The Role of Pharmacotherapy in Endometriosis
RELEASE DATE
September 1, 2023
EXPIRATION DATE
September 30, 2025
FACULTY
Katherine Hale, PharmD, BCPS, MFA
Clinical Pharmacist
Kadlec Regional Medical Center
Richland, Washington
FACULTY DISCLOSURE STATEMENTS
Dr. Hale has no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT
 Pharmacy
Pharmacy
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-23-089-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients’ conditions and possible contraindications or dangers in use, review of any applicable manufacturer's product information, and comparison with recommendations of other authorities.
GOAL
The purpose of this activity is to discuss pharmacotherapy options for the treatment of endometriosis.
OBJECTIVES
After completing this activity, the participant should be able to:
- Review risk factors, clinical presentation, and diagnosis of endometriosis.
- Explain the pathogenesis of endometriosis.
- Identify medication interventions for the management of endometriosis.
- Recognize the role of the pharmacist in endometriosis management.
ABSTRACT: Endometriosis is characterized by deposits of endometrial tissue external to the uterus, impacting many individuals of reproductive age and affecting many areas of life. Presenting symptoms may be ambiguous, with pain as a common factor. Multiple modalities for medical and surgical management exist. Pharmacotherapy options include nonsteroidal anti-inflammatory drugs and medications that affect hormone processes. Gonadotropin-releasing hormone antagonist therapy options are the newest class of medications FDA-approved for endometriosis management. Pharmacists may help improve outcomes by assisting in medication optimization and providing patient and provider education regarding pharmacotherapy options and self-care.
Endometriosis occurs when endometrial tissue is deposited at sites external to the normal uterine cavity, primarily affecting women within reproductive age.1-3 Endometriosis has also been reported in premenarche and postmenopausal females, though rare. Lesion growth is estrogen-dependent and typically deposits on the ovaries, peritoneum, and bowels. Formally, endometriosis is defined as the presence of endometrium-like epithelium and/or stroma outside the endometrium and myometrium, usually with an associated inflammatory process.3
True incidence of endometriosis is challenging to elucidate due to the complexity of its presentation, often with nonspecific or variable symptoms. In individuals evaluated for chronic pelvic pain, endometriosis is estimated to be prevalent in up to 70% of cases.2 Chronic pelvic pain or pressure, dyspareunia, and severe pain during menses are the hallmark symptoms of endometriosis. This benign inflammatory condition may also result in heavy menstrual bleeding, abnormal uterine bleeding, abdominal pain, low back pain, and/ or infertility.2,4 Symptoms may worsen at time of menstruation and improve when menstruation is complete.2,4-6 Risk factors for endometriosis development are summarized in TABLE 1.
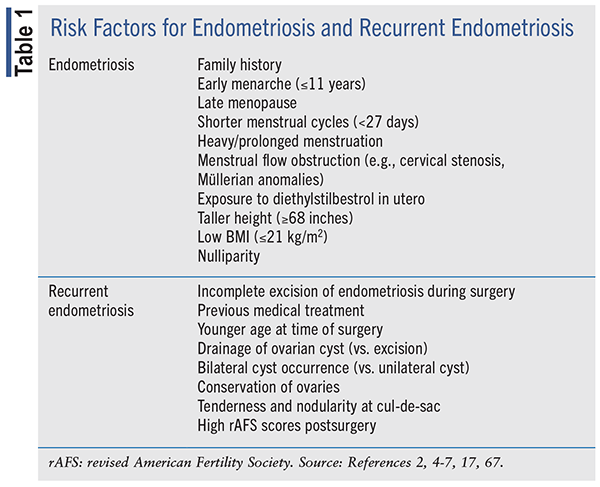
Recurrence of endometriosis after surgical or medical management ranges from 6% to 67% depending on how recurrence is defined.7 Studies define recurrence as relapse of pain (symptom-based), lack of fertility improvement, or lesion revisualization by ultrasound or surgery (imaging-based or laparoscopically proven).3 Studies evaluating recurrence have variable cohort size and duration of follow-up.7 At 2 and 5 years postsurgery, endometriosis has been reported to occur in 21.5% and 40% to 50% of patients, respectively, and also appears to occur at a higher rate in younger age groups.8,9 The cause of recurrent endometriosis is not clear.
