Update on the Management of Asthma
RELEASE DATE
July 1, 2022
EXPIRATION DATE
July 31, 2024
FACULTY
Kamden Benjamin, PharmD Candidate 2023
Western New England University
College of Pharmacy & Health Sciences
Mackenzie Laisure, PharmD Candidate 2022
Western New England University
College of Pharmacy & Health Sciences
Jared L. Ostroff, PharmD, MBA, BCACP, BCGP
Ambulatory Pharmacy Supervisor
Baystate Health
Marissa L. Ostroff, PharmD, BCPS, BCGP
Clinical Associate Professor of Pharmacy Practice—Ambulatory Care
Western New England University
College of Pharmacy & Health Sciences
Springfield, Massachusetts
FACULTY DISCLOSURE STATEMENTS
Kamden Benjamin, Mackenzie Laisure, and Drs. Jared and Marissa Ostroff have no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT
 Pharmacy
Pharmacy
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-22-094-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients’ conditions and possible contraindications or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
GOAL
To give pharmacists an overview of asthma management based on the 2021 update to the Global Initiative for Asthma (GINA) guidelines, recent literature, and emerging asthma therapies.
OBJECTIVES
After completing this activity, the participant should be able to:
- Review the assessment and classification of asthma.
- Identify nonpharmacologic and pharmacologic interventions for patients with asthma.
- Discuss guideline-recommended treatment options and relevant updates to the GINA guidelines.
- Recognize the pharmacist’s role in the treatment of asthma.
ABSTRACT: Asthma is an episodic disease characterized by variable airway inflammation and fluctuating respiratory signs and symptoms such as wheezing, shortness of breath, chest tightness, and cough. In 2021, the Global Initiative for Asthma (GINA) published the latest version of its asthma-management guidelines. Among the updates to these guidelines is a revised definition of severe asthma based on changes GINA has made over time. Nonpharmacologic interventions should supplement pharmacologic therapy in order to optimize symptom control and reduce exacerbation risk. Emerging treatments, including monoclonal antibodies, offer a promising future for patients with severe asthma. Pharmacists play a crucial role in disease-state education, assessment of symptom control, and counseling on medication adherence and nonpharmacologic interventions in patients with asthma.
Asthma is a common chronic respiratory disease characterized by variable airway inflammation and respiratory symptoms.¹ Examples of exacerbating triggers are exercise, allergens, irritants, infections, and environmental changes. Treatment goals for asthma management include achieving adequate symptom control, maintaining normal activity levels, and minimizing the risk of asthma-related death, exacerbations, persistent airflow limitation, and side effects.¹ Inhalers used to manage asthma include short-acting beta-2 agonists (SABAs), inhaled corticosteroids (ICS), ICS plus long-acting beta-2 agonists (ICS-LABAs), ICS plus formoterol (ICS-formoterol), and long-acting muscarinic antagonists (LAMAs). Supportive therapies include leukotriene receptor antagonists, theophylline, and azithromycin. More recently, agents such as anti–immunoglobulin E (IgE) or anti-interleukin (IL) monoclonal antibodies (MABs) and house dust mite sublingual immunotherapy (HDM-SLIT) have received approval for use in asthma. This article will provide an overview of the most recent update (2021) of the Global Initiative for Asthma (GINA) asthma-management guidelines, review recent literature supporting treatment options, and discuss emerging asthma therapies.
DIAGNOSIS
A physical examination and evaluation of the patient’s symptoms and signs (hereafter referred to collectively as “symptoms” for brevity) and expiratory airflow limitation are crucial in making an asthma diagnosis.1 Shortness of breath, wheezing, cough, and chest tightness are cardinal symptoms of asthma, especially if multiple symptoms are present. Constructing a timeline of respiratory symptoms and relating it to past medical history is important for differential diagnosis. Comorbidities such as atopic dermatitis, allergic rhinitis, and eczema increase the probability of an asthma diagnosis. Other factors include worsening status at night or early in the morning, varying time and intensity of respiratory symptoms, and confirmed triggers. On the other hand, isolated cough, chronic sputum production, chest pain, and crackles reduce the suspicion of asthma. Objective tests such as forced expiratory volume in 1 second (FEV1)/forced vital capacity and postbronchodilator reversibility tests allow healthcare providers (HCPs) to quantify limitations, if present.1 As a hallmark of asthma pathophysiology, the measurement of variable airflow limitation is imperative for reaching a diagnosis.
