Update on the Management of Diabetic Kidney Disease
RELEASE DATE
November 1, 2021
EXPIRATION DATE
November 30, 2023
FACULTY
Lynn Luu, PharmD Candidate 2022
Department of Pharmacotherapy
College of Pharmacy and Pharmaceutical Sciences
Washington State University
Spokane, Washington
Bahar Adamzadeh, PharmD Candidate 2022
Department of Pharmacotherapy
College of Pharmacy and Pharmaceutical Sciences
Washington State University
Spokane, Washington
Joshua J. Neumiller, PharmD, CDCES, FADCES, FASCP
Vice-Chair and Professor
Department of Pharmacotherapy
College of Pharmacy and Pharmaceutical Sciences
Washington State University
Spokane, Washington
FACULTY DISCLOSURE STATEMENTS
Ms. Luu and Ms. Adamzadeh have no conflicts of interest in relation to this activity. Dr. Neumiller serves on the Speaker’s Bureau for Dexcom.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT
 Pharmacy
Pharmacy
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-21-129-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients’ conditions and possible contraindications or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
GOAL
To educate pharmacists on recent evidence and guideline updates regarding the management of patients with type 2 diabetes and chronic kidney disease.
OBJECTIVES
After completing this activity, the participant should be able to:
- State current treatment recommendations to prevent and/or slow the progression of diabetic kidney disease (DKD) in patients with type 2 diabetes mellitus.
- Understand recommendations for monitoring of estimated glomerular filtration rate and urinary albumin-to-creatinine ratio.
- Review clinical trial data supporting the use of sodium-glucose cotransporter-2 (SGLT2) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, and finerenone in patients with DKD to improve kidney and cardiovascular outcomes.
- Discuss key patient management considerations when initiating an SGLT2 inhibitor, GLP-1 receptor agonist, and/or finerenone in a patient with DKD.
ABSTRACT: Diabetic kidney disease (DKD) is a complication that occurs in approximately 40% of patients with type 2 diabetes. DKD is associated with significant morbidity and mortality and, additionally, complicates glycemic management. While interventions to prevent and/or slow the progression of DKD have historically been limited, agents from the sodium-glucose cotransporter-2 inhibitor, glucagon-like peptide-1 receptor agonist, and nonsteroidal mineralocorticoid receptor antagonist classes of medication offer new hope for DKD patients. Pharmacists can play a critical role as members of the care team to promote the safe and appropriate use of these kidney-protective agents in appropriate individuals to improve patient outcomes.
The number of people in the United States living with diabetes mellitus (DM) continues to rise. According to most recent estimates from the CDC, over 34 million individuals currently live with DM in the U.S.1 The large majority (90%-95%) of these individuals have type 2 diabetes mellitus (T2D).1 For people living with DM, a major goal of management is preventing and/or delaying progression of microvascular and macrovascular complications.2 One important diabetes-related complication is diabetic kidney disease (DKD). Unfortunately, the prevalence of DKD has increased in parallel with the increasing prevalence of DM.3 Approximately 40% of people with T2D develop DKD.4 DKD is associated with significant morbidity and mortality, including risk for progression to kidney failure and need for dialysis and increased risk for experiencing cardiovascular (CV) and cerebrovascular events.4 While optimizing blood glucose and blood pressure control are recommended to prevent and/or slow the progression of DKD, glycemic management is complicated in the setting of DKD due to altered clearance of many glucose-lowering therapies and an associated increase in risk for hypoglycemia.2,5
Use of renin-angiotensin system (RAS) inhibitors (e.g., angiotensin-converting enzyme [ACE] inhibitors or angiotensin receptor blockers [ARBs]) has been the standard of therapy to slow progression of DKD for decades.6 Despite RAS inhibition, patients with DKD maintain residual risk for progression of DKD and poor outcomes, and until recently there has been a lack of additional therapies with proven kidney benefit to improve outcomes in patients with DKD. This article will review recent evidence and guidelines supporting the use of agents from the sodium-glucose cotransporter-2 (SGLT2) inhibitor, glucagon-like peptide-1 receptor agonist (GLP-1 RA), and nonsteroidal mineralocorticoid receptor antagonist (MRA) medication classes to improve kidney and CV outcomes in patients with DKD.