The impact of endometriosis is felt across multiple domains. Quality of life, mental health, intimate relationships and family planning, work, and education have all shown to be affected. Pain is a contributing factor in all categories.10 The economic burden of endometriosis has been estimated to be as high as $12,000 to $16,000 per patient per year in direct and indirect costs.11,12 One review estimated an annual cost in the United States of approximately $22 billion.13 Endometriosis has been associated with reduced quality of life, with pain having the strongest correlation.14
PATHOGENESIS
Multiple theories exist regarding the pathogenesis of endometriosis.1 Retrograde menstruation, where about 10% of menstrual blood products flows into the peritoneal cavity through the fallopian tubes, is the most commonly accepted theory. Cells and tissue sloughed from the endometrium are thought to then implant outside the uterus and grow via estrogen-mediated processes. Sloughed tissue may even be transported by the vasculature or the lymphatic system to distant sites. Lymphatic and/or hematogenous spread, Müllerian rests, invagination, inflammation, genomic alterations, coelomic metaplasia, and iatrogenic spread are alternative pathogenesis theories.1,15,16
Several causal mechanisms of pain in endometriosis have been proposed. Increased TNF- α, interleukins (1β, 6, 8, and 33), insulin-like growth factor-1, vascular endothelial growth factor, cytokines, and macrophages play a role in stimulating the inflammatory response. Bleeding from endometrial implants, pelvic floor nerve irritation, and infiltration of pelvic floor nerves by endometrial implants are also thought to lead to endometriosis pain.15-17
CLINICAL PRESENTATION
Presenting symptoms of endometriosis are ambiguous. Most frequently reported symptoms include dysmenorrhea, chronic pelvic pain, ovulatory pain, dyspareunia, dysuria, dyschezia, urinary urgency/frequency, low back pain, chronic fatigue, and infertility.2,4
DIAGNOSIS
Diagnosis may be made based on signs/symptoms, physical exam, imaging, ultrasound, visual inspection, cystoscopy or biopsy, or laparoscopic exam. However, these methods are not able to confirm or rule out endometriosis. Diagnosis by surgery is the most definitive.2,17
MANAGEMENT OF ENDOMETRIOSIS
Several guidelines exist regarding the management of endometriosis.18 Most recently updated are the European Society of Human Reproduction and Embryology guidelines in 2022.2 Each guideline provides evidence-based recommendations regarding surgical or pharmacologic symptom management of pain and/or infertility. Because endometriosis is complex, with variable symptom presentation and multiple modalities for management, agreement between the most widely used guidelines is estimated to be about 7%.18
