Treatment of Metastatic Bone Disease in Prostate Cancer
RELEASE DATE
June 1, 2021
EXPIRATION DATE
June 30, 2023
FACULTY
Ashley Barlow, PharmD
PGY2 Oncology Pharmacy Resident
MD Anderson Cancer Center
Houston, Texas
FACULTY DISCLOSURE STATEMENTS
Dr. Barlow has no actual or potential conflict of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT
 Pharmacy
Pharmacy
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-21-064-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients’ conditions and possible contraindications or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
GOAL
To review the treatment options for men with bone metastases associated with metastatic prostate cancer.
OBJECTIVES
After completing this activity, the participant should be able to:
- Discuss the epidemiology of metastatic bone disease in patients with prostate cancer.
- Describe the signs, symptoms, and diagnosis of metastatic bone disease.
- Review the supporting literature for pharmacologic treatment approaches for metastatic bone disease, with a focus on bone-modifying agents and radium-223.
- Review the adverse effects of pharmacologic agents used for metastatic bone disease and patientmanagement considerations.
ABSTRACT: More than 90% of patients with metastatic prostate cancer (PCa) are diagnosed with bone metastases. The presence of metastatic bone disease contributes to decreased quality of life resulting from pain and pathologic fractures, as well as increased treatment cost and overall mortality. A multimodal approach with analgesics, radiation, bonemodifying therapy, surgery, and chemotherapy are used to manage bone metastases from PCa. These treatments can be used independently or concurrently based on patient symptoms, complications of disease, plan for other systemic chemotherapy, and goals of care. Pharmacists are key components of the healthcare team that guide patients’ management by providing counseling on side effects, treatment monitoring, and cost of therapy for the agents used for metastatic PCa.
Prostate cancer (PCa) is the most prevalent cancer in men over the age of 50 years and the second leading cause of death by cancer.1 PCa has a high tendency for metastatic spread to the bone, making it the most common site of distant disease. Two different types of bone metastases exist in prostate cancer: lytic metastases, which refers to disease that infiltrates and destroys the bone tissue, and blastic metastases, which builds new bone but replaces the normal healthy bone tissue with tumor cells. Regardless of the type, outcomes are poor with the emergence of bone metastases. In the early stages of the disease course, patients are often asymptomatic. However, up to 35% to 45% of patients can endure significant bone pain, while about 20% can experience a pathologic fracture and, less commonly, a spinal cord compression.2 In advanced stages, these symptoms are progressive, severe, and can lead to hospitalization for acute pain crises or spinal cord compression.
Approximately 50% of men with metastatic bone disease will experience a skeletal-related adverse event (SRE; including pain, fracture, spinal cord compression, and emergent need for radiation or surgery) within 2 years of diagnosis.1 Collectively, bone metastases can lead to devastating neurologic compromise and decline in physical function. The presence of bone metastases in men with PCa portends a dismal prognosis and is associated with increased mortality.3 Therefore, appropriate management of bone metastases in PCa is vital to improve patient outcomes.
BONE DYNAMICS
The structural integrity of the bone is meticulously regulated by maintaining an equilibrium between bone resorption and bone formation. This process is orchestrated by two critical bone cells: osteoblasts, cells that build new bone, and osteoclasts, those that break down bone. These two cells work in tandem to break down old bone and restore new, healthy bone, resulting in the bone-remodeling process.3 The dynamics of this process are governed by external factors, nutrients, and cytokines that prompt bone remodeling from one process toward another.
To maintain balance during this process, a substance called RANK ligand is secreted and serves as a communication molecule between the osteoclasts and osteoblasts. In response to excess bone formation and the need for turnover, osteoblasts secrete RANK ligand, which binds to the RANK receptor on osteoclasts and promotes proliferation, survival, and growth of osteoclasts to begin bone resorption.4 Relevant to the pathogenesis of weakened bone in men with metastatic PCa, a critical element to the production of healthy, strong bones is the contribution of testosterone. Testosterone serves as a signaling hormone that promotes bone growth and osteoblast activation.5
The normal process of bone remodeling is disturbed in patients with metastatic PCa for a variety of reasons. Androgen deprivation therapy (ADT) is a nearly universal intervention for patients with advanced-stage disease. As these agents work to directly deplete the body’s production of testosterone, initiation of these therapies for a prolonged period results in accelerated bone resorption, leading to bone loss.5 The rate of bone loss for men on continuous ADT is estimated to be as high as 10% over 2 years.6 In addition, PCa is a disease that mainly impacts older males, resulting in naturally decreased bone density and integrity due to older age. Of utmost importance, the contribution of metastatic bone disease results in compromised bone strength, and depending on the extent of disease, patients can also experience nontraumatic pathologic fractures.
