcomes. Cephalalgia. 2004;24(6):483-490.
- Diener HC, Dodick D, Evers S, et al. Pathophysiology, prevention, and treatment of medication overuse headache. Lancet Neurol. 2019;18(9):891-902.
- Freitag FG, Lake A III, Lipton R, et al. Inpatient treatment of headaches: an evidence-based assessment. Headache. 2004;44:342-360.
- Munksgaard SB, Madsen SK, Wienecke T. Treatment of medication overuse headache—a review. Acta Neurol Scand. 2019;139(5):405-414.
- Humphrey PP, Feniuk W, Perren MJ, et al. Serotonin and migraine. Ann NY Acad Sci. 1990;600:587-598.
- Silberstein SD. Serotonin (5-HT) and migraine. Headache. 1994;34:408-417.
- Goldstein DJ, Roon KI, Offen WW, et al. Selective serotonin 1F (5-HT1F) receptor agonist LY334370 for acute migraine: a randomised controlled trial. Lancet. 2001;358:1230-1234.
- Buzzi MG, Moskowitz MA. Evidence for 5-HT1B/1D receptors mediating the antimigraine effect of sumatriptan and dihydroergotamine. Cephalalgia. 1991;11(4):165-168.
- Silberstein SD, McCrory DC. Ergotamine and dihydroergotamine: history, pharmacology, and efficacy. Headache. 2003;43:144-166.
- Bigal ME, Tepper SJ. Ergotamine and dihydroergotamine: a review. Curr Pain Headache Rep. 2003;7:55-62.
- Jamieson DG. The safety of triptans in the treatment of patients with migraine. Am J Med. 2002;112:135-140.
- Tfelt-Hansen PC, Koehler PJ. History of the use of ergotamine and dihydroergotamine in migraine from 1906 and onward. Cephalalgia. 2008;28:877-886.
- Saper JR, Silberstein S. Pharmacology of dihydroergotamine and evidence for efficacy and safety in migraine. Headache. 2006;46(suppl 4):S171-S181.
- D.H.E. 45 (dihydroergotamine mesylate) package insert. East Hanover, NJ: Novartis Pharmaceuticals Corp; July 2002.
- Cafergot (ergotamine tartrate and caffeine) package insert. East Hanover, NJ: Novartis Pharmaceuticals Corp; June 2002.
- Schulman EA, Rosenberg SB. Claudication: an unusual side effect of DHE administration. Headache. 1991;31(4):237-239.
- Ward TN, Scott G. Dihydroergotamine suppositories in a headache clinic. Headache. 1991;31(7):465-466.
- Carleton SC, Shesser RF, Pietrzak MP, et al. Double-blind, multicenter trial to compare the efficacy of intramuscular dihydroergotamine plus hydroxyzine versus intramuscular meperidine plus hydroxyzine for the emergency treatment of acute migraine headaches. Ann Emerg Med. 1998;32:129-138.
- Colman I, Brown MD, Innes GD, et al. Parenteral dihydroergotamine for acute migraine headaches: a systemic review of the literature. Ann Emerg Med. 2005;45(4):393-401.
- Touchon J, Bertin L, Pilgrim AJ, et al. A comparison of subcutaneous sumatriptan and dihydroergotamine nasal spray in the acute treatment of migraine. Neurology. 1996;47:361-365.
- Young WB. Appropriate use of ergotamine tartrate and dihydroergotamine in the treatment of migraine: current perspectives. Headache. 1997;37(suppl 1):S42-S45.
- Gallagher RM. Acute treatment of migraine with dihydroergotamine nasal spray. Dihydroergotamine Working Group. Arch Neurol. 1996;53:1285-1291.
- Migranal (dihydroergotamine) package insert. East Hanover, NJ: Novartis Pharmaceuticals Corp; December 2001.
- Vecsei L, Szok D, Nyari A, Tajti J. Treating status migrainosus in the emergency setting: what is the best strategy? Expert Opin Pharmacother. 2018;19(14):1523-1531.
- Trudhesa (dihydroergotamine) package insert. Seattle, WA: Impel NeuroPharma Inc; September 2021.
- Peroutka SJ. 5-Hydroxytriptamine receptor subtypes and the pharmacology of migraine. Neurology. 1993;43(6 suppl 3):S34-S38.
- Deliganis AV, Peroutka SJ. 5-Hydroxytriptamine1D receptor agonism predicts antimigraine efficacy. Headache. 1991;31(4):228-231.
- Vila-Pueyo M. Targeted 5-HT1F therapies for migraine. Neurotherapeutics. 2018;15(2):291- 303.
- Barbanti P, Aurilia C, Egeo G, et al. Serotonin receptor targeted therapy for migraine treatment: an overview of drugs in phase I and II clinical development. Expert Opin Investig Drugs. 2017;26(3):269-277.
- Tepper SJ, Rapoport AM, Sheftell FD. Mechanisms of action of the 5-HT1B/1D receptor agonists. Arch Neurol. 2002;59(7):1084-1088.
- Fullerton T, Gengo FM. Sumatriptan: a selective 5-hydroxytriptamine receptor agonist for the acute treatment of migraine. Ann Pharmacother. 1992;26:800-808.
- Frova (frovatriptan) package insert. Chadds Ford, PA: Endo Pharmaceuticals Inc; April 2007.
- Axert (almotriptan) package insert. Raritan, NJ: Ortho-McNeil Neurologics; April 2009.
- Imitrex (sumatriptan) package insert. Research Triangle Park, NC: GlaxoSmithKline; November 2013.
- Amerge (naratriptan) package insert. Research Triangle Park, NC: GlaxoSmithKline; November 2016.
- Zomig and Zomig ZMT (zolmitriptan) package insert. Wilmington, DE: AstraZeneca; December 2018.
- Maxalt and Maxalt-MLT (rizatriptan) package insert. Whitehouse Station, NJ: Merck & Co, Inc; October 2019.
- Relpax (eletriptan) package insert. New York: Pfizer; 2006.
- Syed YY. Sumatriptan/naproxen sodium: a review in migraine. Drugs. 2016;76(1):111-121.
- Tfelt-Hansen P, De Vries P, Saxena PR. Triptans in migraine: a comparative review of pharmacology, pharmacokinetics and efficacy. Drugs. 2000;50:1259-1287.
- Pascual J, Muñoz P. Correlation between lipophilicity and triptan outcomes. Headache. 2005;45(1):3-6.
- Göbel H, Winter P, Boswell D, et al. Comparison of naratriptan and sumatriptan in recurrence- prone migraine patients. Naratriptan International Recurrence Study Group. Clin Ther. 2000;22:981-989.
- Rapoport AM, Tepper SJ, Bigal ME, Sheftell FD. The triptan formulations: how to match patients and products. CNS Drugs. 2003;17:431-447.
- Dixon R, French S, Kemp J, et al. Effect of hepatic impairment on the pharmacokinetics of zolmitriptan. J Clin Pharmacol. 1998;38: 694-701.