Nonsteroidal anti-inflammatory drugs (NSAIDs), combined hormonal contraceptives (CHCs), and progestin-only medications are mainstays in empiric therapy. Gonadotropin-releasing hormone (GnRH) agonists, GnRH antagonists, and danazol may also be used and are considered second- and third-line therapies. Aromatase inhibitors are not currently FDA approved for this indication but have been evaluated in the reduction of pain associated with endometriosis.2,4-6,17,18
Treatment goals include improvement of quality of life by decreasing overall pain and symptoms, decreasing or slowing endometriosis growth, decreasing the chance of or delay recurrence, and maintaining or restoring fertility.2,4,5,17
Pharmacotherapy options for medical management of endometriosis are summarized in TABLE 2.
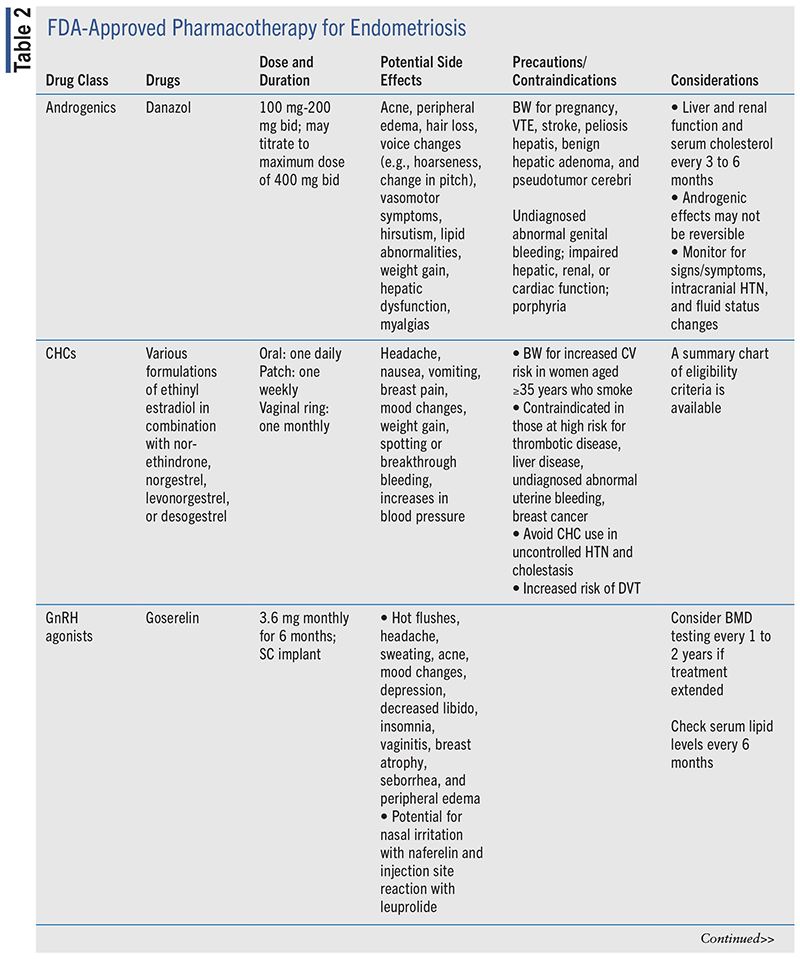
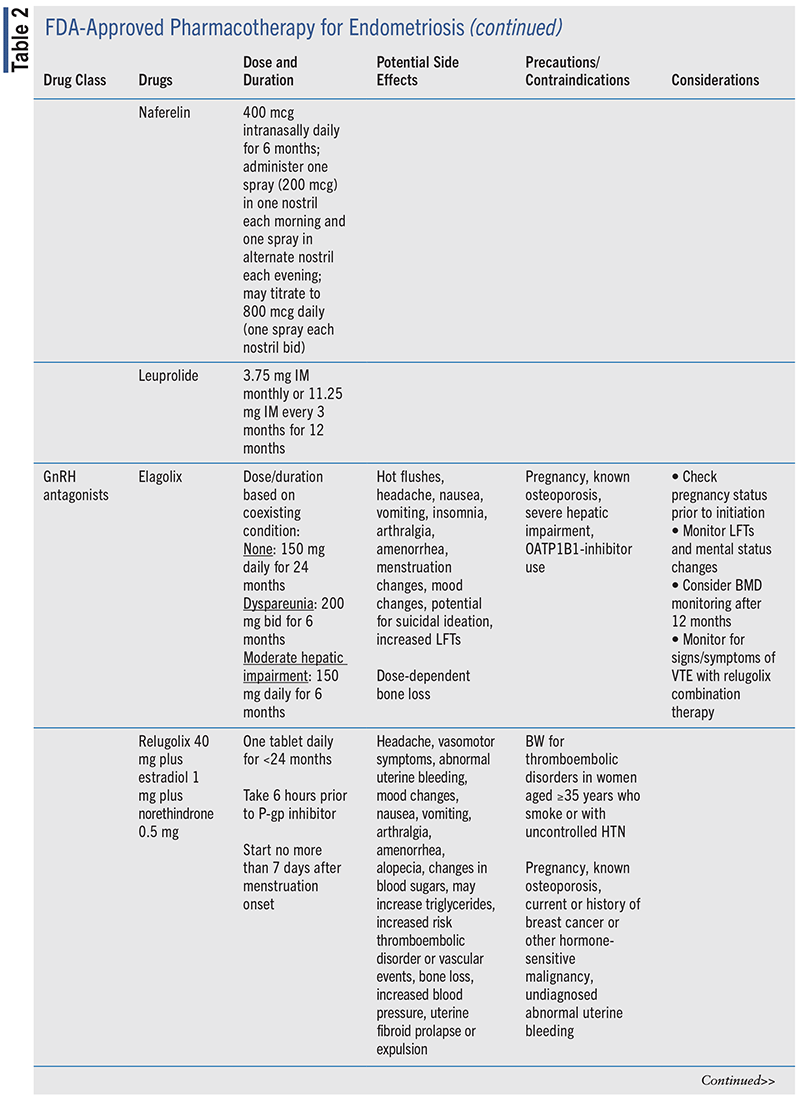
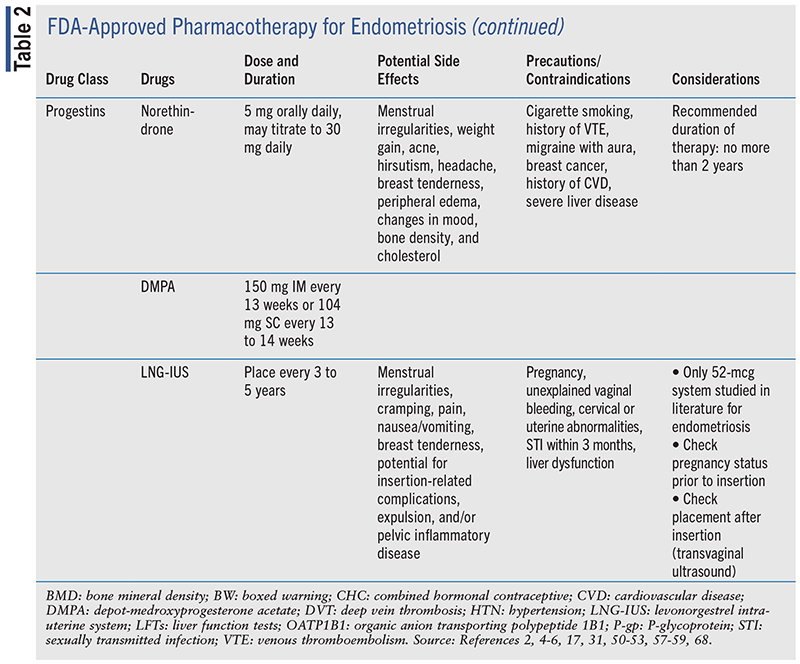
NSAIDs
By reversibly inhibiting cyclooxygenase (COX)-1 and COX-2 enzymes, NSAIDs block endometrial prostaglandin E2 (PGE2) and E1 production, decreasing pain and swelling associated with dysmenorrhea.4,19,20 NSAID therapy should start 1 to 2 days prior to menstruation and continue through the first 2 to 3 days for best efficacy.6 Low-dose ibuprofen and naproxen sodium are available OTC, while alternate NSAID therapies or higher doses of ibuprofen and naproxen sodium are prescription only. Trial of an alternate NSAID is recommended if the first choice is ineffective.6
While one Cochrane review of 80 randomized, controlled trials (RCTs) identified effective pain reduction with NSAID for dysmenorrhea, a second Cochrane review of NSAID therapy in the treatment of pain associated with endometriosis found a lack of evidence available to support efficacy in this population.19,20
NSAIDs are not without side effects, with nausea, dyspepsia, diarrhea, and constipation being most prominent. This may be mitigated by taking with a meal and maintaining hydration. NSAIDs should be avoided in patients with history of gastric ulcer or peptic ulcer disease, and in patients taking anticoagulants due to increased risk of bleeding. NSAIDs also increase cardiovascular risk and carry a boxed warning identifying increased risk of thrombotic cardiovascular events such as heart attack and stroke. Use cautiously in patients with history of or at risk for renal insufficiency.21,22
Advantages of NSAID use include OTC availability and low cost. NSAIDs, however, may not fully resolve discomfort due to estrogen-mediated effects of endometriosis.