MANIFESTATIONS
SRE refers to the constellation of complications that arise in patients with metastatic PCa to the bone. These SRE include pain, fracture, spinal cord compression, and emergent needs for radiation or surgery.1 Bone pain is the most common manifestation and typically has an insidious onset and progressively worsens over weeks to months. The pain is usually described as aching in the large muscles or joints, including the legs and lower back, but can also include burning or radiating pain in the setting of nerve entrapment.2 Acute pain crises occur more rarely but can include pathologic fracture and, if the metastatic lesion is within the vertebral column, spinal cord compression. Spinal cord compression results when the tumor within the vertebral column puts pressure down onto the spinal cord and causes damage to nerves or leads to paralysis. Manifestations of a spinal cord compression consist of progressive pain in the back or neck, radiating pain down the arms and/or legs, weakness, numbness or tingling in the arms and legs, and urinary incontinence. Uncontrolled pain can result in unnecessary morbidity from fractures, interference with activities of daily living, or hospital admissions.7 Depending on the extent of bone involvement, some patients can experience anemia, elevations in bone turnover markers, and hypercalcemia.4
DIAGNOSIS
The primary indication for evaluation of bone metastases is patients with new-onset or worsening bone pain with or without a concurrent rise in prostatespecific antigen (PSA) or alkaline phosphatase (ALP).1 A radionuclide bone scan is used as an initial step to screen for suspected bone metastasis, as this bone scan allows for the detection of the presence of osteoblastic lesions, their number, and anatomic distribution. Plain radiographs, magnetic resonance imaging, and positron emission computed tomography (PET/CT) scans can also be used to support the diagnosis of metastatic PCa to the bone.1,2 The benefit of PET/CT scans would allow for evaluation of whole-body metastatic sites and extent of bone involvement. If the patient is newly diagnosed, a biopsy may be performed to confirm that the metastatic bone lesions are related to a primary diagnosis of prostate cancer as opposed to an alternate cancer diagnosis. Patients should also be evaluated for common etiologies of bone pain, including rheumatic conditions, skeletal-muscle discomfort, or treatmentrelated complications, such as osteoporosis.1
MANAGEMENT
The goals of treating skeletal metastases are to reduce existing bone-related symptoms and prolong the time to onset of a new bone-related insult to quality of life or overall survival.1,2 The SREs related to metastatic PCa are managed using a multimodal approach of pharmacologic treatments, surgery, and/or radiation.1 For patients with pathologic fractures or spinal cord compression, surgery is indicated for internal fixation along with analgesics as needed as a primary modality. Radiation therapy can assist in ameliorating painful symptoms and reducing the burden of bone disease in patients who are deemed candidates for this therapy.2 Radiation therapy can also be considered for patients without symptoms to minimize future complications if the bone metastases progress.1 When a type of radiation called external beam radiation is used, this modality is highly effective and fast-acting, with pain relief responses in 85% of patients within 2 weeks and complete responses in 50%.7
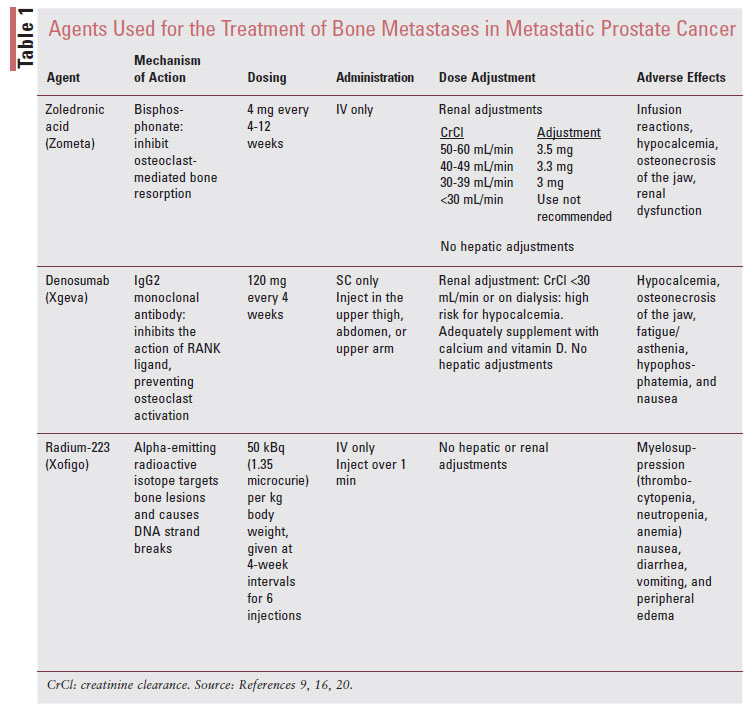
The focus of this article will detail options for pharmacologic treatments that have been studied in patients with metastatic PCa (TABLE 1). Bone-modifying agents, bisphosphonates and denosumab, are indicated for men with bone metastases from castrationresistant (i.e., no longer achieving suppressed PSA while on ADT) prostate cancer, whether current symptoms exist for metastatic bone disease or if it is only present on imaging studies.2,7