CLASSIFICATION
The GINA guidelines combine symptom severity, frequency, and current treatment step to classify asthma as mild, moderate, severe, or difficult-to-treat.1 Diverging from previous versions, the 2021 guidelines no longer differentiate between “intermittent” and “mild persistent” asthma.2
Asthma that is well controlled on step 1 or 2 therapy consisting of as-needed ICS-formoterol, with or without a scheduled low-dose controller, is mild. Asthma that is well controlled on steps 3 or 4 using low-to-medium-dose ICS-LABA is moderate.1 Severe asthma, being more complex than mild and moderate asthma, falls under the umbrella term difficult-totreat. The 2021 GINA guidelines updated the definition of severe asthma to exclude reference to steps 4 and 5 based on changes made over the years.1,2 Before a diagnosis of severe asthma is made, the existence of poor inhaler technique, insufficient medication adherence, comorbidities, or ongoing trigger exposure should be identified and treated.1
In addition to severity, asthma is divided into phenotypes that include but are not limited to allergic, nonallergic, and adult-onset asthma, asthma with obesity, and asthma with persistent airflow limitation.1
ASSESSING ASTHMA CONTROL
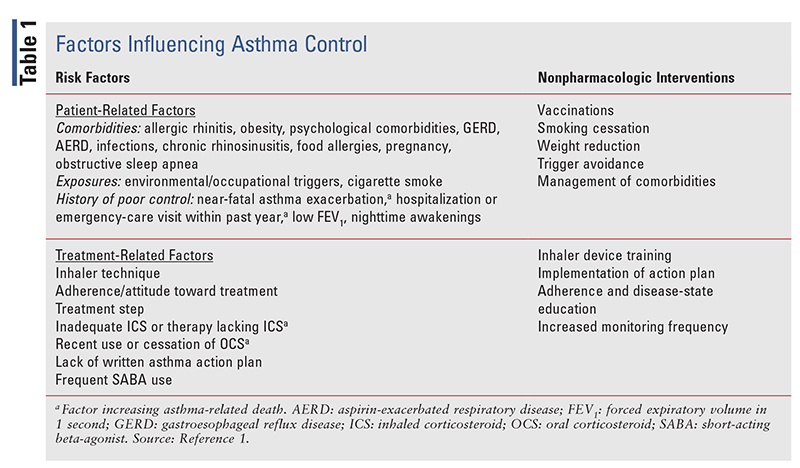
Poor symptom control increases the risk of exacerbations, warranting frequent assessment. Factors influencing asthma control are categorized as either patient-related or treatment-related (TABLE 1).1
Patient interviews that are aimed at determining the degree of asthma control should review symptom severity and frequency, treatment-related issues, lung function, comorbidity management, and overall risk factors for exacerbations over the past 4 weeks (TABLE 1). Specifically, obtaining information on activity limitations, nighttime awakening due to asthma symptoms, and frequency of SABA use is essential.1 Lung function should be measured at the start of treatment, after 3 to 6 months of treatment, and periodically afterward to assess ongoing risk and response to treatment.¹
Tools for evaluating asthma control include the Asthma Control Questionnaire (ACQ) and the Asthma Control Test (ACT). These tools allow HCPs to assign patient scores based on self-reported asthma control. The ACQ assesses asthma symptoms, and SABA assesses reliever use. ACQ scores range from 0 to 6, with 6 representing worst asthma control.¹ The ACT questions patients about symptoms and reliever use, with a score range of 5 to 25 (in contrast to the ACQ, a higher ACT score represents better asthma control).¹
Nonpharmacologic Interventions
The management of risk factors for poor asthma control can significantly reduce a patient’s risk of exacerbation. Nonpharmacologic interventions (TABLE 1). are essential for modifying risk factors. Counseling patients on self-management methodology and on the proper use of their written asthma action plan can help increase patient adherence, reduce oral corticosteroid (OCS) use, and manage comorbidities.1 A randomized clinical trial investigated the effect of comprehensive self-management interventions on outcomes in older adults with asthma. Comprehensive self-management implementation was determined to improve outcomes such as asthma control and emergency-department visits, and it reduced risk factors such as poor adherence and incorrect inhaler technique.3
A written asthma action plan includes patient-specific education, reviews reliever and controller medications, and provides instructions on the use of OCS and when to seek medical attention.1 The sections of a written asthma action plan break down a patient’s asthma status into well-controlled, getting worse, and severe. Escalation of therapy may be required if relievers are needed more often than usual, nighttime symptoms increase, or daily activities become limited.1 Immediate medical help should be sought when a patient enters the severe-asthma category (i.e., experiences dyspnea, needs relievers more than every 3 to 4 hours, or awakens from symptoms at night).1
As recommended by the GINA guidelines and the CDC, patients with asthma should receive immunizations as appropriate for their age and underlying medical conditions. These include annual influenza vaccinations as well as primary and booster coronavirus disease 2019 (COVID-19) series as available.4 However, asthma patients may be at higher risk for contracting pneumococcal diseases; therefore, the risks versus benefits should be considered and discussed.¹ Without proper implementation of nonpharmacologic interventions and modification of risk factors, patients are at increased risk for poor outcomes and exacerbations.1
EXACERBATIONS
Persistently uncontrolled asthma may lead to exacerbations, which are characterized by a progressive increase in symptoms that ultimately leads to a decline in lung function. Typically, exacerbations are caused by the combination of poor controller-medication adherence and exposure to triggers.1 Changes from baseline in symptoms, peak expiratory flow (PEF), or FEV1 are used to classify the degree of deterioration in patients presenting with suspected exacerbations.