CHCs
CHCs consist of a combination of estrogen and progestin and may be used cyclically or continuously to reduce symptoms of dysmenorrhea, dyspareunia, and pelvic pain.2,4,5,17 Estrogens affect the anterior pituitary gland, and progesterone/progestins affect the hypothalamus. This negative feedback loop results in less secretion of GnRH, follicle-stimulating hormone (FSH), and luteinizing hormone (LH). This leads to less menstrual flow, and cell proliferation is inhibited.23,24 Additional effects are progesterone mediated with pseudo-decidualization of the endometrium, inhibition of angiogenesis, and increased cell apoptosis.24
A systematic review of two RCTs evaluating the use of oral CHCs in the treatment of endometriosis found self-reported pain from dysmenorrhea was less with CHC use compared with placebo.25 A cohort study evaluated the efficacy of a contraceptive ring or patch in reducing endometriosis pain in 207 women.26 Participants were treated with 12 months of continuous therapy with a vaginal ring (ethinyl estradiol 15 mcg and etonogestrel 120 mcg daily) or a transdermal patch (ethinyl estradiol 20 mcg and norelgestromin 150 mcg daily). Choice of therapy was per patient preference, with 60% preferring the ring over the patch. Pain symptoms decreased in each group, but rates of irregular bleeding were higher with continuous use. For participants with rectovaginal endometriosis, more pain relief was noted with the ring. Pain during intercourse was also reduced.26 A single double-blind RCT compared low-dose oral CHC with placebo in reducing dysmenorrhea associated with endometriosis in 100 women. Significant reductions in dysmenorrhea as measured by verbal rating scores occurred in the oral CHC group (P <.0001).27
Use of CHCs compared with no treatment, placebo, or an active comparator in the reduction of dysmenorrhea, pelvic pain, and dyspareunia caused by endometriosis was evaluated in a systematic review (n = 9 observational studies; n = 9 RCTs).28 CHC use significantly decreased pain from baseline, and continuous CHC administration appeared more effective; however, authors noted that evidence quality was low, and more studies are needed.28 Continuous CHC use has been shown to reduce pain with few side effects and high satisfaction with CHC therapy.29
While additional studies are needed regarding the use of CHCs in the treatment of endometriosis to further elucidate the effect of CHCs on pain reduction, cyclic or continuous use of oral CHCs, the patch, or the ring is recommended as empiric therapy.2,4-6,17 Advantages of CHC use include lower costs, the variety of options, and availability. Compared with GnRH agonist/antagonist therapies and danazol, CHC use has greater tolerability and no effects on bone mineral density (BMD); however, CHC use may increase risk of venous thromboembolism (VTE).4,25 Additionally, oral formulations must be taken daily, which may affect medication adherence.
Progestins
Due to the negative feedback loop on the hypothalamus, progesterone monotherapy reduces GnRH, FSH, and LH secretion and results in ovulation suppression, a pseudo-pregnancy state, decreased production of PGE2, and atrophy of endometrial implants.30 Patients who are not responding to CHCs or in whom CHC use is contraindicated may find relief with oral, injectable, implantable, or intrauterine progestin-only therapy.
Norethindrone acetate is FDA approved for endometriosis and has been shown to reduce dysmenorrhea and noncyclic pelvic pain.31-33 A retrospective cohort study of 103 women with pain caused by rectovaginal endometriosis evaluated variation in pain symptoms and satisfaction with norethindrone therapy over 5 years.34 A decrease in chronic pelvic pain and dyspareunia was observed (P <.001 vs. baseline at 1 and 5 years). Satisfaction with norethindrone therapy was high, with 68.8% of participants rating satisfied or very satisfied with therapy.34
Medroxyprogesterone acetate (MPA), given orally or as an intramuscular or subcutaneous injection, has been shown to reduce symptoms of endometriosis.35,36 A Cochrane review of 13 RCTs found that compared with placebo, MPA in doses of 100 mg daily was more effective at decreasing endometriosis symptoms for up to 12 months (mean deviation -0.70, 95% CI -8.61 to -5.39; P <.0001).30 An RCT of 300 women treated with 104 mg of subcutaneous depot-medroxyprogesterone acetate (DMPA) compared with leuprolide (3.75 mg or 11.25 mg) every 3 months for 12 months found that DMPA was just as effective as leuprolide in pain reduction and achieved less bone loss.37 A second RCT, also comparing the effectiveness of subcutaneous DMPA to leuprolide, found that DMPA was equivalent to leuprolide in reducing dysmenorrhea, pelvic pain, and dyspareunia after 12 months of follow-up.38 Less bone loss occurred in both studies, but more irregular bleeding was identified.
Administration of 150 mg IM MPA every 3 months was compared with a cyclic monophasic oral CHC (20 mcg ethinyl estradiol and desogestrel 150 mcg) plus 50 mg danazol daily for 21 days of each 28-day cycle. Satisfaction scores and symptom severity were evaluated upon completion of study at 1 year. Satisfaction scores were higher in the MPA group (72.5%) compared with the CHC plus danazol group (57.5%), with fewer dysmenorrhea symptoms in the MPA group.39
Limited studies exist for the use of levonorgestrel-releasing intrauterine system (LNG-IUS) for pain reduction in endometriosis.40 Studies evaluating treatment with LNG-IUS versus expectant management after laparoscopic surgery have found that frequency and severity of dysmenorrhea were reduced. Recurrence of moderate-to-severe dysmenorrhea occurred in 7% to 10% of patients compared with 39% to 45% of surgery-only patients. Therapy satisfaction scores were also higher in the LNG-IUS groups compared with expectant management groups.41,42 Use of postoperative LNG-IUS was compared with the GnRH analogue goserelin in the reduction of chronic pelvic pain in 40 women with severe endometriosis for 12 months. Pain severity decreased in both groups at 3- and 6-month follow-ups but increased to pre-treatment levels at 12 months for the LNG-IUS group. Irregular bleeding was reported at greater frequency with LNG-IUS.43 While LNG-IUS is used postsurgery to reduce endometriosis symptoms, there are a paucity of data regarding its efficacy. Higher-quality studies with larger sample sizes are needed to fully elucidate the effects of LNG-IUS on endometriosis symptoms.40