- Tepper SJ, Millson D. Safety profile of the triptans. Expert Opin Drug Saf. 2003;2:123-132.
- Tepper SJ. Safety and rational use of the triptans. Med Clin North Am. 2001;85:959-970.
- Dodick D, Lipton RB, Martin V, et al. Consensus statement: cardiovascular safety profile of triptans (5-HT agonists) in the acute treatment of migraine. Headache. 2004;44(5):414-425.
- Maassen VanDen Brink A, Reekers M, Bax WA, et al. Coronary side effect potential of current and prospective antimigraine drugs. Circulation. 1998;98:25-30.
- Laine K, Raasakka T, Mäntynen J, Saukko P. Fatal cardiac arrhythmia after oral sumatriptan. Headache. 1999;39:511-512.
- Wammes-van der Heijden EA, Rahimtoola H, Leufkens HG, et al. Risk of ischemic complications related to the intensity of triptan and ergotamine use. Neurology. 2006;67(7):1128- 1134.
- Pacheco-Coronado R, McMullan PW, Galbut BH, et al. Myocardial infarction after taking zolmitriptan. Yale J Biol Med. 2005;78(3):147-150.
- Arora A, Arora S. Spontaneous splenic infarction associated with sumatriptan use. J Headache Pain. 2006;7(4):214-216.
- Fulton JA, Kahn J, Nelson LS, Hoffman RS. Renal infarction during the use of rizatriptan and zolmitriptan: two case reports. Clin Toxicol (Phila). 2006;44(2):177-180.
- Repaka A, Wenger J, Sitaraman SV. A case of sumatriptan-induced intestinal ischemia. J Gastroenterol. 2006;41(2):177-178.
- Martin VT, Goldstein JA. Evaluating the safety and tolerability profile of acute treatments for migraine. Am J Med. 2005;228(118 suppl 1):36S-44S.
- Hilaire ML, Cross LB, Eichner SF. Treatment of migraine headaches with sumatriptan in pregnancy. Ann Pharmacother. 2004;38(10):1726-1730.
- Yu DK. The contribution of P-glycoprotein to pharmacokinetic drug-drug interactions. J Clin Pharmacol. 1999;39:1203-1211.
- Evans RW, Tepper SJ, Shapiro RE, et al. The FDA alert on serotonin syndrome with use of triptans combined with selective serotonin reuptake inhibitors or selective serotonin- norepinephrine reuptake inhibitors: American Headache Society position paper. Headache. 2010;50(6):1089-1099.
- Gardner DM, L ynd LD. Sumatriptan contraindications and the serotonin syndrome. Ann Pharmacother. 1998;32:33-38.
- Shapiro RE, Tepper SJ. The serotonin syndrome, triptans, and the potential for drug-drug interactions. Headache. 2007;47:266-269.
- Wooltorton E. Triptan migraine treatment and antidepressants: risk of serotonin syndrome. CMAJ. 2006;175(8):874.
- Eadie MJ. Clinically significant drug interactions with agents specific for migraine attacks. CNS Drugs. 2001;15(2):105-118.
- Goldberg MR, Lowry RC, Musson DG, et al. Lack of pharmacokinetic and pharmacodynamic interaction between rizatriptan and paroxetine. J Clin Pharmacol. 1999;39:192-199.
- Michalets EL. Update: clinically significant cytochrome P-450 drug interactions. Pharmacotherapy. 1998;18(1):84-112.
- Goldberg MR, Sciberras D, De Smet M, et al. Influence of beta-adrenoceptor antagonists on the pharmacokinetics of rizatriptan, a 5-HT1B/1D agonist: differential effects of propranolol, nadolol and metoprolol. Br J Clin Pharmacol. 2001;52(1):69-76.
- Martin VT, Loder E, Taylor K, et al. Eletriptan treatment of migraine in patients switching from barbiturate-containing analgesics: results from a multiple attack study. Cephalalgia. 2005;25(9):726- 734.
- Pascual J, García-Moncó C, Roig C, et al. Rizatriptan 10-mg wafer versus usual non-triptan therapy for migraine: analysis of return to function and patient preference. Headache. 2005;45(9):1140-1150.
- Friedman BW, Hochberg M, Esses D, et al. A clinical trial of trimethobenzamide/diphenhydramine versus sumatriptan for acute migraines. Headache. 2006;46(6):934-941.
- Friedman BW, Corbo J, Lipton RB, et al. A trial of metoclopramide vs. sumatriptan for the emergency department treatment of migraines. Neurology. 2005;64(3):463-468.
- Brandes JL, Kudrow D, Stark SR, et al. Sumatriptan-naproxen for acute treatment of migraine: a randomized trial. JAMA. 2007;297(13):1443-1454.
- Dahlöf CG. Non-oral formulations of triptans and their use in acute migraine. Curr Pain Headache. 2005;9(3):206-212.
- Diamond S, Freitag FG, Feoktistov A, Nissan G. Sumatriptan 6 mg subcutaneous as an effective migraine treatment in patients with cutaneous allodynia who historically fail to respond to oral triptans. J Headache Pain. 2007;8(1):13-18.
- Dahlöf C. Sumatriptan nasal spray in the acute treatment of migraine: a review of clinical studies. Cephalalgia. 1999;19:769-778.
- Ensink FB. Subcutaneous sumatriptan in the acute treatment of migraine. J Neurol. 1991;238(2 suppl 1):S66-S69.
- Winner P, Adelman J, Aurora S, et al. Efficacy and tolerability of sumatriptan injection for the treatment of morning migraine: two multicenter, prospective, randomized, double-blind, controlled studies in adults. Clin Ther. 2006;28(10):1582-1591.
- Winner P, Rothner AD, Wooten JD, et al. Sumatriptan nasal spray in adolescent migraineurs: a randomized, double-blind, placebo-controlled, acute study. Headache. 2006;46(2):212-222.
- Goadsby PJ, Yates R. Zolmitriptan intranasal: a review of the pharmacokinetics and clinical efficacy. Headache 2006;46(1):138-149.
- Dowson AJ, MacGregor EA, Purdy RA, et al. Zolmitriptan orally disintegrating tablet is effective in the acute treatment of migraine. Cephalalgia. 2002;22(2):101-106.
- Dodick DW. Triptan nonresponder studies: implications for clinical practice. Headache. 2005;45(2):156-162.
- Marcus SC, Shewale AR, Silberstein SD, et al. Comparison of healthcare resource utilization and costs among patients with migraine with potentially adequate and insufficient triptan response. Cephalalgia. 2020;40(7):639-649.
- Leroux E, Buchanan A, Lombard L, et al. Evaluation of patients with insufficient efficacy and/or tolerability to triptans for the acute treatment of migraine: a systematic literature review. Adv Ther. 2020;37(12):4765-4796.
- Dodick DW, Silberstein S, Dahlöf CG. Is there a preferred triptan? Headache. 2002;42:1-7.