Assessment of the severity of an exacerbation in the primary care setting should include history, physical examination, and objective measurements. In patient interviews, vocabulary such as “flare-up” or “attack” may be used to avoid confusion.1 In the pharmacy, patients should be reminded of symptoms of exacerbations and when to seek medical help.
Follow-up with the primary HCP after self-managed exacerbations should occur within 1 to 2 weeks, preferably before cessation of OCS.1 Resolution of exacerbation symptoms, the need for scheduled reliever dosing, step-down of controller therapy, risk factors, and the patient’s understanding of the action plan should be reviewed on follow-up. Reeducation upon changes in treatment is imperative to successful therapy.
PHARMACOLOGIC MANAGEMENT
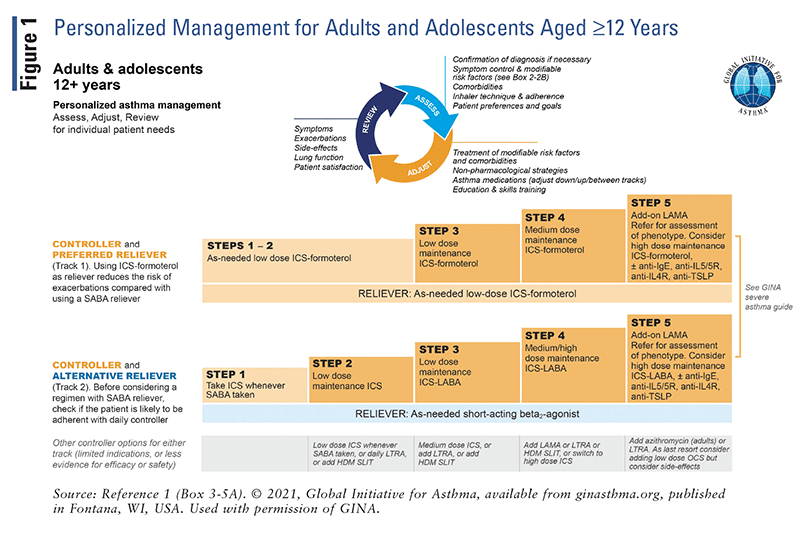
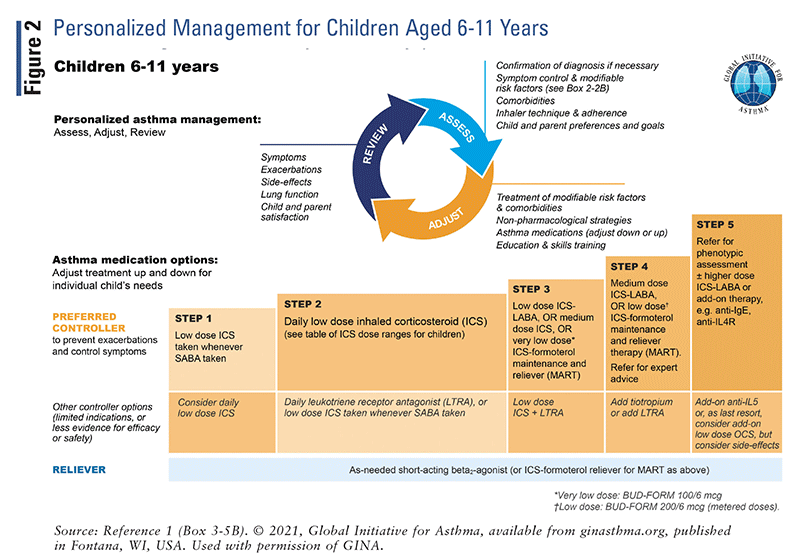
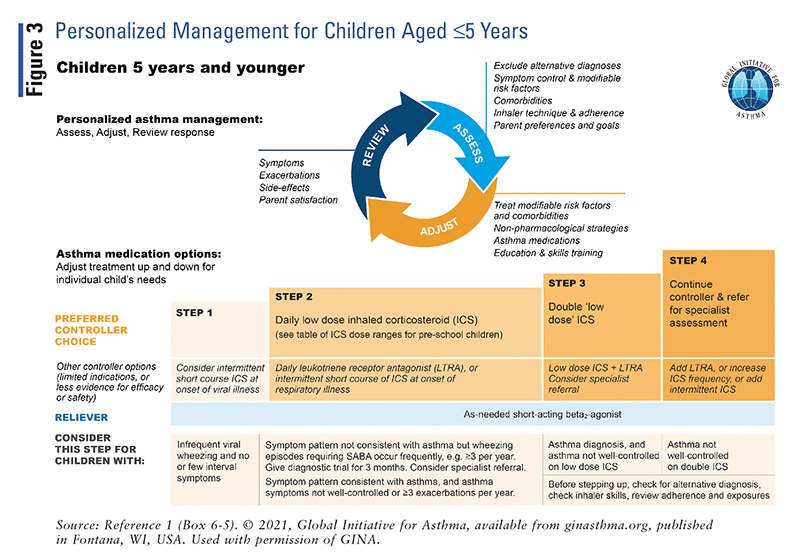
The updated GINA guidelines include treatment “tracks” for the management of adults and adolescents.² Track 1 is the preferred approach recommended by GINA, whereas track 2 is an alternative approach.² Asthma treatment consists of controller medications such as ICS, reliever medications such as ICS-formoterol or as-needed SABAs, and add-on therapies for severe asthma. It is important to be aware that maintenance and reliever therapy (MART) with low-dose ICS-formoterol is recommended in the track 1 approach for adults and adolescents. MART was found to reduce the risk of exacerbations with similar or better symptom control compared with the combination of maintenance inhaler plus SABA.¹ Refer to FIGURES 1–3 for a detailed overview of asthma management for steps 1 through 5.
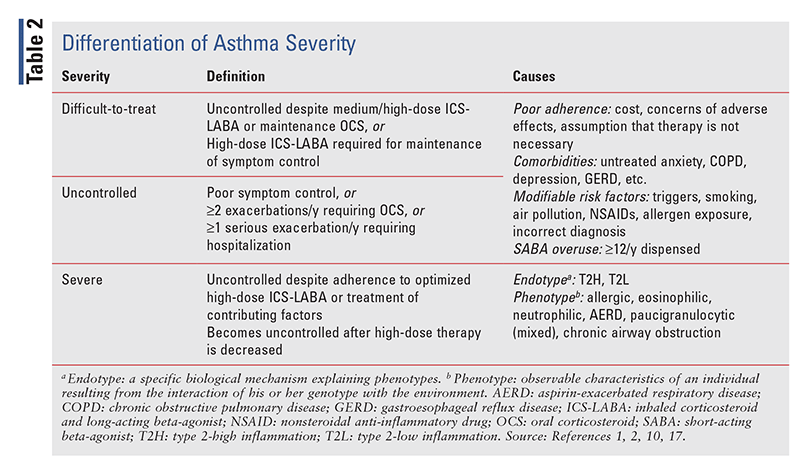
DIFFICULT-TO-TREAT ASTHMA