Few studies evaluating the effects of implantable progestins are available. One small, open, prospective RCT in 41 participants compared the effect of etonogestrel implant to depot MPA on pain reduction in endometriosis at 1 year. Both groups had pain reduction (68% etonogestrel and 53% DMPA) with similar side-effect profiles and patient satisfaction scores.44 A comparison of etonogestrel implant to LNG-IUS found that dysmenorrhea and chronic pelvic pain were significantly reduced over the 2-year study duration.45
The multiple options for medication administration and improved side-effect profile compared with alternate therapies are advantages of progestin-only therapy. There is less risk of bone loss and VTE. Disadvantages include potential for insertion-related complications and increased risk of pelvic inflammatory disease with LNG-IUS.4
GnRH Agonists
GnRH agonists are considered second- or third-line therapy in individuals aged 18 years or older who do not respond to CHCs or progestin-only therapy and may be used in individuals aged 16 years or older for pain not responsive to conservative surgical management and/or CHC or progestin-only therapies.2,5,17,46 GnRH agonists inhibit FSH and LH secretion by binding to pituitary GnRH receptors. During the first 10 days of therapy, FSH and LH are actually stimulated, which can increase pain symptoms of endometriosis (flare effect). After this, flare downregulation of GnRH receptors occurs, leading to suppression of FSH, LH, and estrogen levels. Lesion regression due to amenorrhea provides symptom relief.4,24
A Cochrane systematic review of 41 trials (N = 4,935) comparing GnRH agonists with placebo determined GnRH agonists to be more efficacious in symptom relief of pelvic tenderness and overall endometriosis symptom severity score. No difference was found between GnRH agonists and danazol or LNG-IUS. No difference was found between choice of GnRH agonists or method of delivery (nasal vs. injection).47
Six months of GnRH agonist therapy following laparoscopic surgery, followed by continuous monophasic CHC use for 6 months, was found to reduce pain scores and need for hysterectomy.48 Treatment with GnRH agonist therapy is expected to provide 50% to 90% reduction in pain, especially for deep or extrapelvic endometriosis.49
Despite the effectiveness of GnRH agonist therapy in reduction of symptoms of endometriosis, use is limited by side-effect profiles and return of pain after stopping therapy. Use of GnRH agonists results in bone loss, vasomotor symptoms (e.g., hot flushes, vaginal dryness), sleep disturbances, urogenital atrophy, and mood changes.4,46,50-53 To improve tolerability, add-back therapy with an estrogen, progestin, or combination of the two may be used to continue providing endometriosis symptom relief and improve adherence while preventing growth of new endometrial tissue.4,46 Norethindrone 2.5 mg to 5 mg daily is recommended for progestin-only add-back therapy, where conjugated estrogens 0.625 mg daily may be used for estrogen-only add-back. Higher doses of estrogen, such as estradiol 1 mg daily or conjugated estrogen 1.25 mg daily, were found to reduce side effects of GnRH agonist therapy but resulted in increased pelvic pain.46,54 For women who are not able to tolerate higher doses of progestin-only therapy, a combination of norethindrone and low-dose estrogen has been studied, though it is not a currently approved regimen by the FDA.46
Duration of GnRH agonist therapy is limited to 6 to 12 months depending on preparation due to concerns for bone loss.50-53
The efficacy of GnRH agonists and longer intervals needed between medication dose administration are advantages to GnRH agonist use; however, duration of use is limited, and significant vasomotor symptoms may occur. Vasomotor symptoms and bone loss can be addressed by using add-back therapy. Additionally, medications may be cost prohibitive, and no oral formulations are available.
GnRH Antagonists
Perhaps the newest therapies for the treatment of endometriosis are oral GnRH antagonists. These medica-tions act within the pituitary to suppress gonadotropin hormone production by direct antagonism of GnRH receptors. This direct antagonism quickly decreases pituitary secretion of gonadotropin, and the flare effect that occurs with GnRH agonists is avoided.24,46 The effect of GnRH antagonists is dose dependent, producing partial or full estrogen suppression, and is quickly reversible.46,55
Elagolix (approved in 2018) and relugolix (approved in 2020) have been shown to reduce moderate or severe endometriosis-associated pain in women with surgically diagnosed endometriosis.55-58 The Elaris EM-I and Elaris EM-II phase III RCTs evaluated elagolix at low (150 mg daily) and high (200 mg twice daily) doses in the reduction of pain associated with dysmenorrhea and nonmenstrual pelvic pain at 3-month follow-up in 872 and 817 women, respectively.55 Low-dose elagolix decreased dysmenorrhea in 43% to 46% and nonmenstrual pelvic pain in 50% of women compared with placebo (P <.001 and P = .003). High-dose elagolix achieved a greater response in both dysmenorrhea (72%-76%) and nonmenstrual pelvic pain (54%-58%) compared with placebo (P <.001).55
SPIRIT 1 and SPIRIT 2 were phase III RCTs (N = 638 and N = 623, respectively) evaluating treatment of moderate-to-severe endometriosis pain with once-daily relugolix combination therapy (relugolix 40 mg, estradiol 1 mg, norethisterone acetate 0.5 mg) or delayed relugolix combination therapy (12 weeks of relugolix 40-mg monotherapy followed by 12 weeks of relugolix combination therapy) compared with placebo.56 Similar reductions in dysmenorrhea and nonmenstrual pelvic pain occurred in both SPIRIT 1 and SPIRIT 2 across both treatment groups. Reduction of dysmenorrhea symptoms occurred in 72% to 75% of those treated with relugolix combination therapy (delayed or not) compared with 30% of placebo (P <.0001). Nonmenstrual pelvic pain was reduced in 53% to 66% of participants compared with 40% for placebo (P <.0001).56
Prior to starting GnRH-antagonist therapy, several factors should be considered. Pregnancy should be excluded prior to start of therapy. Duration of GnRH-antagonist use is limited by potential for dose-dependent bone loss to occur. Nonhormonal contraception during elagolix therapy and for 28 days upon completion of therapy is recommended. Relugolix combination therapy should be discontinued if gallbladder disease develops. Monitor for potential drug-drug interactions with GnRH antagonists. Watch for strong cytochrome P450 3A4 (CYP3A4) inducers and P-glycoprotein (P-gp) inhibitors with relugolix combination therapy and for P-gp, CYP3A4, and cytochrome P450 2C19 (CYP2C19) inhibition with elagolix.57,58
Compared with GnRH agonists, the GnRH antagonists are available as oral formulations and have no flare effect upon start. Similarly, GnRH antagonists may cause significant vasomotor symptoms and bone loss and also have limited durations of use.