- Rapoport AM, Tepper SJ, Sheftell FD, et al. Which triptan for which patient? Neurol Sci. 2006;27(suppl 2):S123-S129.
- Díez FI, Straube A, Zanchin G. Patient preference in migraine therapy. A randomized, open- label, crossover clinical trial of acute treatment of migraine with oral almotriptan and rizatriptan. J Neurol. 2007;254(2):242-249.
- Diener HC, Gendolla A, Gebert I, Beneke M. Almotriptan in migraine patients who respond poorly to oral sumatriptan: a double-blind, randomized trial. Headache. 2005;45(7):874-882.
- Dowson AJ, Mathew NT, Pascual J. Review of clinical trials using acute intervention with oral triptans for migraine management. Int J Clin Pract. 2007;60(6):698-706.
- Adelman JU, Lipton RB, Ferrari MD, et al. Comparison of rizatriptan and other triptans on stringent measures of efficacy. Neurology. 2001;57:1377-1383.
- Goadsby PJ, Massiou H, Pascual J, et al. Almotriptan and zolmitriptan in the acute treatment of migraine. Acta Neurol Scand. 2007;115(1):34-40.
- Mandema JW, Cox E, Alderman J, et al. Therapeutic benefit of eletriptan compared to sumatriptan for the acute relief of migraine pain—results of a model-based meta-analysis that accounts for encapsulation. Cephalagia. 2005;25(9):715-725.
- Goldstein J, Tiseo PT, Albert KS, et al. Eletriptan in migraine patients reporting unsatisfactory response to rizatriptan. Headache. 2006;46(7):1142-1150.
- Diener HC. Efficacy of almotriptan 12.5 mg in achieving migraine related composite endpoints: a double-blind, randomized, placebo-controlled study in patients with previous poor response to sumatriptan 50 mg. Curr Med Res Opin. 2005;21(10):1603-1610.
- Dahlöf CG. Infrequent or non-response to oral sumatriptan does not predict response to other triptans: review of four trials. Cephalalgia. 2006;26(2):98-106.
- Láinez MJ, Evers S, Kinge E, et al. Preference for rizatriptan 10-mg wafer vs. eletriptan 40-mg tablet for acute treatment of migraine.Cephalalgia. 2006;26(3):246-256.
- Evers S, Afra J, Frese A, et al. EFNS guideline on the drug treatment of migraine—revised report of an EFNS task force. Eur J Neurol. 2009;16(9):968-981.
- Cutrer FM, Goadsby PJ, Ferrari MD, et al. Priorities for triptan treatment attributes and the implications for selecting an oral triptan for acute migraine: a study of U.S. primary care physicians. Clin Ther. 2004;26(9):1533-1545.
- Katsarava Z, Fritsche G, Muessig M, et al. Clinical features of withdrawal headache following overuse of triptans and other headache drugs. Neurology. 2001;57:1694-1698.
- Reyvow (lasmiditan) package insert. Indianapolis, IN: Lilly USA, LLC; October 2019.
- Cohen ML, Schenck K. 5-Hydroxytryptamine1F receptors do not participate in vasoconstriction: lack of vasoconstriction to LY344864, a selective serotonin1F receptor agonist in rabbit saphenous vein. J Pharmacol Exp Ther. 1999;290(3):935-939.
- Goadsby PJ. New targets in the acute treatment of headache. Curr Opin Neurol. 2005;18(3):283-288.
- Kuca B, Silberstein SD, Wietecha L, et al; COL MIG-301 Study Group. Lasmiditan is an effective acute treatment for migraine: a phase 3 randomized study. Neurology. 2018;91(24):e2222-e2232.
- Goadsby PJ, Wietecha LA, Dennehy EB, et al. Phase 3 randomized, placebo-controlled, double-blind study of lasmiditan for acute treatment of migraine. Brain. 2019;142(7):1894-1904.
- Oswald JC, Schuster NM. Lasmiditan for the treatment of acute migraine: a review and potential role in clinical practice. J Pain Res. 2018;11:2221-2227.
- Krege JH, Rizzoli PB, Liffick E, et al. Safety findings from Phase 3 lasmiditan studies for acute treatment of migraine: results from SAMURAI and SPARTAN. Cephalalgia. 2019;39(8):957-966.
- Loo LS, Plato BM, Turner IM, et al. Effect of a rescue or recurrence dose of lasmiditan on efficacy and safety in the acute treatment of migraine: findings from the phase 3 trials (SAMURAI and SPARTAN). BMC Neurol. 2019 13;19(1):191.
- Dowson AJ, D’Amico D, Tepper SJ, et al. Identifying patients who require a change in their current acute migraine treatment: the Migraine Assessment of Current Therapy (Migraine-ACT) questionnaire. Neurol Sci. 2004;25(suppl 3):S276-S278.
- Negro A, Martelletti P. Gepants for the treatment of migraine. Expert Opin Investig Drugs. 2019;28(6):555-567.
- Ubrelvy (ubrogepant) package insert. Madison, NJ: Allergan USA, Inc; December 2019.
- Nurtec ODT (rimegepant) package insert. New Haven, CT: Biohaven Pharmaceuticals, Inc; May 2021.
- Edvinsson L, Haanes KA, Warfvinge K, Krause DN. CGRP as the target of new migraine therapies—successful translation from bench to clinic. Nat Rev Neurol. 2018;14(6):338-350.
- Schuster NM, Rapoport AM. Calcitonin gene-related peptide-targeted therapies for migraine and cluster headache: a review. Clin Neuropharmacol. 2017;40(4):169-174.
- Dodick DW, Lipton RB, Ailani J, et al. Ubrogepant for the treatment of migraine. N Engl J Med. 2019;381(23):2230-2241.
- Lipton RB, Dodick DW, Ailani J, et al. Effect of ubrogepant vs placebo on pain and the most bothersome associated symptom in the acute treatment of migraine: the ACHIEVE II randomized clinical trial. JAMA. 2019;322(19):1887-1898.
- Voss T, Lipton RB, Dodick DW, et al. A phase IIb randomized, double-blind, placebo- controlled trial of ubrogepant for the acute treatment of migraine. Cephalalgia. 2016;36(9):887- 898.
- Ailani J, Lipton RB, Hutchinson S, et al. Long-term safety evaluation of ubrogepant for the acute treatment of migraine: phase 3, randomized, 52-week extension trial. Headache. 2020;60(1):141-152.
- Dodick DW, Lipton RB, Ailani J, et al. Ubrogepant, an acute treatment for migraine, improved patient-reported functional disability and satisfaction in 2 single-attack phase 3 randomized trials, ACHIEVE I and II. Headache. 2020;60(4):686-700.
- Ankrom W, Bondiskey P, Li CC, et al. Ubrogepant is not associated with clinically meaningful elevations of alanine aminotransferase in healthy adult males. Clin Transl Sci. 2020;13(3):462-472.