There is an important distinction between uncontrolled and difficult-to-treat asthma and severe asthma (TABLE 2). Difficult-to-treat asthma is frequently due to modifiable risk factors. Although uncontrolled and difficult-to-treat asthma are defined differently, they share the same causes.1 Severe asthma—as noted earlier, a subset of difficult-to-treat asthma—is typically caused by the patient’s endotype or phenotype. Pharmacotherapeutic options for patients with difficult-to-treat asthma include HDM-SLIT, LAMAs, combination ICS-LABA-LAMAs, and biological agents. See TABLES 2–5 for more information.
Sublingual Allergen Immunotherapy
SLIT may be used as an add-on controller treatment for adults and adolescents with comorbid allergic rhinitis that is sensitized to HDM in steps 2 though 4 of either track.1 At this time, Odactra is the only HDM-SLIT product available in the United States; it is FDA approved for HDM-sensitive allergic rhinitis as confirmed by IgE tests, suboptimal symptom control on low- to high-dose ICS, and predicted FEV1 >70%. Although its mechanism is not fully understood, HDM-SLIT acts by inducing a T-cell switch, resulting in decreased IL-4 and IL-5 and increased IL-10 signaling.5 Systematic reviews exploring the efficacy of SLIT found modest reductions in ICS during use.¹ Odactra is a once-daily formulation taken at least 5 minutes before or after food consumption. The most common adverse effects include throat irritation, pruritus of the ear and mouth, oropharyngeal swelling, and severe allergic reactions. Based on a black box warning for severe allergic reactions, patients using HDM-SLIT should be prescribed an epinephrine autoinjector, and all first doses must be administered under HCP supervision followed by at least 30 minutes of observation.5,6 Contraindications to severe, unstable, or uncontrolled asthma, history of severe systemic allergic reactions, and history of eosinophilic esophagitis are the reasons that this agent is not recommended for use in patients on treatment step 5.1 GINA is currently reviewing evidence on SLIT therapy and will present further recommendations based on these findings in its next update.