Danazol
Once the mainstay for treatment of endometriosis, danazol is now recommended by most guidelines as last-line therapy if treatment failure results with CHCs, progestins, GnRH agonists, or GnRH antagonists.2,4,5,18 This is primarily due to the significant side effects that occur in patients taking danazol. Side effects include acne, weight gain, menstrual spotting or amenorrhea, hirsutism, hair loss, potentially irreversible voice changes (e.g., hoarseness, pitch changes, voice deepening), vasomotor symptoms, and hepatic dysfunction.59 A Cochrane review of five studies identified that danazol therapy decreased pain in endometriosis but the occurrence of androgenic side effects limited its role in therapy.60
Aromatase Inhibitors
Aromatase inhibitors have been studied in patients for whom alternate therapies have failed and continued pain and symptoms occur; however, they are not currently FDA-approved for the treatment of endometriosis.2,4,17,61-65 Aromatase P450 is an enzyme that catalyzes estrogen formation from androgens. Individuals without endometriosis have no aromatase activity in the endometrial tissue, yet aromatase activity has been found to be present at higher levels in endometriosis and stimulated by PGE2, which regulates estrogen synthesis in endometriosis causing inflammation.61,62
A systematic review of 10 studies (N = 251) assessed the efficacy of aromatase inhibitors in endometriosis pain reduction.63 When used in combination with a progestin or CHC, aromatase inhibitors were found to improve quality of life and decrease pain. Combining aromatase inhibitors with GnRH agonists reduced the recurrence of endometriosis after surgery.63 In a small, prospective trial of 14 premenopausal patients with refractory endometriosis and chronic pelvic pain, significant pain reduction of 40% occurred at 6 months when treated with anastrozole 1 mg daily plus CHC (ethinyl estradiol 20 mcg, levonorgestrel 0.1 mg).64 Letrozole combined with norethindrone decreased nonmenstrual pelvic pain with few side effects.65
Current guidelines identify aromatase inhibitors for use in refractory endometriosis not responsive to alternate medical or surgical therapies. Aromatase inhibitors may be used in conjunction with CHCs, progestins, GnRH agonists, or GnRH antagonists.2,18 Letrozole is administered as 2.5 mg orally daily, while anastrozole is 1 mg orally daily. Potential side effects include hot flushes, bone loss, arthralgias, myalgias, and decreased BMD. If treatment is extended beyond 2 years, BMD testing should be considered.2,4,49
Surgery
Surgical management of endometriosis (typically laparoscopy) should be considered for adolescents and adults if pain persists despite maximally tolerated doses of NSAIDs or hormone-based therapies.2,66 Surgery should also be considered for those who have medication contraindications, need a tissue-diagnosis of endometriosis, need to exclude malignancy in an adnexal mass, or have bowel or urinary tract obstruction due to endometriosis.2,61 While surgery can be used to evaluate endometriosis and provide a histologic diagnosis or assessment for potential malignancy of cysts or masses, it also reduces pain by destroying endometrial implants.17,66
ROLE OF THE PHARMACIST
Pharmacists are highly accessible heathcare providers to patients and caregivers in the community. Pharmacists are well positioned to provide counseling and education regarding self-care in endometriosis treatment and refer patients to resources to assist with the assessment of dysmenorrhea and/or chronic pelvic pain. With the hormone focus of current pharmacotherapies, pharmacists can provide needed education regarding medication adherence and problem-solving in the event of a missed medication dose.
In both the inpatient and outpatient care settings, pharmacists can assist providers in medication optimization and evaluate efficacy, safety, and tolerability of pharmacotherapy regimens. Pharmacists can assist in determining the appropriateness of a therapy given patient-specific factors, provide recommendations for initial or alternate therapies, assist with prior authorizations, and optimize medication choices per insurance formularies.
Pharmacists can also educate patients and providers regarding potential medical misinformation and assist in reducing any stigma or bias associated with chronic pelvic pain, dyspareunia, and dysmenorrhea.
CONCLUSION
Endometriosis is a complex, chronic health condition that may present with a constellation of symptoms or no symptoms at all. Pharmacotherapy options, including NSAIDs, CHCs, progestin-only therapies, GnRH agonists, and GnRH antagonists may be initiated based on presenting symptoms, endometriosis recurrence, and patient/provider preference. Pharmacists have the opportunity to assist in medication optimization, improving outcomes, and optimizing care for the endometriosis patient.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
1. Swiersz LM, Giudice LC. Endometriosis. In: DeGroot LJ, et al, eds. Endocrinology. 5th ed. Philadelphia, PA: Elsevier; 2006.
2. European Society of Human Reproduction and Embryology Endometriosis Guideline Development Group. Endometriosis: guideline of European Society of Human Reproduction and Embryology. 2022. www.eshre.eu/Guideline/Endometriosis. Accessed August 10, 2023.
3. Tomassetti C, Johnson NP, Petrozza J, et al; International Working Group of AAGL, ESGE, ESHRE, and WES. An international terminology for endometriosis, 2021. J Minim Invasive Gynecol. 2021;28(11):1849-1859.
4. Sturpe DA, Pincus KJ. Endometriosis. In: DiPiro JT, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 9th ed. New York, NY: McGraw-Hill Education; 2014.