- Lipton LB, Conway CM, Stock EG, et al. Efficacy, safety, and tolerability of rimegepant 75 mg, an oral CGRP receptor antagonist, for the acute treatment of migraine: results from a phase 3, double-blind, randomized, placebo-controlled trial, study 301 (PS123LB) [abstract]. Headache. 2018;58:1287-1337.
- Croop R, Goadsby PJ, Stock DA, et al. Efficacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: a randomised, phase 3, double- blind, placebo-controlled trial. Lancet. 2019;394(10200):737-745.
- Ho TW, Mannix LK, Fan X et al; MK-0974 Protocol 004 study group. Randomized controlled trial of an oral CGRP receptor antagonist, MK-0974, in acute treatment of migraine. Neurology. 2008;70(16):1304-1312.
- Lipton RB, Croop R, Stock EG, et al. Rimegepant, an oral calcitonin gene-related peptide receptor antagonist, for migraine. N Engl J Med. 2019;381(2):142-149.
- Becker WJ. Acute migraine treatment in adults. Headache. 2015;55(6):778-793.
- Jesani J, Simerson D. Pharmacologic management of acute migraines in the emergency department. Adv Emerg Nurs J. 2019;41(2):150-162.
- Kelley NE, Tepper DE. Rescue therapy for acute migraine, part 3: opioids, NSAIDs, steroids, and post-discharge medications. Headache. 2012;52(3):467-482.
- Orr SL, Aubé M, Becker WJ, et al. Canadian Headache Society systematic review and recommendations on the treatment of migraine pain in emergency settings. Cephalalgia. 2015;35(3):271-284.
- Dodson H, Bhula J, Eriksson S, Nguyen K. Migraine treatment in the emergency department: alternatives to opioids and their effectiveness in relieving migraines and reducing treatment times. Cureus. 2018;10(4):e2439.
- Dolgorukova A, Osipchuk AV, Murzina AA, Sokolov AY. The influence of metoclopramide on trigeminovascular nociception: possible anti-migraine mechanism of action. Neuroscience. 2020;425:123-133.
- Golikhatir I, Cheraghmakani H, Bozorgi F, et al. The efficacy and safety of prochlorperazine in patients with acute migraine: a systematic review and meta-analysis. Headache. 2019;59(5):682-700.
- Kelly AM, Walcynski T, Gunn B. The relative efficacy of phenothiazines for the treatment of acute migraine: a meta-analysis. Headache. 2009;49(9):1324-1332.
- Colman I, Brown MD, Innes GD, et al. Parenteral metoclopramide for acute migraine: meta- analysis of randomised controlled trials. BMJ. 2004;329(7479):1369-1373.
- Salazar G, Fragoso M, Vergez L, et al. Metoclopramide as an analgesic in severe migraine attacks: an open, single-blind, parallel control study. Recent Pat CNS Drug Discov. 2011;6(2):141-145.
- Friedman BW, Mulvey L, Esses D, et al. Metoclopramide for acute migraine: a dose- finding randomized clinical trial. Ann Emerg Med. 2011;57(5):475-482.
- Jiang C, Wang T, Qiu ZG, et al. Efficacy of metoclopramide for the treatment of acute migraine. Medicine (Baltimore). 2019;98(37):e17065.
- Eken C. Critical reappraisal of intravenous metoclopramide in migraine attack: a systematic review and meta-analysis. Am J Emerg Med. 2015;33(3):331-337.
- Dogan NO, Pekdemir M, Yılmaz S, et al. Intravenous metoclopramide in the treatment of acute migraines: a randomized, placebo-controlled trial. Acta Neurol Scand. 2019;139(4):334- 339.
- Richman PB, Allegra J, Eskin B, et al. A randomized clinical trial to assess the efficacy of intramuscular droperidol for the treatment of acute migraine headaches. Am J Emerg Med. 2002;20:39-42.
- Richman P, Reischel U, Ostrow A, et al. Droperidol for acute migraine headache. Am J Emerg Med. 1999;17:398-400.
- Fisher H. A new approach to emergency department therapy of migraine headaches with intravenous haloperidol: a case series. J Emerg Med. 1995;13(1):119-122.
- Wang SJ, Silberstein SD, Young WB. Droperidol treatment of status migrainosus and refractory migraine. Headache. 1997;37(6):377-382.
- Shrestha M, Singh R, Moreden J, Hayes JE. Ketorolac vs. chlorpromazine in the treatment of acute migraine without aura. A prospective, randomized, double-blind trial. Arch Intern Med. 1996;156(15):1725-1728.
- Seim MB, March JA, Dunn KA. Intravenous ketorolac vs. intravenous prochlorperazine for the treatment of migraine headaches. Acad Emerg Med. 1998;5(6):573-576.
- Tokola RA, Neuvonen PJ. Effects of migraine attack and metoclopramide on the absorption of tolfenamic acid. Br J Clin Pharmacol. 1984;17(1):67-75.
- Coppola M, Yealy DM, Leibold RA. Randomized, placebo-controlled evaluation of prochlorperazine versus metoclopramide for emergency department treatment of migraine headache. Ann Emerg Med. 1995;26:541-567.
- BET 1: metoclopramide or prochlorperazine for headache in acute migraine? Emerg Med J. 2013;30(7):595-596.
- Jones J, Pack S, Chun E. Intramuscular prochlorperazine versus metoclopramide as single- agent therapy for the treatment of acute migraine. Ann Emerg Med. 1996;14(3):262-264.
- Friedman BW, Esses D, Solorzano C, et al. A randomized controlled trial of prochlorperazine versus metoclopramide for treatment of acute migraine. Ann Emerg Med. 2008;52(4):399-406.
- Cameron JD, Lane PL, Speechley M. Intravenous chlorpromazine vs intravenous metoclopramide in acute migraine headache. Acad Emerg Med. 1995;2(7):597-602.
- Gaffigan ME, Bruner DI, Wason C, et al. A randomized controlled trial of intravenous haloperidol vs. intravenous metoclopramide for acute migraine therapy in the emergency department. J Emerg Med. 2015;49(3):326-334.
- Klapper JA, Stanton JS. Ketorolac versus DHE and metoclopramide in the treatment of migraine headaches. Headache. 1991;31(8):523-524.
- Khazaei M, Hosseini Nejad Mir N, Aghdam FY, et al. Effectiveness of intravenous dexamethasone, metoclopramide, ketorolac, and chlorpromazine for pain relief and prevention of recurrence in the migraine headache: a prospective double-blind randomized clinical trial. Neurol Sci. 2019;40(5):1029-1033.
- Talebian MT, Mirbaha S, Davarinezhad-Moghadam E, et al. Comparing the therapeutic effects of dexamethasone-metoclopramide with ketorolac in relieving headache in patients with acute migraine attacks presenting to the emergency department. Adv J Emerg Med. 2019;9;3(2):e17.
- Talabi S, Masoumi B, Azizkhani R, Esmailian M. Metoclopramide versus sumatriptan for treatment of migraine headache: a randomized clinical trial. J Res Med Sci. 2013;18(8):695-698.