PATHOPHYSIOLOGY
The pathophysiology of DKD is quite complex and involves metabolic, hemodynamic, inflammatory, and fibrotic changes that lead to structural and functional abnormalities in the diabetic kidney.4 The presence of hyperglycemia itself contributes to the production of advanced glycation end products (AGE) and accumulation of reactive oxygen species that contribute to cellular injury.7 Glomerular hyperfiltration, a hemodynamic change that occurs in up to 40% of patients with T2D, is also a major contributor to the development and progression of DKD.4 Glomerular hyperfiltration is driven in part by systemic hypertension and obesity, thus highlighting blood pressure and weight management as important in the prevention and management of DKD. Overactivation of the MR is also linked to the promotion of inflammation and fibrosis in DKD.8 Importantly, many of the pathophysiological drivers of DKD are also detrimental to the CV system, with DKD patients at increased risk for CV disease and associated morbidity and mortality.9 With these pathophysiological factors in mind, a wholistic treatment approach that addresses key modifiable risk factors is recommended to prevent and/or delay the progression of DKD.
DIAGNOSIS AND MONITORING
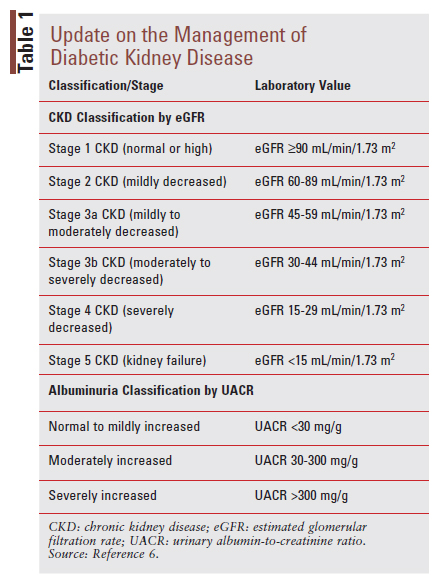
Chronic kidney disease (CKD) is defined as abnormalities of kidney structure or function and is generally diagnosed by the presence of persistently reduced estimated glomerular filtration rate (eGFR), persistently elevated urinal albumin excretion, or both, for greater than 3 months.6 CKD is classified based on its cause, GFR category, and albuminuria category.6 TABLE 1 provides a summary of classification terms for patients with CKD based on eGFR and albuminuria.6 It is important to consider that both lower eGFR and increased albuminuria category place people at higher risk for negative kidney and CV outcomes.6 DKD is a term often used to refer to CKD occurring in people with DM, although some people with DM may have other etiologies (e.g., beyond diabetes) contributing to their kidney impairment.6 For this reason, the terms DKD and CKD in T2D are sometimes used interchangeably as the exact etiology of an individual’s kidney disease is not always known. The American Diabetes Association (ADA) recommends screening urinary albumin (via urinary albumin-to-creatinine ratio [UACR]) and eGFR at least annually in all patients with T2D.2 For patients who have an established UACR >300 mg/g and/or an eGFR of 30-60 mL/ min/1.73 m2, it is recommended that they be monitored at least twice annually to guide therapy.2
TREATMENT OPTIONS TO PREVENT AND/OR DELAY PROGRESSION OF DKD
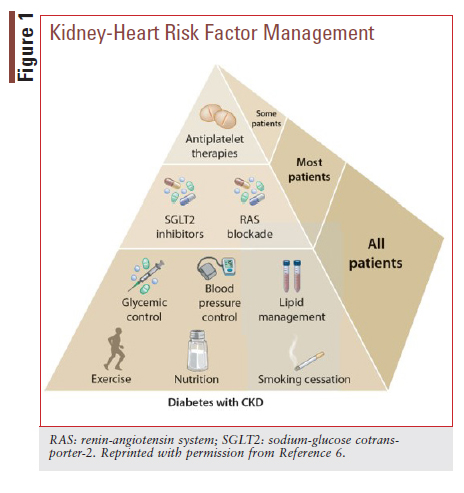
A multifaceted approach that targets key modifiable risk factors for kidney and CV disease progression is recommended by the Kidney Disease: Improving Global Outcomes (KDIGO) 2020 Clinical Practice Guideline for Diabetes Management in CKD.6 KDIGO recommends that all patients with DKD be encouraged to engage in lifestyle interventions (e.g., physical activity and nutrition management) and receive guideline-directed therapies to optimize glycemic, blood pressure, and lipid control (FIGURE 1).6
KDIGO further recommends that most patients receive treatment with a RAS inhibitor and an SGLT2 inhibitor, provided they do not have a contraindication and are able to tolerate these therapies.6 Antiplatelet therapy is recommended as indicated for CV protection.