Bisphosphonates
Bone-modifying treatment with the bisphosphonate zoledronic acid has been shown to reduce the incidence of SREs.1 Bisphosphonates inhibit osteoclastmediated bone resorption.8 They attach to the hydroxyapatite binding site on osteoclasts and interfere with the mevalonate metabolism by blocking the enzymes responsible for cholesterol synthesis, thereby promoting osteoclast apoptosis. Of the bisphosphonates, zoledronic acid has been the most extensively evaluated to prevent bone loss in patients with PCa undergoing ADT and reduce the incidence of SRE in patients with metastatic PCa.9
One of the most robust studies to date exists from a prospective, randomized, placebo-controlled phase III study of zoledronic acid 4 mg once every 4 weeks versus placebo in 643 patients with metastatic PCa.10 At 15 months, treatment with zoledronic acid reduced the proportion of patients with ≥1 SRE (defined as radiation to bone, pathologic fracture, spinal cord compression, surgery to bone, or change in antineoplastic therapy) versus placebo (44.2% vs. 33.2%, respectively; P = .021). After 2-year followup, zoledronic acid reduced the time to first SRE (321 vs. 488 days; P = .0009). Other bisphosphonates that have been studied in this setting include ibandronate and pamidronate. Ibandronate has been shown to be as effective as radiotherapy in reducing the pain associated with metastatic bone disease, although it has not been shown to elicit the same benefit observed with zoledronic acid and is therefore not recommended.1
Zoledronic acid was originally approved at a dose of 4 mg every 3 to 4 weeks. It was subsequently studied every 12 weeks in patients with metastatic breast cancer and was shown to be equally safe and effective. In a multi-institutional, open-label, randomized trial, 1,822 patients with bone metastases associated with prostate cancer, multiple myeloma, and breast cancer were randomized to zoledronic acid every 4 weeks or every 12 weeks. The study found that the regimens were noninferior regarding prevention of SRE, and 28.6% and 29.5% experienced at least one SRE within 2 years of randomization with the every-12-week and every-4-week regimens, respectively.11 Less frequent treatment can reduce the burden of healthcare costs for treatment, improve patient convenience, and reduce the incidence of side effects due to lower cumulative doses. Either regimen is recommended in the National Comprehensive Cancer Network (NCCN) guidelines, and patient convenience, cost, and institution policies should be considered when determining the dosing frequency.1
Adverse Effects: Bisphosphonates are generally well tolerated. Acute infusion reactions occur in about 30% of patients, manifesting as a flu-like syndrome (chills, flushing, bone pain, and arthralgias).8 These symptoms are transient typically within 3 days following the infusion, and resolution is expected by about 3 days after symptom onset but may take up to 14 days.8 This reaction should reduce in frequency and severity by the second or third infusion. In addition, prophylactic acetaminophen can be used for prevention. Prolonged hypocalcemia can occur in patients who are not receiving adequate supplementation while on treatment. Patients should have their calcium monitored prior to each dose and receive counseling about the importance of preventative calcium and vitamin D supplementation throughout the duration of therapy. Bisphosphonate use has been shown to contribute to renal dysfunction, and accumulation can occur in patients with impaired renal clearance. The effects on renal function are known to be dose and infusion-rate dependent. Therefore, doses of zoledronic acid should be modified for patients with an estimated glomerular filtration rate (eGFR) <60 mL/min, and use is contraindicated if the eGFR is <30 mL/min.8 Clinicians may elect to switch to an alternative bone-modifying agent, such as denosumab, or monitor closely with adjusted doses.