Long-Acting Muscarinic Antagonists
In previous GINA guidelines, LAMAs were recommended in treatment steps 4 and 5 as a tiotropium (Spiriva Respimat) inhaler separate from an ICSLABA. In 2021, the recommendation was expanded to include ICS-LABA-LAMA (i.e., triple therapy) in steps 4 and 5 for patients aged 18 years and older.1,2 GINA suggests that triple therapy provides modest improvement in lung function, but symptomimprovement data are inconclusive. It is important to note that the addition of a LAMA in a patient with exacerbations is not recommended until the HCP ensures that the patient is receiving an adequate trial of at least a medium-dose ICS-LABA.1
Trelegy Ellipta, the only triple-therapy product available in the U.S., is indicated as add-on maintenance treatment to ICS-LABAs in patients aged 6 years and older. This medication should be taken as 1 actuation by mouth daily. Pharmacists and HCPs should counsel patients concerning correct technique. The patient should be instructed to open the cover fully until a click is heard, fully exhale away from the mouthpiece, breathe the medication in steadily and deeply, and hold the breath for at least 3 to 4 seconds after inhalation.7 After each use, the patient should rinse the mouth in order to prevent oropharyngeal candidiasis. Additional adverse effects commonly associated with the use of Trelegy Ellipta are upper respiratory infection, sinusitis, influenza, headache, and back pain.7
In the randomized, double-blind, active-controlled, phase IIIa CAPTAIN trial, Trelegy Ellipta was compared with Breo Ellipta regarding increases in FEV1 and reductions in annualized exacerbation rate in patients with inadequately controlled asthma on medium- to high-dose ICS-LABA therapy.8 Patients were excluded if they had uncontrolled asthma symptoms within 6 weeks of starting the trial, had comorbid chronic obstructive pulmonary disease or other lung disease, or were smokers. The primary efficacy outcome was the change in FEV1 from baseline at 24 weeks, and the primary safety outcome was the annualized moderate or severe exacerbation rate at 52 weeks. Trelegy Ellipta (strengths: 100/25/31.25 mcg, 200/25/31.25 mcg, 100/25/62.5 mcg, 200/25/62.5 mcg; Trelegy 100 and 200) was compared with Breo Ellipta (strengths: 100/25 mcg, 200/25 mcg; Breo 100 and 200) for both end points. Trelegy 100 resulted in a 110-mL increase in FEV1 versus Breo 100 (95% CI, 66-153 mL; P <.001). Trelegy 200 elicited a 92-mL increase in FEV1 versus Breo 200 (95% CI, 49-135 mL; P <.001).8 The mean annualized rate of exacerbation was 0.31 for both Trelegy and Breo 100 and 200, with a 2.6% rate reduction (95% CI, –26.2-24.9) for Trelegy compared with Breo.8
SEVERE ASTHMA