Glycemic Control
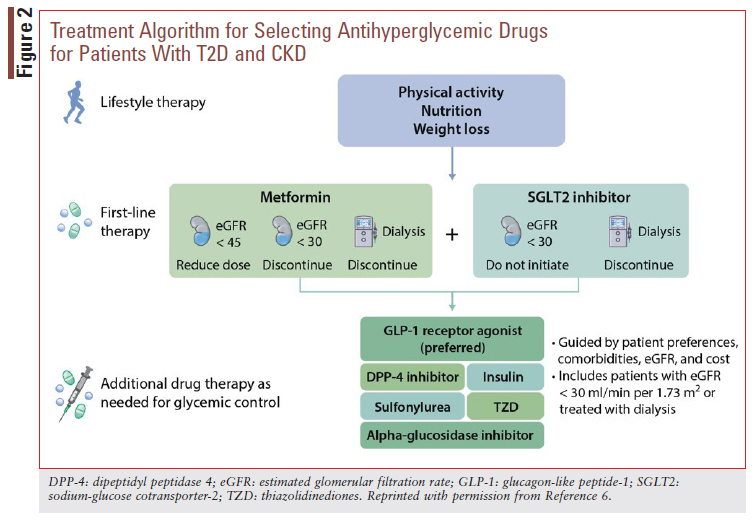
As emphasized in FIGURE 1, glycemic control is a critical component of DKD management. Indeed, the ADA recommends optimization of glucose control to reduce the risk or slow progression of CKD.2 Glycemic targets should be individualized, however, as discussed in more detail below. To achieve individualized glycemic targets, KDIGO recommends first-line combination pharmacotherapy with metformin plus an SGLT2 inhibitor in patients who can tolerate these medications and have sufficient kidney function for initiation (FIGURE 2).6 For metformin, it is recommended that the dose be reduced (typically to a half-maximal target dose of 1,000 mg daily) in patients with an eGFR <45 mL/ min/1.73 m2 and discontinued once the eGFR falls below 30 mL/min/1.73 m2 or the patient starts dialysis.6 For patients with DKD not meeting individualized glycemic targets despite recommended first-line therapy, KDIGO recommends adding additional glucose-lowering therapies as needed. A long-acting GLP-1 RA is preferentially recommended as the next agent due to evidence suggesting benefit of GLP-1 RAs on kidney outcomes (see GLP-1 RAs section below).6
Glycemic Targets
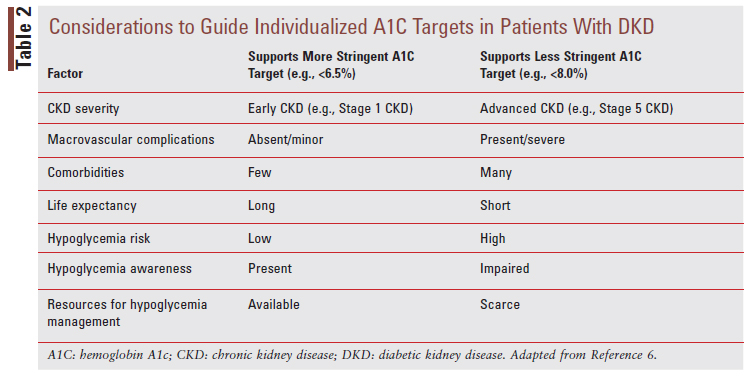
The KDIGO guideline recommends an individualized glycated hemoglobin A1c (A1C) target in the general range of <6.5% to <8.0% in patients not receiving dialysis.2 Targeting an A1C <6.5% may be appropriate in younger people with earlier DKD when prevention of additional complications is a major consideration. Conversely, a less stringent A1C target of <8.0% may be more appropriate in patients with longstanding DM, multiple comorbidities, or a high risk of hypoglycemia.6 TABLE 2 provides considerations to guide A1C target selection in patients with DKD.6 When A1C may not be a reliable indicator of glycemic control in advanced stages of DKD and the use of continuous glucose monitoring (CGM) is an option, CGM metrics such as time in range (defined as time spent between 70 mg/dL and 180 mg/dL) and time spent in hypoglycemia (defined as time spent <70 mg/dL) may be helpful metrics to guide treatment decisions and therapeutic changes.6
Glycemic Monitoring
While A1C has limitations in the setting of DKD, it is the recommended method for long-term glycemic monitoring in DKD.2,6 It is important to consider that A1C values can measure as falsely low or high in patients with DKD. A1C values can be falsely decreased by factors that reduce the erythrocyte lifespan such as anemia, red blood cell transfusions, and use of erythrocyte-stimulating agents or iron replacement.6 Conversely, A1C values may be falsely increased