A more rare, serious adverse effect from bisphosphonate treatment is the emergence of osteonecrosis of the jaw (ONJ). ONJ is caused by a disruption of vascular supply or avascular necrosis that results in progressive bone destruction in the maxillofacial region of patients.12 The risk of ONJ is influenced by duration of therapy, timing of administration of bone-modifying treatments relative to dental surgery (e.g., tooth extraction, dental implants), concomitant chemotherapy (angiogenesis inhibitors or corticosteroids), radiation to the mandible, ill-fitting dentures, and underlying diseases.13 Patients should be screened for relative risk factors and informed of them prior to initiating bisphosphonate therapy in order to improve communication about dental plans patients may have while on treatment. Current guidelines recommend preventing ONJ with oral hygiene, baseline dental evaluation for high-risk individuals, and avoidance of invasive dental surgery during bisphosphonate therapy.14
Denosumab
Denosumab (Xgeva) is a fully human monoclonal IgG2 antibody that binds RANK ligand with high affinity and specificity, which prevents the activation of the RANK receptor in the surface of osteoclasts. PCa cells secrete growth factors that stimulate osteoblast activity to increase the concentration of RANK ligand, which results in the maturation and differentiation of osteoclasts. These osteoclasts further increase production of substances that promote the growth of tumor cells, resulting in a continuous cycle of tumor growth and bone destruction.15 By blocking the downstream effect of RANK ligand, denosumab can halt osteoclast-mediated bone resorption that is overactivated in patients with bone metastases. Denosumab was approved in November 2010 by the FDA for the prevention of SREs in patients with bone metastases from solid tumors, based on superior results in phase III clinical trials that compared denosumab with zoledronic acid.16
Denosumab 120 mg SC every 4 weeks was compared with zoledronic acid 4 mg IV every 4 weeks in a randomized, double-blind phase III study of 1,904 patients with metastatic PCa. Denosumab delayed the time to first SRE by 3.6 months compared with zoledronic acid and numerically reduced pain severity scores.17 The median time to first SRE was 20.7 months in patients treated with denosumab and 17.1 months in patients with zoledronic acid (P = .002). The rates of SRE were similar between denosumab and zoledronic acid, respectively: spinal cord compression (3% vs. 4%), radiation (19% vs. 21%), and pathologic fracture (14% vs.15%). A post hoc analysis calculated that the number needed to treat was five for prevention of the first or subsequent SRE.18 Due to the results of this trial, NCCN guidelines prefer the use of denosumab over zoledronic acid but recommend considering comorbidities, cost, renal function, and underlying conditions when making the decision between treatments.1
Adverse Effects: Denosumab is well-tolerated, with the most common adverse effects being fatigue, asthenia, hypophosphatemia, and nausea.16 Similar to bisphosphonates, hypocalcemia can also occur with denosumab. Rates of hypocalcemia appear to be twofold higher with denosumab compared with bisphosphonates and tend to occur within the first 6 months of treatment. In the head-to-head trial comparing zoledronic acid with denosumab, the rate of hypocalcemia was 13% in patients treated with denosumab compared with 6% in patients taking zoledronic acid.17 Although the use of denosumab is touted to have a safer profile in patients with renal dysfunction, the risk of hypocalcemia and hypophosphatemia is further increased in patients with a creatinine clearance (CrCl) <30 mL/ min or those on dialysis.16 The NCCN states that denosumab should not be used in patients with CrCl <30mL/min.1 Denosumab is not excreted through the kidneys and does not require dose adjustments for the presence of renal dysfunction. All patients, regardless of baseline calcium, should be provided recommendations for calcium and vitamin D supplementation throughout treatment, with vigilant monitoring prior to each dose. The risk of ONJ is also present with denosumab. When compared in a phase III trial for patients with metastatic castrate-resistant PCa, the incidence of ONJ was 2.3% in patients receiving denosumab compared with 1.3% in patients receiving zoledronic acid (P = .09).19
SYSTEMIC ANTICANCER THERAPY
Over the past decade, the landscape of treatments for metastatic prostate cancer has evolved, with several treatment options demonstrating an overall survival benefit in metastatic PCa, including ADT, chemotherapy, and vaccines. Radium-223 dichloride (Xofigo) is a newly approved agent that specifically targets bone metastases in metastatic castrationresistant PCa.