5. Practice Bulletin No. 114: Management of endometriosis. Obstet Gynecol. 2010;116(1):223-236.
6. ACOG Committee Opinion No. 760: Dysmenorrhea and endometriosis in the adolescent. Obstet Gynecol. 2018;132(6):e249-e258.
7. Bozdag G. Recurrence of endometriosis: risk factors, mechanisms, and biomarkers. Womens Health (Lond). 2015:11(5):693-699.
8. Tandoi I, Somigliana E, Riparini J. High rate of endometriosis recurrence in young women. J Pediatr Adolesc Gynecol. 2011;24:376-379.
9. Guo SW. Recurrence of endometriosis and its control. Hum Reprod Update. 2009;15:441-461.
10. Culley L, Law C, Hudson N, et al. The social and psychological impact of endometriosis on women’s lives: a critical narrative review. Human Reprod Update. 2013;19(6):625-639.
11. Soliman AM, Yang H, Du XE, et al. The direct and indirect costs associated with endometriosis: a systematic literature review. Hum Reprod. 2016;31(4):712-722.
12. Soliman AM, Surrey E, Bonafede M, et al. Real-world evaluation of direct and indirect economic burden among endometriosis patients in the United States. Adv Ther. 2018;35(3):408-423.
13. Simoens S, Hummelshoj L, D’Hooghe T. Endometriosis: cost estimates and methodological perspective. Hum Reprod Update. 2007;13(4):395-404.
14. Jia SZ, Leng JH, Shi JH, et al. Health-related quality of life in women with endometriosis: a systematic review. J Ovarian Res. 2012;5(1):29.
15. Chen LH, Lo WC, Huang HY, Wu HM. A lifelong impact on endometriosis: pathophysiology and pharmacological treatment. Int J Mol Sci. 2023;24:7503.
16. Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511-519.
17. Practice Committee of the American Society for Reproductive Medicine. Treatment of pelvic pain associated with endometriosis: a committee opinion. Fertil Steril. 2014;101:927-935.
18. Kalaitzopoulos DR, Samartzis N, Kolovos GN, et al. Treatment of endometriosis: a review with comparison of 8 guidelines. BMC Womens Health. 2021;21:397.
19. Brown J, Crawford TJ, Allen C, et al. Nonsteroidal anti-inflammatory drugs for pain in women with endometriosis. Cochrane Database Syst Rev. 2017;1:CD004753.
20. Marjoribanks J, Ayeleke RO, Farquhar C, Proctor M. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev. 2015;7:CD001751.
21. Ibuprofen package insert. Livonia, MI: Cardinal Health 107, LLC; December 2022.
22. Naproxen package insert. Farmingdale, NY: Time-Cap Labs, Inc; October 2018.
23. Cooper DB, Patel P, Mahdy H. Oral contraceptive pills. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
24. Capezzuoli T, Rossi M, La Torre F, et al. Hormonal drugs for the treatment of endometriosis. Curr Opin Pharmacol. 2022;67:102311.
25. Brown J, Crawford TJ, Datta S, Prentice A. Oral contraceptives for pain associated with endometriosis. Cochrane Database Syst Rev. 2018;5:CD001019.
26. Vercellini P, Barbara G, Somigliana E, et al. Comparison of contraceptive ring and patch for the treatment of symptomatic endometriosis. Fertil Steril. 2010;93:2150-2161.
27. Harada T, Momoeda M, Taketani Y, et al. Low-dose oral contraceptive pill for dysmenorrhea associated with endometriosis: a placebo-controlled, double-blind, randomized trial. Fertil Steril. 2008;90:1583-1588.
28. Jensen JT, Schlaff W, Gordon K. Use of combined hormonal contraceptives for the treatment of endometriosis-related pain: a systematic review of the evidence. Fertil Steril. 2018;110:137-152.
29. Vercellini P, Frontino G, De Giorgi O, et al. Continuous use of an oral contraceptive for endometriosis-associated recurrent dysmenorrhea that does not respond to a cyclic pill regimen. Fertil Steril. 2003;80:560-563.
30. Brown J, Kives S, Akhtar M. Progestagens and anti-progestagens for pain associated with endometriosis. Cochrane Database Syst Rev. 2012;3:CD002122.
31. Norethindrone package insert. New York, NY: Amneal Pharmaceuticals; December 2022.
32. Muneyyirci-Delale O, Karacan M. Effect of norethindrone acetate in the treatment of symptomatic endometriosis. Int J Fertil Womens Med. 1998;43(1):24-27.
33. Vercellini P, Bracco B, Mosconi P, et al. Norethindrone acetate or dienogest for the treatment of symptomatic endometriosis: a before and after study. Fertil Steril. 2016;105:734-743.e3.
34. Morotti M, Venturini PL, Biscaldi E, et al. Efficacy and acceptability of long-term norethindrone acetate for the treatment of rectovaginal endometriosis. Eur J Obstet Gynecol Reprod Biol. 2016;213:4-10.
35. Luciano AA, Turksoy RN, Carleo J. Evaluation of oral medroxyprogesterone acetate in the treatment of endometriosis. Obstet Gynecol. 1988;72(3 Pt 1):323-327.
36. Telimaa S, Puolakka J, Rönnberg L, Kaupplia A. Placebo-controlled comparison of danazol and high-dose medroxyprogesterone acetate in the treatment of endometriosis. Gynecol Endocrinol. 1987;1:13-23.
37. Crosignani PG, Luciano A, Bergqvist A. Subcutaneous depot medroxyprogesterone acetate versus leuprolide acetate in the treatment of endometriosis-associated pain. Hum Reprod. 2006;21(1):248-256.
38. Schlaff WD, Carson SA, Luciano A, et al. Subcutaneous injection of depot medroxyprogesterone acetate compared with leuprolide acetate in the treatment of endometriosis-associated pain. Fertil Steril. 2006;85:314-325.