by the presence of metabolic acidosis and through AGE formation in advanced stages of DKD.6 Given these limitations, self-monitoring of blood glucose and/or CGM is recommended to inform daily treatment decisions. When the validity of the A1C is questionable, CGM can be used to generate a glucose management indicator (GMI) value. The GMI is derived from CGM data to provide an average glucose value expressed as a percentage to correlate with A1C measurements.10
Blood Pressure Control
ADA recommends optimization of blood pressure control to reduce the risk or slow the progression of CKD.2 For patients with DM and hypertension at lower risk for CV disease (10-year atherosclerotic cardiovascular disease [ASCVD] risk of <15%), ADA recommends a target blood pressure of <140/90 mmHg. For patients at higher risk (existing ASCVD or 10-year ASCVD risk ≥15%), a target of <130/80 mmHg may be appropriate if it can be safely achieved.2 The general preferred antihypertensive classes recommended by ADA include ACE inhibitors, ARBs, dihydropyridine calcium channel blockers, and thiazide-like diuretics.2 In the presence of hypertension and albuminuria, an ACE inhibitor or an ARB is specifically recommended for kidney protection.2
SPECIFIC AGENTS TO MITIGATE KIDNEY RISK
While general optimization of glycemic and blood pressure control are central elements of DKD management, both the ADA and KDIGO recommend the use of specific therapies with demonstrated benefit in large CV and kidney outcome trials.2,6
RAS Inhibitors
RAS inhibition with an ACE inhibitor or an ARB in patients with DM, hypertension, and albuminuria is recommended by both the ADA and KDIGO.2,6 KDIGO specifically recommends that the RAS inhibitor be titrated to the highest approved dose that is tolerated to achieve maximal benefit.6 RAS inhibitors provide benefit in DKD by reducing blood pressure, lowering glomerular capillary pressure, and decreasing albuminuria. While combination antihypertensive therapy is often required to meet individualized blood pressure targets, combinations of ACE inhibitors, ARBs, and/or direct renin inhibitors (e.g., aliskiren) should not be used due to a lack of additive benefits with an increased risk for hyperkalemia and acute kidney injury.6 While RAS inhibition has been considered a standard of therapy for several decades, RAS inhibitors are unfortunately underutilized in patients with CKD.11 Notably, newer therapies that have received indications to improve outcomes in patients with DKD (select SGLT2 inhibitors and finerenone) were studied as add-on to background optimized ACE inhibitor or ARB therapy to address residual kidney and CV risk in these patients.