Radium-223
Radium-223 is an alpha-emitting, bone-seeking calcium mimetic radiopharmaceutical specifically approved for patients with metastatic castrate-resistant PCa and symptomatic bone metastases in the absence of known visceral metastases.1,20 Radium-223 is highly selective to bone tissue and binds to areas of increased bone turnover in bone metastases. Once incorporated into bone, radium-223 emits radiation and elicits a cytotoxic effect through causing double-stranded DNA breaks. Unlike previous radiopharmaceuticals approved in the metastatic PCa landscape, samarium and strontium, radium-223 emits alpha particles of short range, which limits off-targeted effects at adjacent tissues or organ sites.20
Previous therapies were less selective, would have broad effects on organs, and were highly bone-marrow suppressive. This was trivial in this patient population given patients with metastatic PCa are heavily pretreated with little to no bone marrow reserve. In contrast to bone-modifying therapies that primarily act to reduce the associated pain or resulting SRE, radium-223 can provide therapeutic benefit for modifying the disease course of metastatic PCa.
Radium-223 was FDA approved based on the results of the ALSYMPCA trial, a randomized phase III trial of 921 men with symptomatic metastatic castrate-resistant PCa, two or more bone metastases, and no known visceral disease randomized in a 2:1 ratio to six radium-223 IV injections dosed at 50 kBq per kilogram of body weight every 4 weeks or best supportive care. Radium-223 significantly improved overall survival, median 14.9 months versus 11.3 months (CI, 0.058–0.83; P <.00185) and prolonged time to first skeletal-related event (median 15.6 vs. 9.8 months) compared with best supportive care.21
Importantly, in contrast to previous therapies, there were no observed differences in grade 3 or 4 hematologic adverse effects. Moreover, a remarkable advantage was observed in favor of radium-223 for delaying the time to first SRE: The time to the first SRE with radium-223 occurred at a median of 13.6 months compared with 8.4 months with placebo; P = .00046. The NCCN guidelines state that the use of radium-223 is a category 1 recommendation for patients who cannot receive the preferred first-line chemotherapy or hormonal therapy agents or have already received these therapies without optimal response.1 This applies only if the patient does not have visceral disease and is not receiving concurrent chemotherapy. Concurrent ADT can be recommended on radium-223, and the use of concurrent bone-modifying agents is strongly encouraged.1
Adverse Effects: Due to the cytotoxic effects of radium-223, grade 1 and 2 hematologic toxicities are common with treatment, while grade 3 to 4 is rarer. Prior to the initial dose of radium-223, platelets must be >100 x 109/L, absolute neutrophil count >1.5 x 109/L, and hemoglobin >10 g/dL.20 For subsequent doses, platelets must be >50 x 109/L and absolute neutrophil count >1 x 109/L.20 Other nonhematologic toxicities are uncommon but can include nausea, vomiting, and diarrhea. The use of concurrent chemotherapy is contraindicated due to the additive risk of myelosuppression, and abiraterone use also comes with an increased risk of fracture.20 Concomitant use of bone-modifying agents is recommended for additive pain relief and bone-strengthening potential.1 As a radioactive isotope, radium-223 may impair fertility in males. Male patients should be advised to use barrier protection during sexual intercourse and inform their female partners of reproductive potential to use effective contraception during and for 6 months after completing treatment with radium Ra 223 dichloride.