- Schulman EA, Dermott KF. Sumatriptan plus metoclopramide in triptan-nonresponsive migraineurs. Headache. 2003;43(7):729-733.
- Barleycorn D. Systematic review: is metoclopramide more effective than sumatriptan in relieving pain from migraine in adults in the emergency department (ED) setting? Int Emerg Nurs. 2016;27:51-55.
- Wijemanne S, Jankovic J, Evans RW. Movement disorders from the use of metoclopramide and other antiemetics in the treatment of migraine. Headache. 2016;56(1):153-161.
- Griffith JD, Mycyk MB, Kyriacou DN. Metoclopramide versus hydromorphone for the emergency department treatment of migraine headache. J Pain. 2008;9(1):88-94.
- Najjar M, Hall T, Estupinan B. Metoclopramide for acute migraine treatment in the emergency department: an effective alternative to opioids. Cureus. 2017;9(4):e1181.
- Orr SL, Friedman BW, Christie S, et al. Management of adults with acute migraine in the emergency department: the American Headache Society evidence assessment of parenteral pharmacotherapies. Headache. 2016;56(6):911-940.
- Hamilton KT, Robbins MS. Migraine treatment in pregnant women presenting to acute care: a retrospective observational study. Headache. 2019;59(2):173-179.
- Mauskop A, Altura BM. Role of magnesium in the pathogenesis and treatment of migraines. Clin Neurosci. 1998;5(1):24-27.
- Choi H, Parmar N. The use of intravenous magnesium sulphate for acute migraine: meta- analysis of randomized controlled trials. Eur J Emerg Med. 2014;21(1):2-9.
- Bigal ME, Bordini CA, Tepper SJ, Speciali JG. Intravenous magnesium sulphate in the acute treatment of migraine without aura and migraine with aura. A randomized, double-blind, placebo-controlled study. Cephalalgia. 2002;22(5):345-353.
- Demirkaya S, Vural O, Dora B, et al. Efficacy of intravenous magnesium sulfate in the treatment of acute migraine attacks. Headache. 2001;41(2):171-177.
- Miller AC, Pfeffer BK, Lawson MR, et al. Intravenous magnesium sulfate to treat acute headaches in the emergency department: a systematic review. Headache. 2019;59(10):1674- 1686.
- Xu F, Arakelyan A, Spitzberg A, et al. Experiences of an outpatient infusion center with intravenous magnesium therapy for status migrainosus. Clin Neurol Neurosurg. 2019;178:31-35.
- Shahrami A, Assarzadegan F, Hatamabadi HR, et al. Comparison of therapeutic effects of magnesium sulfate vs. dexamethasone/metoclopramide on alleviating acute migraine headache. J Emerg Med. 2015;48(1):69-76.
- Cete Y, Dora B, Ertan C, et al. A randomized prospective placebo-controlled study of intravenous magnesium sulphate vs. metoclopramide in the management of acute migraine attacks in the Emergency Department. Cephalalgia. 2005;25(3):199-204.
- Baratloo A, Mirbaha S, Delavar Kasmaei H, et al. Intravenous caffeine citrate vs. magnesium sulfate for reducing pain in patients with acute migraine headache; a prospective quasi-experimental study. Korean J Pain. 2017;30(3):176-182.
- Delavar Kasmaei H, Amiri M, Negida A, et al. Ketorolac versus magnesium sulfate in migraine headache pain management; a preliminary study. Emerg (Tehran). 2017;5(1):e2.
- Waberzinek G, Marková J, Mastik J. Safety and efficacy of intravenous sodium valproate in the treatment of acute migraine. Neuro Endocrinol Lett. 2007;28(1):59-64.
- Reiter PD, Nickisch J, Merritt G. Efficacy and tolerability of intravenous valproic acid in acute adolescent migraine. Headache. 2005;45(7):899-903.
- Norton J. Use of valproate in status migraine. Headache. 2000;40:755-757.
- Edwards KR, Norton J, Behnke M. Comparison of intravenous valproate versus intramuscular dihydroergotamine and metoclopramide for acute treatment of migraine headache. Headache. 2001;41(10):976-980.
- Bakhshayesh B, Seyed Saadat SM, Rezania K, et al. A randomized open-label study of sodium valproate vs sumatriptan and metoclopramide for prolonged migraine headache. Am J Emerg Med. 2013;31(3):540-544.
- Friedman BW, Garber L, Yoon A, et al. Randomized trial of IV valproate vs metoclopramide vs ketorolac for acute migraine. Neurology. 2014;82(11):976-983.
- Maizels M, Geiger AM. Intranasal lidocaine for migraine: a randomized trial and open-label follow-up. Headache. 1999;39(8):543-551.
- Buckley R, McCurry T, Gera J. Intranasal lidocaine for migraine using a metered-dose spray. Headache. 2000;40(6):498.
- Avcu N, Dogan NÖ, Pekdemir M, et al. Intranasal lidocaine in acute treatment of migraine: a randomized controlled trial. Ann Emerg Med. 2017;69(6):743-751.
- Chi PW, Hsieh KY, Chen KY, et al. Intranasal lidocaine for acute migraine: a meta-analysis of randomized controlled trials. PLoS One. 2019;14(10):e0224285.
- Pfaffenrath V, Fenzl E, Bregman D, Färkkila M. Intranasal ketorolac tromethamine (SPRIX®) containing 6% of lidocaine (ROX-828) for acute treatment of migraine: safety and efficacy data from a phase II clinical trial. Cephalalgia. 2012;32(10):766-777.
- Ayulo MA Jr, Phillips KE, Tripathi S. Safety and efficacy of IV lidocaine in the treatment of children and adolescents with status migraine. Pediatr Crit Care Med. 2018;19(8):755-759.
- Amparán-Estrada S. Management of acute migraine headache with lidocaine. Plast Reconstr Surg. 2005 Jul;116(1):335.
- Ebied AM, Nguyen DT, Dang T. Evaluation of occipital nerve blocks for acute pain relief of migraines. J Clin Pharmacol. 2020;60(3):378-383.
- Latev A, Friedman BW, Irizarry E, et al. A randomized trial of a long-acting depot corticosteroid versus dexamethasone to prevent headache recurrence among patients with acute migraine who are discharged from an emergency department. Ann Emerg Med. 2019;73(2):141- 149.
- Giuliano C, Smalligan RD, Mitchon G, Chua M. Role of dexamethasone in the prevention of migraine recurrence in the acute care setting: a review. Postgrad Med. 2012;124(3):110-115.
- Friedman BW, Greenwald P, Bania TC, et al. Randomized trial of IV dexamethasone for acute migraine in the emergency department. Neurology. 2007;69(22):2038-2044.
- Singh A, Alter HJ, Zaia B. Does the addition of dexamethasone to standard therapy for acute migraine headache decrease the incidence of recurrent headache for patients treated in the emergency department? A meta-analysis and systematic review of the literature. Acad Emerg Med. 2008;15(12):1223-1233.