SGLT2 Inhibitors
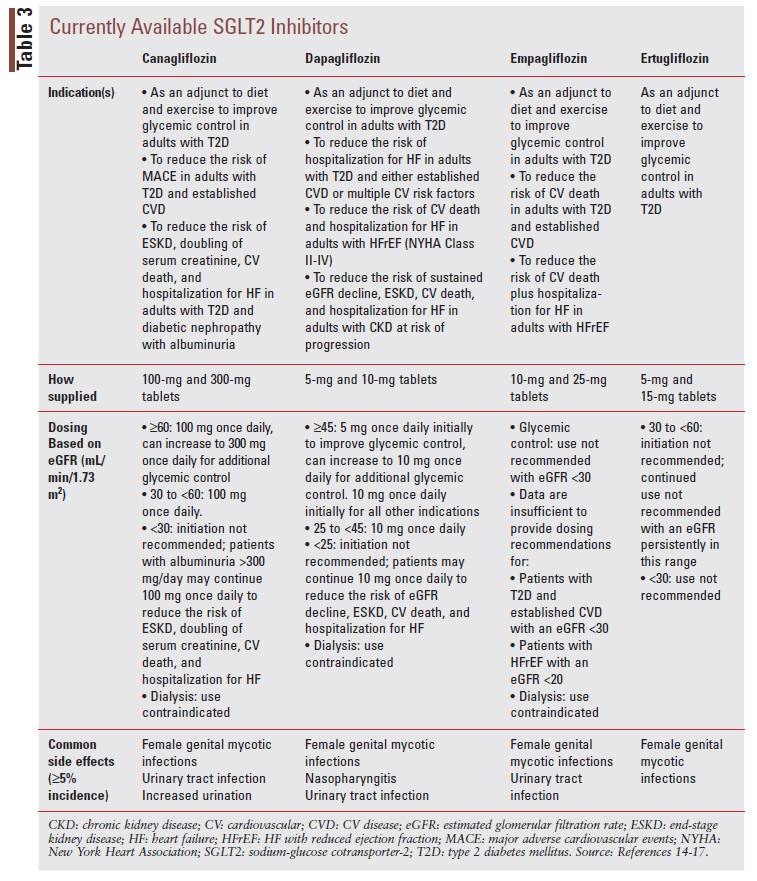
KDIGO recommends first-line combination therapy of metformin plus an SGLT2 inhibitor in patients with T2D and DKD (see FIGURE 2).6 While initially approved as glucose-lowering agents to treat T2D, SGLT2 inhibitors have emerged as agents that can improve CV, heart failure (HF), and kidney outcomes in patients with and without DM. In the context of patients with T2D and DKD, the kidneyprotective effects of SGLT2 inhibitors likely occur through several mechanisms.12 Notably, SGLT2 inhibitors are believed to protect the kidney by reducing glomerular hyperfiltration and thus address the deleterious hemodynamic changes that occur in CKD.12 SGLT2 inhibitors also improve important DKD risk factors, including blood glucose, blood pressure, and obesity.12 Nonetheless, SGLT2 inhibitors have demonstrated benefits in nondiabetic kidney disease, suggesting their kidney-protective effects are not dependent on glucose reduction.13 This notion is likewise supported by the fact that the glucose-lowering effects of SGLT2 inhibitors are less robust in patients with low eGFR.5 There are currently four SGLT2 inhibitors commercially available in the U.S., inclusive of canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin (TABLE 3).14-17
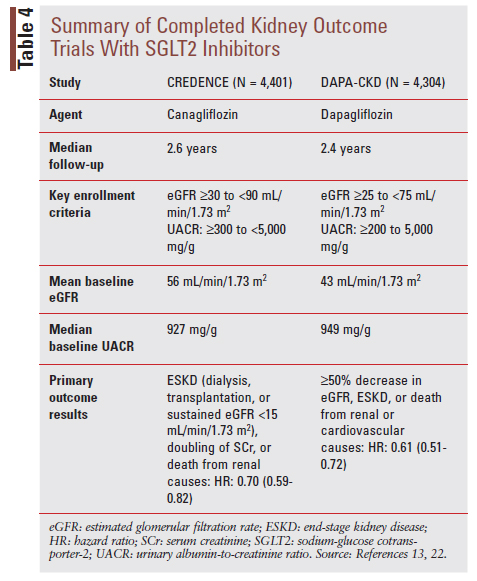
The first signal for kidney benefit with SGLT2 inhibitors came from secondary outcomes reported in large cardiovascular outcome trials (CVOTs) with canagliflozin, dapagliflozin, and empagliflozin showing reduced risk of DKD onset and progression.18-21 Based on these results, dedicated kidney outcome trials were conducted to confirm these findings. To date, results have been reported for the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) and Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) trials (TABLE 4).13,22 A kidney outcome trial with empagliflozin is ongoing and expected to complete in 2022.23
The CREDENCE and DAPA-CKD trials both reported a significant benefit of SGLT2 inhibitor use on a primary kidney composite outcome.13,22 Importantly, given the risk of CV disease in patients with DKD, these trials also showed benefit on prespecified CV outcomes. Canagliflozin treatment in the CREDENCE trial, for example, decreased risk for the CV composite outcomes of CV death or hospitalization for HF (hazard ratio [HR]: 0.69, 95% CI:0.57-0.83; P <.01) and CV death, myocardial infarction (MI), or stroke (HR: 0.80; 95% CI: 0.67-0.95; P = .01). The risk of hospitalization for HF was also reduced with canagliflozin (HR: 0.61; 95% CI: 0.47-0.80; P <.001).22 The DAPA-CKD trial added to our understanding of SGLT2 inhibitor impact on CKD by enrolling patients with and without T2D and patients with lower baseline eGFR values (as low as 25 mL/min/1.73m2).13 Importantly, both trials observed these benefits on top of background optimized RAS inhibitor therapy.