Patients should be counseled about the necessary precautions to minimize unwanted exposure to the radiopharmaceutical. Radium-223 at measurable levels may be present in blood, urine, and fecal matter, as only 63% is excreted within the first week after treatment. Moreover, given that radium-223 targets areas of active bone turnover, low concentrations will be found in bone in the weeks after treatment. However, since it is an alpha-emitting radiation therapy, the exposure risk is minimal for caregivers they would encounter during treatment. Caregivers should wear gloves and wash hands after handling any body fluids of the patient actively receiving radium-223, wash any clothing soiled with radium-223 promptly and separately from other nonsoiled clothing, flush the toilet several times after use, and wash hands thoroughly after urination.20 All of these precautions should be followed for a minimum 1 week after receiving treatment. Radium-223 may be administered on an outpatient basis, because the alpha-particles emitted by radium-223 only travel a fraction of a millimeter within the body and people who encounter patients receiving treatment should not be at harm.20
THE ROLE OF THE PHARMACIST
An important aspect of the ordering process of denosumab for patients with bone metastases is the distinction between brand names that are used for different indications. Denosumab is sold under two brand names, Prolia and Xgeva. Xgeva is FDA approved for the treatment of hypercalcemia of malignancy as well as bone metastases.16,22 In addition, zoledronic acid is available under two brand names, Reclast and Zometa, which carry different indications.9,23 For the treatment of metastatic bone disease, Zometa carries the FDA approval and should be the only formulation ordered for this indication.9 Zoledronic acid dosing adjustments must be distinguished between oncologic indications. For bone metastases, there are strict adjustments for renal function due to the concern for accumulation with repeated dosing.9 In contrast, dose adjustments are not required for hypercalcemia of malignancy that can be observed in patients with metastatic PCa as well.9 Close attention should be paid to the differences in dosing and indication for these products to ensure that the indication is clarified and in accordance with the selected product for a given patient. In addition, the cost of denosumab is substantially higher than that of zoledronic acid.
Pharmacists should practice oncology stewardship by minimizing the inpatient administration of denosumab and deferring its use for the outpatient setting to reduce hospital costs. In the setting where an inpatient order for treatment with denosumab is requested, a pharmacist can explain that these agents take multiple doses and continued administration to obtain benefit in reducing the risk of SREs, and therefore, the immediate administration is not imperative in the hospital setting and can be deferred. Zoledronic acid is a hazardous drug according to the National Institute for Occupational Safety and Health, and institutions are to use appropriate precautions for receiving, handling, storing, preparing, dispensing, transporting, administering, and disposing this agent.9 Pharmacists can enhance the safety of bisphosphonate and denosumab use by preemptively counseling patients about the risk of ONJ and encouraging patients to maintain good dental hygiene.
Pharmacists can also aid in the process of selecting candidates who would obtain the greatest benefit with radium-223. Radium-223 is selected for patients with symptomatic bone metastases. The term symptomatic in the ALSYMPCA clinical trial was defined as requiring regular use of analgesic medication or recent use of external beam radiation therapy for cancer-related bone pain.21 In the clinical trial, patients benefited whether they were minimally symptomatic (not on active analgesia) or moderately symptomatic; therefore, there is no need to delay treatment for severe, debilitating bone-pain symptoms. It is crucial to ensure the patient will not receive additional cytotoxic therapy while on radium-223 and that there is an adequate wash-out period prior to starting. Moreover, since radium-223 does not directly impact androgen synthesis, PSA is not an accurate marker of treatment effectiveness. In the ALSYMPCA trial, only 16% of patients had a PSA reduction by >30%; however, overall survival was still improved. Treatment efficacy should be evaluated with imaging (PET, CT, or MRI) to assess the stability of bone lesions. Another consideration is the cost of radium-223. The cost of a single dose of treatment can be considerable, with the average wholesale price of approximately $12,220 per dose for a 70-kg patient.20 Assistance programs are available for patients who qualify through Xofigo Access Services.