As noted previously, although it is a subset of difficult-to-treat asthma, severe asthma differs in its cause and treatment (TABLE 2). Based on its definition, severe asthma is a retrospective diagnosis that has often been mislabeled as severe refractory asthma. The 2021 GINA guidelines changed this terminology to avoid confusion.1
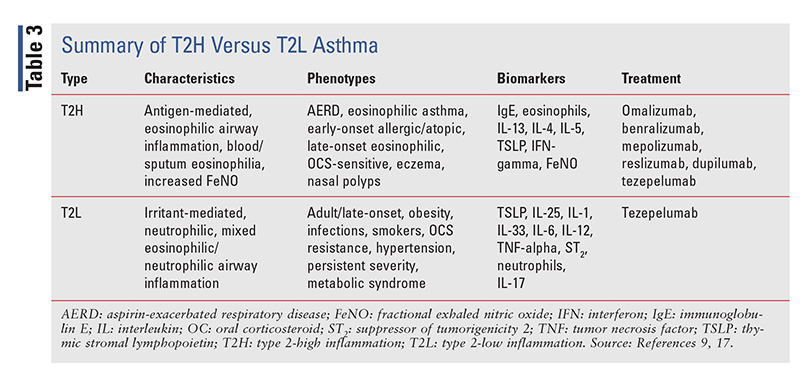
Severe asthma is heterogeneous, resulting in many observable clinical and pathophysiological attributes, or phenotypes.1 Although they are not fully understood, many clinical phenotypes have been identified and investigated for targeted therapy with MABs. Two distinct biological mechanisms underlying the phenotypes have been discovered: type 2-high inflammation (T2H) and type 2-low inflammation (T2L; TABLE 3). Further investigation is needed before phenotyping can be relied on as a method for treating severe asthma; however, MABs are a promising look into the future of therapy.1
Monoclonal Antibodies
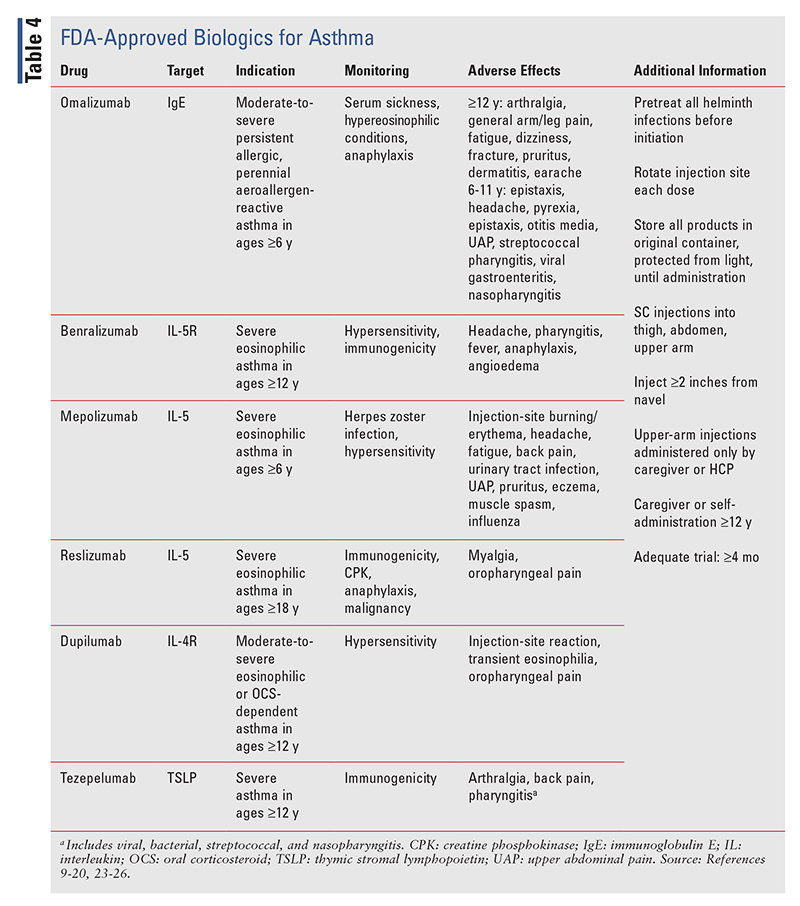
Biologics are recommended for patients who remain uncontrolled on step 5 after expert referral and phenotyping.1 Because asthma is heterogeneous, numerous inflammatory pathways have become possible drug targets.9 Although the targeting of specific inflammatory biomarkers is effective in theory, it is not indicative of an efficacious response. MABs pose much greater risks than standard treatment. Manufacturers provide programs designed to assist patients with access and support. After initiation of biological therapy, patient reassessment should occur after 3 to 4 months and every 3 to 6 months thereafter.1
All MABs carry similar risks despite their different targets (TABLE 4). Patients should receive appropriate counseling prior to initiation and throughout treatment. As a consequence of inhibiting immunomediated inflammatory pathways, patients treated with MABs are at increased risk for contracting infections.1,10 Appropriate patient education is necessary on important precautions to take, such as routine vaccinations and proper hygiene, and on when to contact a doctor. Patients on maintenance OCS may attempt to abruptly discontinue their regimen upon initiation of biologics. HCPs should work closely with patients starting any biologic for asthma and gradually taper OCS to prevent adrenal insufficiency.1,10
Anti-IgE Monoclonal Antibody
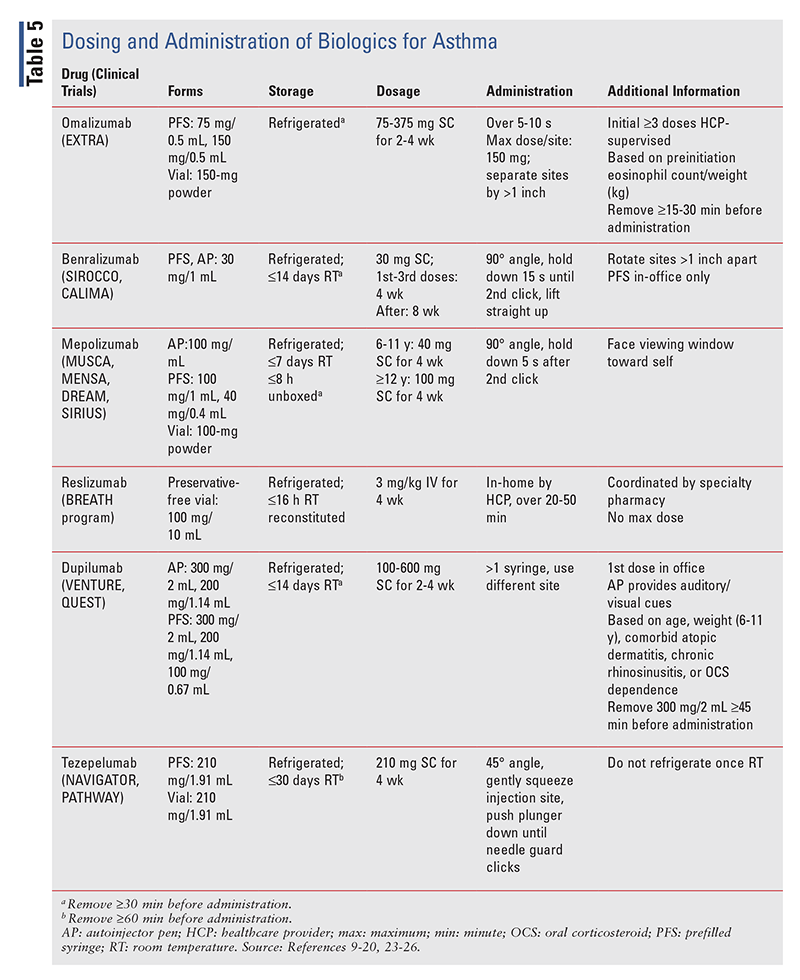