When initiating an SGLT2 inhibitor in patients with T2D and DKD, it is important to consider potential tolerability and safety issues. Such considerations include the potential for genital mycotic infections, diabetic ketoacidosis (DKA), hypoglycemia (if added to background insulin or sulfonylurea therapy), and hypovolemia.6 The following are key practice points offered by KDIGO for SGLT2 inhibitor use in patients with T2D and DKD6:
• Treating patients with T2D, CKD, and an eGFR ≥30 mL/min/1.73 m2 with an SGLT2 inhibitor is recommended.
• For patients in whom additional glucose-lowering may increase risk for hypoglycemia (e.g., those treated with insulin or sulfonylureas and currently meeting glycemic targets), it may be necessary to stop or reduce the dose of an antihyperglycemic drug other than metformin to facilitate addition of an SGLT2 inhibitor.
• The choice of an SGLT2 inhibitor should prioritize agents with documented kidney or CV benefits and take eGFR into account.
• It is reasonable to withhold SGLT2 inhibitors during times of prolonged fasting, surgery, or critical medical illness (when patients may be at greater risk for DKA).
• If a patient is at risk for hypovolemia, consider decreasing thiazide or loop diuretic dosages before commencement of SGLT2 inhibitor treatment, advise patients about symptoms of volume depletion and low blood pressure, and follow up on volume status after drug initiation.
• Once an SGLT2 inhibitor is initiated, it is reasonable to continue an SGLT2 inhibitor even if the eGFR falls below 30 mL/min/1.73 m2, unless it is not tolerated, or kidney replacement therapy is initiated.
Of note, dapagliflozin is indicated for initiation in patients with DKD down to an eGFR of 25 mL/ min/1.73 m2 as supported by the DAPA-CKD trial.15 The KDIGO recommendation to initiate an SGLT2 inhibitor down to an eGFR of 30 mL/min/1.73 m2 was established prior to publication of the DAPACKD trial findings. It is anticipated that recommendations from KDIGO and ADA will be updated to reflect findings from DAPA-CKD and other recently reported trials where SGLT2 inhibitors were initiated at eGFRs below 30 mL/min/1.73 m2.
GLP-1 RAs
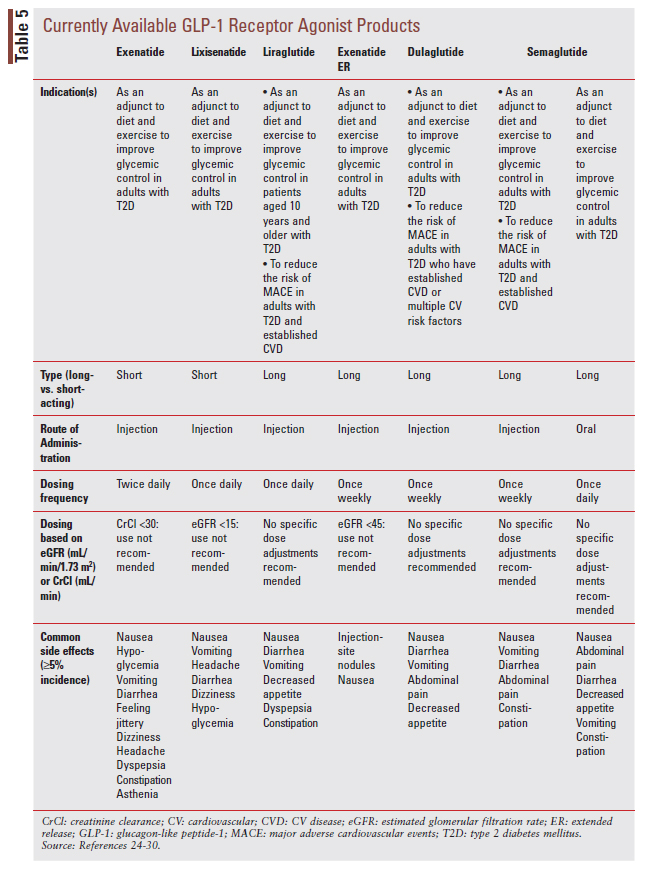
Like the SGLT2 inhibitor class, GLP-1 RAs were developed as glucose-lowering agents for the treatment of T2D. There are currently seven stand-alone GLP-1 RA products on the market (TABLE 5).24-30
GLP-1 RAs can be used to improve glycemic control even in advanced DKD, and several agents in the class have demonstrated benefit for CV outcomes (see expanded CV indications listed in TABLE 5).2,6
GLP-1 RAs are theorized to benefit patients with T2D and DKD through their glucose-lowering and anti-inflammatory effects.31 As previously noted, KDIGO preferentially recommends the addition of a long-acting GLP-1 RA in patients with T2D and DKD who need additional glucose lowering despite recommended first-line therapy (FIGURE 2).6 While primary kidney outcome data are currently lacking, secondary kidney outcome data from CVOTs with dulaglutide, liraglutide, and injectable semaglutide have reported benefits of treatment on albuminuria.32-34 An additional clinical trial in patients T2D and CKD noted a benefit of dulaglutide treatment on eGFR decline.35 An ongoing kidney outcome trial is underway testing the impact of injectable semaglutide on primary kidney outcomes in patients with T2D and CKD.36 The following are KDIGO practice points related to GLP-1 RA use in DKD, including recommendations to minimize risks for gastrointestinal side effects and treatment-emergent hypoglycemia6:
• In patients with T2D and CKD who have not achieved individualized glycemic targets despite use of metformin and SGLT2 inhibitor treatment, or who are unable to use those medications, we recommend a long-acting GLP-1 RA.
• The choice of GLP-1 RA should prioritize agents with documented CV benefits.
• To minimize gastrointestinal side effects, start with a low dose of GLP-1 RA, and titrate up slowly.
• The risk of hypoglycemia is generally low with GLP-1 RAs when used alone, but risk is increased when GLP-1 RAs are used concomitantly with other medications such as sulfonylureas or insulin. The doses of sulfonylurea and/or insulin may need to be reduced.
Nonsteroidal Mineralocorticoid Receptor Antagonists
As mentioned earlier, overactivation of the MR has been implicated in the pathogenesis of DKD.8 Finerenone, a novel nonsteroidal MRA, was recently approved by the FDA to reduce the risk of sustained eGFR decline, end-stage kidney disease, CV death, nonfatal MI, and hospitalization for HF in adults with CKD associated with T2D.37 Finerenone acts as a bulky, passive antagonist at the MR which, in conjunction with its unique physicochemical properties, differentiates it from steroidal MRAs such as spironolactone and eplerenone.8 Of importance, finerenone has demonstrated a lower risk for contributing to hyperkalemia when compared with steroidal MRAs.8 The approval of finerenone is supported by two large outcome trials studying finerenone as add-on to background RAS inhibitor therapy in patients with T2D and CKD. The Effect of Finerenone on Chronic Kidney Disease Outcomes in the Type 2 Diabetes (FIDELIODKD) trial enrolled over 5,000 participants.38 Finerenone use reduced risk of the composite primary kidney disease outcome (kidney failure, a sustained decrease in eGFR of ≥40%, or death from kidney disease) by 18% (HR: 0.82; 95% CI: 0.73-0.93; P = .001). Event rates for hyperkalemia were overall low in the trial (2.3% vs. 0.9% in the finerenone and placebo groups, respectively).38
The Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease (FIGARO-DKD) trial was also positive for the primary CV composite outcome inclusive of death from CV causes, nonfatal MI, nonfatal stroke, and hospitalization for HF (HR: 0.87; 95% CI: 0.76-0.98; P = .03).39 Together, findings from these two trials substantiate finerenone as an effective intervention to improve kidney and CV outcomes in patients with T2D and DKD.
TABLE 6 provides a summary of key product and monitoring information for finerenone.37 Given the recent approval of finerenone (July 2021), recommendations regarding its use are not reflected in current guideline documents from ADA and KDIGO, although updates to recommendations from these organizations are forthcoming and will undoubtedly provide guidance on use of finerenone in patients with DKD.