- Taheraghdam AA, Amiri H, Shojaan H, et al. Intravenous dexamethasone versus morphine in relieving of acute migraine headache. Pak J Biol Sci. 2011;14(12):682-687.
- Rowe BH, Colman I, Edmonds ML, et al. Randomized controlled trial of intravenous dexamethasone to prevent relapse in acute migraine headache. Headache. 2008;48(3):333-340.
- Donaldson D, Sundermann R, Jackson R, Bastani A. Intravenous dexamethasone vs placebo as adjunctive therapy to reduce the recurrence rate of acute migraine headaches: a multicenter, double-blinded, placebo-controlled randomized clinical trial. Am J Emerg Med. 2008;26(2):124- 130.
- Fiesseler FW, Shih R, Szucs P, et al. Steroids for migraine headaches: a randomized double- blind, two-armed, placebo-controlled trial. J Emerg Med. 2011;40(4):463-468.
- Wasiak J, Anderson JN. Is dexamethasone effective in treating acute migraine headache? Med J Aust. 2002;176(2):83.
- Tobin J, Flitman S. Treatment of migraine with occipital nerve blocks using only corticosteroids. Headache. 2011;51(1):155-159.
- Ramachandran R. Neurogenic inflammation and its role in migraine. Semin Immunopathol. 2018;40(3):301-314.
- Amiri H, Ghodrati N, Nikuyeh M, et al. Granisetron and metoclopramide in the treatment of pain and emesis in migraine patients: a randomized controlled trial study. Turk J Emerg Med. 2017;17(2):61-64.
- Wider B, Pittler MH, Ernst E. Feverfew for preventing migraine. Cochrane Database Syst Rev. 2015;4(4):CD002286.
- Rafieian-Kopaei M, Hasanpour-Dehkordi A, Lorigooini Z, et al. Comparing the effect of intranasal lidocaine 4% with peppermint essential oil drop 1.5% on migraine attacks: a double- blind clinical trial. Int J Prev Med. 2019;10:121.
- Cady RK, Schreiber CP, Beach ME, Hart CC. Gelstat Migraine® (sublingually administered feverfew and ginger compound) for acute treatment of migraine when administered during the mild phase. Med Sci Monit. 2005;11(9):165-169.
- Friedman BW, Cabral L, Adewunmi V, et al. Diphenhydramine as adjuvant therapy for acute migraine: an emergency department-based randomized clinical trial. Ann Emerg Med. 2016;67(1):32-39.
- Patel PS, Minen MT. Complementary and integrative health treatments for migraine. J Neuroophthalmol. 2019;39(3):360-369.
- Wells RE, Beuthin J, Granetzke L. Complementary and integrative medicine for episodic migraine: an update of evidence from the last 3 years. Curr Pain Headache Rep. 2019;23(2):10.
- Giamberardino MA, Affaitati G, Costantini R, et al. Acute headache management in emergency department. A narrative review. Intern Emerg Med. 2020;15(1):109-117.
- Goldner JA, Levitt LP. Treatment of complicated migraine with sublingual nifedipine. Headache. 1987;27:484-486.
- Yu W, Horowitz SH. Familial hemiplegic migraine and its abortive therapy with intravenous verapamil. Neurology. 2001;57:1732.
- Hsu DA, Stafstrom CE, Rowley HA, et al. Hemiplegic migraine: hyperperfusion and abortive therapy with intravenous verapamil. Brain Dev. 2008;30(1):86-90.
- Hoffert MJ, Scholz MJ, Kanter R. A double-blind controlled study of nifedipine as an abortive treatment in acute attacks of migraine with aura. Cephalalgia. 1992;12(5):323-324.
- Silberstein SD. Preventive migraine treatment. Continuum (Minneap Minn). 2015;21(4 Headache):973-989.
- Silberstein SD. Practice parameter: evidence based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754-762.
- Dodick DW, Silberstein SD. Migraine prevention. Pract Neurol. 2007;7:383-393.
- Pringsheim T, Davenport W, Mackie G, et al. Canadian Headache Society guideline for migraine prophylaxis. Can J Neurol Sci. 2012;39(suppl 2):S1-S59.
- Loder E, Rizzoli P. Pharmacologic prevention of migraine: a narrative review of the state of the art in 2018. Headache. 2018;58(suppl 3):218-229.
- Kouremenos E, Arvaniti C, Constantinidis TS; Hellenic Headache Society. Consensus of the Hellenic Headache Society on the diagnosis and treatment of migraine. J Headache Pain. 2019;20(1):113.
- Blumenfeld AM, Bloudek LM, Becker WJ, et al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: results from the second international burden of migraine study (IBMS-II). Headache. 2013;53:644-655.
- Rizzoli P. Preventive pharmacotherapy in migraine. Headache. 2014;54:364-369.
- Estemalik E, Tepper S. Preventive treatment in migraine and the new US guidelines. Neuropsychiatr Dis Treat. 2013;9:709-720.
- Loder E, Burch R, Rizzoli P. The 2012 AHS/AAN guidelines for prevention of episodic migraine: a summary and comparison with other recent clinical practice guidelines. Headache. 2012;52(6):930-945.
- Ha H, Gonzalez A. Migraine headache prophylaxis. Am Fam Physician. 2019;99(1):17-24.
- Schwedt TJ. Preventive therapy of migraine. Continuum (Minneap Minn). 2018;24(4 Headache):1052-1065.
- Sinclair AJ, Sturrock A, Davies B, Matharu M. Headache management: pharmacological approaches. Pract Neurol. 2015;15(6):411-423.
- Gronseth GH, Cox J, Getchius TSD. Amendments to the 2011 American Academy of Neurology clinical practice guideline process manual. www.aan.com/siteassets/home- page/policy-and-guidelines/guidelines/about-guidelines/15processmanualamendment_v607.pdf Accessed September 2, 2021.
- Sprenger T, Viana M, Tassorelli C. Current prophylactic medications for migraine and their potential mechanisms of action. Neurotherapeutics. 2018;15(2):313-323.
- Levin M, Silberstein SD, Gilbert R, et al. Basic considerations for the use of monoclonal antibodies in migraine. Headache. 2018;58(10):1689-1696.
- Croop R, Lipton RB, Kudrow D, et al. Oral rimegepant for preventive treatment of migraine: a phase 2/3, randomised, double-blind, placebo-controlled trial. Lancet. 2021;397(10268):51-60.
- Jackson JL, Kuriyama A, Kuwatsuka Y, et al. Beta-blockers for the prevention of headache in adults, a systematic review and meta-analysis. PLoS One. 2019 Mar 20;14(3):e0212785.
- Silberstein SD. Topiramate in migraine prevention: a 2016 perspective. Headache. 2017;57(1):165-178.
- Freitag FG, Collins SD, Carlson HA, et al; Depakote ER Migraine Study Group. A randomized trial of divalproex sodium extended-release tablets in migraine prophylaxis. Neurology. 2002;58(11):1652-1659.
- Parikh SK, Silberstein SD. Current status of antiepileptic drugs as preventive migraine therapy. Curr Treat Options Neurol. 2019;21(4):16.