Omalizumab (Xolair) was the first biological agent to be FDA approved for the treatment of allergic asthma in adults (2003) and children (2016).11 Additional indications are for chronic idiopathic urticaria and nasal polyposis in adults and adolescents aged 12 years and older. Omalizumab reduces blood and tissue eosinophilia and allergen-induced early- and late-phase asthmatic responses. By binding free IgE, it causes dendritic cells, mast cells, and basophils to remain inactivated. Over time, IgE receptors on these cells become downregulated to prevent future allergic responses.11 Resources that increase the accessibility of omalizumab for patients include XOLAIR Access Solutions, Genentech Patient Foundation, and the XOLAIR copay program.
Monoclonal Antibodies Against IL-5 and IL-5R
Three MABs targeting IL-5 treat uncontrolled severe asthma in GINA steps 4 and 5. Potential predictors of a positive response to these medications are higher blood eosinophils, increased frequency of severe exacerbations in the past year, adult-onset asthma, nasal polyps, baseline maintenance OCS, and poor lung function. Strong predictors of a positive response are blood eosinophil count and the number of severe exacerbations in the previous year.1 Patient resources for all three drugs include AstraZeneca Access 360, the FASENRA Savings Program and Dose Reminder Program, Gateway to NUCALA, Bridge to NUCALA, NUCALA Copay Program, NUCALA Patient-Assistance Program, TEVA Support Solutions, and CINQAIR Cost Support Program.
Benralizumab: This agent targets the IL-5 receptor, depleting serum eosinophils and basophils through antibody-dependent cell-mediated cytotoxicity and ultimately reducing eosinophilic inflammation.12 Benralizumab (Fasenra) was FDA approved in 2017 for use as add-on maintenance treatment in patients aged 12 years and older with severe eosinophilic asthma; it currently has no other approved indications. In the phase III SIROCCO and CALIMA trials, 13% of patients treated with benralizumab developed antidrug antibodies, and 12% developed neutralizing antibodies during the 48- to 56-week treatment period.12
Mepolizumab and Reslizumab: Both agents reduce eosinophilic inflammation by targeting freely circulating IL-5 and reducing the growth, differentiation, recruitment, activation, and survival of eosinophils. Mepolizumab (Nucala) and reslizumab (Cinqair) are FDA approved for add-on maintenance treatment in patients with severe eosinophilic asthma—in patients aged 6 years and older for mepolizumab, and patients aged 18 years and older for reslizumab.13,14 Among 1,028 patients in six trials, three cases of anaphylaxis were observed in those who were treated with reslizumab, leading to its black box warning.13-15
Anti–IL-4/IL-4R Monoclonal Antibody
Dupilumab (Dupixent) is the only anti–IL-4/IL-4R MAB approved for asthma. This drug has several other indications, including atopic dermatitis, eosinophilic esophagitis, and chronic rhinosinusitis with nasal polyposis.1,15,16 Support programs include DUPIXENT MyWay and CoverMyMeds.