CONCLUSION
DKD is an important complication in patients with T2D. While therapeutic options to prevent and slow the progression of DKD have historically been limited, recent evidence supporting the benefits of novel therapies provide new treatment options to improve kidney and CV outcomes in this high-risk population. Pharmacists can play an important role in helping optimize use of these important therapies by recommending their use in appropriate patients and educating patients and caregivers on their appropriate use.
REFERENCES
- Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. www.cdc.gov/diabetes/library/features/diabetes-stat-report. html. Accessed September 1, 2021.
- American Diabetes Association. Standards of Medical Care in Diabetes – 2021. Diabetes Care. 2021;44(Suppl. 1):S1-S232.
- de Boer IH, Rue TC, Hall YN, et al. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305(24):2532-2539.
- Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032-2045.
- Neumiller JJ, Alicic RZ, Tuttle KR. Therapeutic considerations for antihyperglycemic agents in diabetic kidney disease. J Am Soc Nephrol. 2017;28(8):2263-2274.
- Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020;98(4S):S1-S115.
- Pichler R, Afkarian M, Dieter BP, et al. Immunity and inflammation in diabetic kidney disease: translating mechanisms to biomarkers and treatment targets. Am J Physiol Renal Physiol. 2017;312:F716-F731.
- Agarwal R, Kolkhof P, Bakris G, et al. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J. 2021;42:152-161.
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709-733.
- Bergenstal RM, Beck RW, Close KL, et al. Glucose management indicator (GMI): a new term for estimating A1C from continuous glucose monitoring. Diabetes Care. 2018; 41:2275-2280.
- Tuttle KR, Alicic RZ, Duru OK, et al. Clinical characteristics of an risk factors for chronic kidney disease among adults and children: an analysis of the CURE-CKD Registry. JAMA Netw Open. 2019;2(12):e1918-169.
- Alicic RZ, Neumiller JJ, Johnson EJ, et al. Sodium-glucose cotransporter 2 inhibition and diabetic kidney disease. Diabetes. 2019; 68:248-257.
- Heerspink HJL, Stefansson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436-1446.
- Invokana (canagliflozin) tablets [product information]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2020.
- Farxiga (dapagliflozin) tablets [product information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2021.
- Jardiance (empagliflozin) tablets [product information]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.; 2021.
- Steglatro (ertugliflozin) tablets [product information]. Whitehouse Station, NJ: Merck & Co., Inc.; 2021.
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:21172128.
- Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323-334.
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657.
- Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
- Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295-2306.
- U.S. National Library of Medicine. EMPA-KIDNEY (The Study of Heart and Kidney Protection With Empagliflozin). https://www.clinicaltrials.gov/ct2/show/NCT03594110. Accessed September 3, 2021.
- Byetta (exenatide) injection [product information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2021.
- Adlyxin (lixisenatide) injection [product information]. Bridgewater, NJ: Sanofi-aventis U.S. LLC.; 2019.
- Victoza (liraglutide) injection [product information]. Plainsboro, NJ: Novo Nordisk Inc.; 2020.
- Bydureon (exenatide extended-release) injection [product information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2020.
- Trulicity (dulaglutide) injection [product information]. Indianapolis, IN: Eli Lilly and Co; 2021.
- Ozempic (semaglutide) injection [product information]. Plainsboro, NJ: Novo Nordisk, Inc.; 2021.
- Rybelsus (semaglutide) tablets [product information]. Plainsboro, NJ: Novo Nordisk, Inc.; 2021.
- Alicic RZ, Cox EJ, Neumiller JJ, et al. Incretin drugs in diabetic kidney disease: biological mechanisms and clinical evidence. Nature Rev Nephrol. 2021;17(4):227-244.
- Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
- Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
- Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomized placebo-controlled trial. Lancet. 2019;394:121-130.
- Tuttle KR, Lakshmanan MC, Rayner B, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018;6:605-617.
- U.S. National Library of Medicine. A Research Study to See How Semaglutide Works Compared to Placebo in People With Type 2 Diabetes and Chronic Kidney Disease (FLOW). https://clinicaltrials.gov/ct2/show/ NCT03819153?term=semaglutide+FLOW&draw=2&rank=1. Accessed September 4, 2021.
- Kerendia (finerenone) tablets [product information]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc.; 2021.
- Bakris GL, Agarwal R, Anker SD, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383:2219-2229.
- Pitt B, Filippatos G, Agarwal R, et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med. 2021 Aug 28. [Online ahead of print].