- Young WB, Siow HC, Silberstein SD. Anticonvulsants in migraine. Curr Pain Headache Rep. 2004;8(3):244-250.
- Romoli M, Costa C, Siliquini S, et al. Antiepileptic drugs in migraine and epilepsy: who is at increased risk of adverse events? Cephalalgia. 2018;38(2):274-282.
- Burch R. Antidepressants for preventive treatment of migraine. Curr Treat Options Neurol. 2019;21(4):18.
- Xu XM, Liu Y, Dong MX, et al. Tricyclic antidepressants for preventing migraine in adults. Medicine (Baltimore). 2017 Jun;96(22):e6989.
- Stovner LJ, Linde M, Gravdahl GB, et al. A comparative study of candesartan versus propranolol for migraine prophylaxis: a randomised, triple-blind, placebo-controlled, double cross-over study. Cephalalgia. 2014;34(7):523-532.
- Diener HC, Gendolla A, Feuersenger A, et al. Telmisartan in migraine prophylaxis: a randomized, placebo-controlled trial. Cephalalgia. 2009;29(9):921-927.
- Dorosch T, Ryon K, Ganzer C, et al. Angiotensin receptor blockers and ACE inhibitors for migraine prophylaxis—a systematic review. Presented at: 2019 American Academy of Neurology Annual Meeting. May 4-10, 2019; Philadelphia, PA.
- Dorosch T, Ganzer CA, Lin M, Seifan A. Efficacy of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in the preventative treatment of episodic migraine in adults. Curr Pain Headache Rep. 2019 12;23(11):85.
- Simpson DM, Hallett M, Ashman EJ, et al. Practice guideline update summary: botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2016;86(19):1818-1826.
- Botox (onabotulinumtoxin A) package insert. Madison, NJ: Allergan USA, Inc; July 2021.
- Tassorelli C, Aguggia M, De Tommaso M, et al. Onabotulinumtoxin A for the management of chronic migraine in current clinical practice: results of a survey of sixty-three Italian headache centers. J Headache Pain. 2017;18(1):66.
- Herd CP, Tomlinson CL, Rick C, et al. Cochrane systematic review and meta-analysis of botulinum toxin for the prevention of migraine. BMJ Open. 2019;9(7):e027953.
- Bendtsen L, Sacco S, Ashina M, et al. Guideline on the use of onabotulinumtoxinA in chronic migraine: a consensus statement from the European Headache Federation. J Headache Pain. 2018 Sep 26;19(1):91.
- Ornello R, Guerzoni S, Baraldi C, et al. Sustained response to onabotulinumtoxin A in patients with chronic migraine: real-life data. J Headache Pain. 2020;21(1):40.
- Vyepti (eptinezumab-jjmr) package insert. Bothell, WA: Lundbeck Seattle BioPharmaceuticals, Inc; September 2021.
- Ajovy (fremanezumab-vfrm) package insert. North Wales, PA: Teva Pharmaceuticals USA Inc; September 2021.
- Aimovig (erenumab-aooe) package insert. Thousand Oaks, CA: Amgen Inc; November 2021.
- Emgality (galcanezumab-gnlm) package insert. Indianapolis, IN: Eli Lilly and Co; June 2019.
- Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377(22):2123-2132.
- Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16(6):425-434.
- Dodick DW, Ashina M, Brandes JL, et al. ARISE: a phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia. 2018;38(6):1026-1037.
- Reuter U, Goadsby PJ, Lanteri-Minet M, et al. Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: a randomised, double-blind, placebo-controlled, phase 3b study. Lancet. 2018;392(10161):2280-2287.
- Tepper SJ, Diener HC, Ashina M, et al. Erenumab in chronic migraine with medication overuse: subgroup analysis of a randomized trial. Neurology. 2019;92(20):e2309-e2320.
- Saely S, Croteau D, Jawidzik L, et al. Hypertension: a new safety risk for patients treated with erenumab. Headache. 2021;61(1):202-208.
- Detke HC, Goadsby PJ, Wang S, et al. Galcanezumab in chronic migraine: the randomized, double-blind, placebo-controlled REGAIN study. Neurology. 2018;91(24):e2211-e2221.
- Stauffer VL, Dodick DW, Zhang Q, et al. Evaluation of galcanezumab for the prevention of episodic migraine: the EVOLVE-1 randomized clinical trial. JAMA Neurol. 2018;75(9):1080- 1088.
- Skljarevski V, Oakes TM, Zhang Q, et al. Effect of different doses of galcanezumab vs placebo for episodic migraine prevention: a randomized clinical trial. JAMA Neurol. 2018;75(2):187-193.
- Ament M, Day K, Stauffer VL, et al. Effect of galcanezumab on severity and symptoms of migraine in phase 3 trials in patients with episodic or chronic migraine. J Headache Pain. 2021;22:6.
- Ford JH, Ayer DW, Zhang Q, et al. Two randomized migraine studies of galcanezumab: effects on patient functioning and disability. Neurology. 2019;93(5):e508-e517.
- Skljarevski V, Matharu M, Millen BA, et al. Efficacy and safety of galcanezumab for the prevention of episodic migraine: results of the EVOLVE-2 phase 3 randomized controlled clinical trial. Cephalalgia. 2018;38(8):1442-1454.
- Mulleners WM, Kim BK, Láinez MJ, et al. Safety and efficacy of galcanezumab in patients for whom previous migraine preventive medication from two to four categories had failed (CONQUER): a multicentre, randomised, double-blind, placebo-controlled, phase 3b trial. Lancet Neurol. 2020;19(10):814-825.
- Brandes JL, Kudrow D, Yeung PP, et al. Effects of fremanezumab on the use of acute headache medication and associated symptoms of migraine in patients with episodic migraine. Cephalalgia. 2020;40(5):470-477.
- Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med. 2017;377(22):2113-2122.
- Winner PK, Spierings EL, Yeung PP, et al. Early onset of efficacy with fremanezumab for the preventive treatment of chronic migraine. Headache. 2019 ;59(10):1743-1752.
- Ferrari MD, Diener HC, Ning X, et al. Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): a randomised, double-blind, placebo-controlled, phase 3b trial. Lancet. 2019;394(10203):1030-1040.
- Ashina M, Saper J, Cady R, et al. Eptinezumab in episodic migraine: a randomized, double- blind, placebo-controlled study (PROMISE-1). Cephalalgia. 2020;40(3):241-254.
- Smith TR, Janelidze M, Chakhava G, et al. Eptinezumab for the prevention of episodic migraine: sustained effect through 1 year of treatment in the PROMISE-1 study. Clin Ther. 2020;42(12):2254-2265.
- Lipton RB, Goadsby PJ, Smith J, et al. Efficacy and safety of eptinezumab in patients with chronic migraine: PROMISE-2. Neurology. 2020;94(13):e1365-e1377.