Duration of therapy for radium-223 is a definitive six treatments; however, the recommended duration for bisphosphonates and denosumab is not well defined. Patients on radium-223 should be counseled on the importance of adhering to treatment schedules as a post hoc analysis of the ALSYMPCA trial demonstrated greater median overall survival in patients who were able to remain on treatment for five to six cycles compared with one to four cycles.24 For bisphosphonates and denosumab, treatment in most studies continued for 2 years. The NCCN does not make any specific recommendations for the duration of treatment; therefore, it is up to clinical discretion and patient preference.
CONCLUSION
Bone metastases in patients with metastatic PCa are associated with significant morbidity and mortality. Patients should be managed with a multimodal approach, including analgesics, bone-modifying therapy with a bisphosphonate or denosumab, and consideration for additional therapies such as radium-223 or traditional radiation therapy. Pharmacists can improve the care of patients undergoing treatment for metastatic PCa by providing in-depth counseling on the adverse effects, monitoring parameters, and schedule of treatments employed.
REFERENCES
- National Comprehensive Cancer Network. Prostate Cancer (Version2.2021). www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed February 20, 2021.
- Autio KA, Scher HI, Morris MJ. Therapeutic strategies for bone metastases and their clinical sequelae in prostate cancer. Curr Treat Options Oncol. 2012;13(2):174-188.
- Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655-1664.
- Cathomas R, Bajory Z, Bouzid M, et al. Management of bone metastases in patients with castration-resistant prostate cancer. Urol Int. 2014;92(4):377-386.
- Mohamad N-V, Soelaiman I-N, Chin K-Y. A concise review of testosterone and bone health. Clin Interv Aging. 2016;11:1317-1324.
- Ross RW, Small EJ. Osteoporosis in men treated with androgen deprivation therapy for prostate cancer. J Urol. 2002;167:1952-1956.
- Jehn CF, Diel IJ, Overkamp F, et al. Management of metastatic bone disease algorithms for diagnostics and treatment. Anticancer Res. 2016;36(6):2631-2637.
- D’Oronzo S, Coleman R, Brown J, Silvestris F. Metastatic bone disease: pathogenesis and therapeutic options: up-date on bone metastasis management. J Bone Oncol. 2019;15:100205.
- Zoledronic acid injection [package insert]. Schaumberg, IL: Sagent Pharmaceuticals; October 2020.
- Saad F, Gleason DM, Murray R, et al. Zoledronic Acid; Prostate Cancer Study Group: Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormonerefractory prostate cancer. J Natl Cancer Inst. 2004;96:879-882.
- Himelstein AL, Foster JC, Khatcheressian JL, et al. Effect of longer-interval vs standard dosing of zoledronic acid on skeletal events in patients with bone metastases: a randomized clinical trial. JAMA. 2017;317(1):48-58.
- Rosella D, Papi P, Giardino R, et al. Medication-related osteonecrosis of the jaw: clinical and practical guidelines. J Int Soc Prev Community Dent. 2016;6(2):97-104.
- Bamias A, Kastritis E, Bamia C, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol. 2005;23:8580-8587.
- Ruggiero S, Gralow J, Marx RE, et al. Practical guidelines for the prevention, diagnosis, and treatment of osteonecrosis of the jaw in patients with cancer. J Oncol Pract. 2006;2(1):7-14.
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337-342.
- Xgeva (denosumab) [package insert]. Thousand Oaks, CA: Amgen Inc; January 2021.
- Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomized, double-blind study. Lancet. 2011;377(9768):813-822.
- Miller K, Fizazi K, Smith M, et al. Benefit of denosumab therapy in patients with bone metastases from castrate resistant prostate cancer: a number-needed-to-treat (NNT) analysis. J Urol. 2011;185:e262.
- Smith M, Saad F, Coleman R, et al. Denosumab and bonemetastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379:39-46.
- Xofigo (radium Ra 223 dichloride) [prescribing information]. Whippany, NJ: Bayer HealthCare Pharmaceuticals, Inc; December 2019.
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213-223.
- Prolia (denosumab) [package insert]. Thousand Oaks, CA: Amgen Inc; January 2021.
- Reclast (zoledronic acid) [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; March 2021.
- Sartor O, Coleman RE, Morris MJ, et al. Baseline characteristics, number of radium-223 (Ra-223) injections, and overall survival (OS) in US Expanded Access Program (EAP) and ALSYMPCA. Eur J Cancer. 2015;51:S484-S485.