39. Vercellini P, De Giorgi O, Oldani S, et al. Depot medroxyprogesterone acetate versus an oral contraceptive combined with very-low-dose danazol for long-term treatment of pelvic pain associated with endometriosis. Am J Obstet Gynecol. 1996;175:396-401.
40. Gibbons T, Georgiou EX, Cheong YC, Wise MR. Levonorgestrel-releasing intrauterine device (LNG-IUD) for symptomatic endometriosis following surgery. Cochrane Database Syst Rev. 2021;12:CD005072.
41. Vercellini P, Frontino G, De Giorgi O, et al. Comparison of a levonorgestrel-releasing intrauterine device versus expectant management after conservative surgery for symptomatic endometriosis: a pilot study. Fertil Steril. 2003;80:305-309.
42. Tanmahasamut P, Rattanachaiyanont M, Angsuwathana S, et al. Postoperative levonorgestrel-releasing intrauterine system for pelvic endometriosis-related pain: a randomized controlled trial. Obstet Gynecol. 2012;119:519-526.
43. Tekin YB, Dilbaz B, Altinbas SK, Dilbaz S. Postoperative medical treatment of chronic pelvic pain related to severe endometriosis: levonorgestrel-releasing intrauterine system versus gonadotropin-releasing hormone analogue. Fertil Steril. 2011;95:492-496.
44. Walch K, Unfried G, Huber J, et al. Implanon versus medroxyprogesterone acetate: effects on pain scores in patients with symptomatic endometriosis–a pilot study. Contraception. 2009;79:29-34.
45. Margatho D, Carvalho NM, Bahamondes L. Endometriosis-associated pain scores and biomarkers in users of the etonogestrel-releasing subdermal implant or the 52-mg levonorgestrel-releasing intrauterine system for up to 24 months. Eur J Contracept Reprod Health Care. 2020;25(2):133-140.
46. Hornstein MD, Gibbons WE. Endometriosis: long-term treatment with gonadotropin-releasing hormone agonists. In: Post TW, ed. Waltham, MA: UpToDate. 2023.
47. Brown J, Pan A, Hart RJ. Gonadotrophin-releasing hormone analogues for pain associated with endometriosis. Cochrane Database Syst Rev. 2010;12:CD008475.
48. Szendei G, Hernadl Z, Devenyl N, Csapo Z. Is there any correlation between stages of endometriosis and severity of chronic pelvic pain? Possibilities of treatment. Gynecol Endocrinol. 2006;21:93-100.
49. Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol. 2018;131:557-571.
50. Zoladex (goserelin implant) package insert. Deerfield, IL: TerSera Therapeutics; 2023.
51. Synarel (nafarelin acetate) package insert. New York, NY: Pfizer Labs; 2023.
52. Lupron Depot (leuprolide acetate for depot suspension 3.75mg) package insert. North Chicago, IL: AbbVie, Inc; 2023.
53. Lupron Depot (leuprolide acetate for depot suspension 11.25mg) package insert. North Chicago, IL: AbbVie, Inc; 2020.
54. DiVasta AD, Feldman HA, Sadler Gallagher J, et al. Hormonal add-back therapy for females treated with gonadotropin-releasing hormone agonist for endometriosis: a randomized controlled trial. Obstet Gynecol. 2015;126(3):617-627.
55. Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
56. Giudice LC, As-Sanie S, Arjona Ferreira JC, et al. Once daily oral relugolix combination therapy versus placebo in patients with endometriosis-associated pain: two replicate phase 3, randomized, double-blind, studies (SPIRIT 1 and 2). Lancet. 2022;399:2267-2279.
57. Orilissa (elagolix) package insert. North Chicago, IL: AbbVie, Inc; 2023.
58. Myfembree (relugolix, estradiol, and norethindrone acetate) package insert. Brisbane, CA: Myovant Sciences, Inc; 2022.
59. Danazol package insert. Parsippany, NJ: Teva Pharmaceuticals USA, Inc; 2022.
60. Farquhar C, Prentice A, Singla AA, Selak V. Danazol for pelvic pain associated with endometriosis. Cochrane Database Syst Rev. 2007;4:CD000068.
61. Attar E, Bulun S. Aromatase inhibitors: the next generation of therapeutics for endometriosis? Fertil Steril. 2006;85:1307-1318.
62. Noble LS, Takayama K, Zeitoun KM, et al. Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J Clin Endocrinol Metab. 1997;82:600-606.
63. Ferrero S, Gillott DJ, Venturini PL, Remorgida V. Use of aromatase inhibitors to treat endometriosis-related pain symptoms: a systematic review. Reprod Biol Endocrinol. 2011;9:89.
64. Amsterdam LL, Gentry W, Jobanputra S, et al. Anastrazole and oral contraceptives: a novel treatment for endometriosis. Fertil Steril. 2005;84:300-304.
65. Ferrero S, Venturini PL, Gillott DJ, et al. Letrozole and norethisterone acetate versus letrozole and triptorelin in the treatment of endometriosis related pain symptoms: a randomized controlled trial. Reprod Biol Endocrinol. 2011;9:88-95.
66. Lebovic DI. Endometriosis: surgical management of pelvic pain. In: Post TW, ed. Waltham, MA: UpToDate. 2023.
67. Hediger ML, Hartnett HJ, Buck Louis GM. Association of endometriosis with body size and figure. Fertil Steril. 2005;84(5):1366-1374.
68. CDC. Summary chart of U.S. medical eligibility criteria for contraceptive use. www.cdc.gov/reproductivehealth/contraception/pdf/summary-chart-us-medical-eligibility-criteria_508tagged.pdf. Accessed August 10, 2023.