- Silberstein S, Diamond M, Hindiyeh NA, et al. Eptinezumab for the prevention of chronic migraine: efficacy and safety through 24 weeks of treatment in the phase 3 PROMISE-2 (prevention of migraine via intravenous ALD403 safety and efficacy-2) study. J Headache Pain. 2020;21(1):120.
- Cohen JM, Ning X, Kessler Y, et al. Immunogenicity of biologic therapies for migraine: a review of current evidence. J Headache Pain. 2021;22:3.
- Borkum JM. CGRP and brain functioning: cautions for migraine treatment. Headache. 2019;59(8):1339-1357.
- Kudrow D, Pascual J, Winner PK, et al. Vascular safety of erenumab for migraine prevention. Neurology. 2020;94:e497-e510.
- Ashina M, Goadsby PJ, Reuter U, et al. Long-term safety and tolerability of erenumab: three-plus year results from a five-year open-label extension study in episodic migraine. Cephalalgia. 2019;39(11):1455-1464.
- Depre C, Antalik L, Starling A, et al. A randomized, double-blind, placebo-controlled study to evaluate the effect of erenumab on exercise time during a treadmill test in patients with stable angina. Headache. 2018;58(5):715-723.
- Oakes TM, Kovacs R, Rosen N, et al. Evaluation of cardiovascular outcomes in adult patients with episodic or chronic migraine treated with galcanezumab: data from three phase 3, randomized, double-blind, placebo-controlled EVOLVE-1, EVOLVE-2, and REGAIN studies. Headache. 2020;60(1):110-123.
- Tringali G, Navarra P. Anti-CGRP and anti-CGRP receptor monoclonal antibodies as antimigraine agents. Potential differences in safety profile postulated on a pathophysiological basis. Peptides. 2019;116:16-21.
- In brief: hypertension with erenumab (Aimovig). Med Lett Drugs Ther. 2021;63(1621):56.
- Noseda R, Bedussi F, Gobbi C, Zecca C. Safety profile of erenumab, galcanezumab and fremanezumab in pregnancy and lactation: analysis of the WHO pharmacovigilance database. Cephalalgia. 2021;41:789-798.
- Qulipta (atogepant) package insert. Dublin, Ireland: Forest Laboratories Ireland Ltd; October 2021.
- Shanmugam S, Karunaikadal K, Varadarajan S, Krishnan M. Memantine ameliorates migraine headache. Ann Indian Acad Neurol. 2019;22(3):286-290.
- Mistry VM, Morizio PL, Pepin MJ, et al. Role of memantine in the prophylactic treatment of episodic migraine: a systematic review. Headache. 2021;61:1207-1213.
- Peikert A, Wilimzig C, Köhne-Volland R. Prophylaxis of migraine with oral magnesium: results from a prospective, multi-center, placebo-controlled and double-blind randomized study. Cephalalgia. 1996;16(4):257-263.
- Pascual J, Caminero AB, Mateos V, et al. Preventing disturbing migraine aura with lamotrigine: an open study. Headache. 2004;44(10):1024-1028.
- Thompson DF, Saluja HS. Prophylaxis of migraine headaches with riboflavin: a systematic review. J Clin Pharm Ther. 2017;42(4):394-403.
- Schoenen J, Jacquy J, Lenaerts M. Effectiveness of high-dose riboflavin in migraine prophylaxis. A randomized controlled trial. Neurology. 1998;50(2):466-470.
- Evans RW, Pascual J, Láinez MJ, Leira R. Bending the rule of monotherapy for migraine prevention? Headache. 2005;45(6):748-750.
- Pfaffenrath V, Kellhammer U, Pöllmann W. Combination headache: practical experience with a combination of a beta-blocker and an antidepressive. Cephalalgia. 1986;6(6 suppl 5):25- 32.
- Pascual J, Leira R, Láinez JM. Combined therapy for migraine prevention? Clinical experience with a beta-blocker plus sodium valproate in 52 resistant migraine patients. Cephalalgia. 2003;23(10):961-962.
- Pascual J, Rivas MT, Leira R. Testing the combination beta-blocker plus topiramate in refractory migraine. Acta Neurol Scand. 2007;115(2):81-83.
- Silberstein SD, Dodick DW, Lindblad AS, et al; Chronic Migraine Treatment Trial Research Group. Randomized, placebo-controlled trial of propranolol added to topiramate in chronic migraine. Neurology. 2012;78(13):976-984.
- Wong SL, Cavanaugh J, Shi H, Awni WM, Granneman GR. Effects of divalproex sodium on amitriptyline and nortriptyline pharmacokinetics. Clin Pharmacol Ther. 1996;60(1):48-53.
- Ganji R, Majdinasab N, Hesam S, et al. Does atorvastatin have augmentative effects with sodium valproate in prevention of migraine with aura attacks? A triple-blind controlled clinical trial. J Pharm Health Care Sci. 2021;7(1):12.
- Khani S, Hejazi SA, Yaghoubi M, Sharifpour E. Comparative study of magnesium, sodium valproate, and concurrent magnesium-sodium valproate therapy in the prevention of migraine headaches: a randomized controlled double-blind trial. J Headache Pain. 2021;22:21.
- Powers SW, Kashikar-Zuck SM, Allen JR, et al. Cognitive behavioral therapy plus amitriptyline for chronic migraine in children and adolescents: a randomized clinical trial. JAMA. 2013;310(24):2622-2630.
- Blumenfeld AM, Frishberg BM, Schim JD, et al. Real-world evidence for control of chronic migraine patients receiving CGRP monoclonal antibody therapy added to onabotulinumtoxinA: a retrospective chart review. Pain Ther. 2021;10:809-826.
- Dodick DW, Silberstein SD. Migraine prevention. Pract Neurol. 2007;7:383-393.
- Stauffer VL, Wang S, Voulgaropoulos M, et al. Effect of galcanezumab following treatment cessation in patients with migraine: results from 2 randomized phase 3 trials. Headache. 2019;59(6):834-847.
- Hepp Z, Dodick DW, Varon SF, et al. Adherence to oral migraine-preventive medications among patients with chronic migraine. Cephalalgia. 2015;35:478-488.
- Hepp Z, Dodick DW, Varon SF, et al. Persistence and switching patterns of oral migraine prophylactic medications among patients with chronic migraine: a retrospective claims analysis. Cephalalgia. 2017;37(5):470-485.
- Lipton RB, Stewart WF. Acute migraine therapy: do doctors understand what migraine patients want from therapy? Headache. 1999;39(suppl 2):S20-S26.
- Malik SN, Hopkins M, Young WB, Sliberstein SD. Acute migraine treatment: patterns of use and satisfaction in a clinical population. Headache. 2006;46:773-780.
- Lipton RB, Dodick D, Sadovsky R, et al; ID Migraine validation study. A self-administered screener for migraine in primary care: the ID Migraine validation study. Neurology. 2003;61(3):375-382.
- Blumenfeld A, Tischio M. Center of excellence for headache care: group model at Kaiser Permanente. Headache. 2003;43(5):431-440.