Uniquely, dupilumab inhibits both IL-4 and IL-13 signaling by binding to the shared IL-4 receptor, preventing inflammatory mediator pathways and reducing airway inflammation.1,16-18 A strong predictive factor for a positive response is increased serum eosinophils. Patients with higher fractional exhaled nitric oxide may experience better results, but this is not strongly predictive.1,18 In the VENTURE trial, the efficacy of dupilumab for reducing the use of maintenance OCS in patients aged 12 years and older was evaluated with a primary end point of the percent reduction in dose. The mean percent reduction in daily OCS use was 70% from a mean baseline of 11 mg in the dupilumab group compared with 42% from a mean baseline of 12 mg in the placebo group.15,18,19
Anti–Thymic Stromal Lymphopoietin Monoclonal Antibody
Tezepelumab (Tezspire), the newest MAB approved for asthma (in 2021),is the first and only biologic to reduce downstream inflammatory pathways by inhibiting thymic stromal lymphopoietin (TSLP) release and downstream inflammation due to blood and airway eosinophils, IgE, IL-5, and IL-13.10,17,20 Thus far, no other MAB has elicited a statistically significant reduction in exacerbations in patients with eosinophils <300 cells/mL. This makes tezepelumab a prospect for the treatment of T2L asthma.10,17,20 TEZSPIRE Together is a program designed to assist patients with administration, accessibility, and counseling.
FUTURE MANAGEMENT APPROACHES
Compared with T2H, T2L asthma has remained a mystery, preventing the development of efficacious drugs. Standard treatments for severe asthma typically target T2H biomarkers such as IgE, IL-4, IL-13, and IL-5. With the recent approval of tezepelumab, epithelial cytokines may be the future of drug development for T2L asthma. Tezepelumab’s success in clinical trials has generated alternative approaches to the treatment of severe asthma. Currently, astegolimab and itepekimab are attempting to follow this agent’s lead in clinical trials.10,21,22
Astegolimab: This agent is a suppressor of tumorigenicity 2 (ST2) inhibitor, ultimately preventing IL-33 signaling and reducing the activation of airway inflammatory cascades. In the phase IIb, double-blind, placebo-controlled ZENYATTA trial, astegolimab was compared with placebo.21 Patients received SC injections of 70 mg, 210 mg, or 490 mg astegolimab or placebo every 4 weeks for a total of 52 weeks. Efficacy was measured by the percent rate reduction in annualized exacerbation rates in patients with both high (≥300 cells/mcL) and low (<300 cells/mcL) eosinophil counts at week 54. Overall, the 70-mg, 210-mg, and 490-mg doses elicited rate reductions of 36.9%, 34.9%, and 39.9%, respectively.21
Itepekimab: This IL-33 inhibitor prevented the same airway inflammatory cascades as astegolimab. In a phase II proof-of-concept trial, itepekimab monotherapy, itepekimab plus dupilumab, dupilumab monotherapy, and placebo were compared to assess the effect on loss-of-asthma-control events.22 Events were defined as a 30% reduction in morning PEF from baseline on two consecutive days, >6 puffs of reliever needed in 24 hours, or an exacerbation requiring systemic glucocorticoids, hospitalization, or emergency-department care. Overall odds ratios (ORs) for itepekimab monotherapy and itepekimab plus dupilumab combination therapy were 0.42 (95% CI, 0.20-0.88; P = .02) and 0.52 (95% CI, 0.26-1.06; P = .07).25 For patients with eosinophil counts <300 cells/mcL, ORs were 0.46 (95% CI, 0.15-1.41) for monotherapy and 0.92 (95% CI, 0.33-2.56) for combination therapy. These values were not analyzed for statistical significance.21
The preliminary clinical impact of both astegolimab and itepekimab warrants further investigation into the role of epithelial cytokines in patients with severe T2L asthma.10
THE PHARMACIST’S ROLE
Pharmacists can make a substantial impact on asthma management. As accessible HCPs, they have a unique opportunity to identify and prevent inadequate asthma control. In the course of carrying out their daily functions, pharmacists can identify trends in inhaler use or refills, new medications, and gaps in patient education.
An increase in SABA refills or a decrease in maintenance refills should prompt the pharmacist to educate the patient on adherence and possibly also provide an appropriate HCP referral. The delivery of patient-friendly information aiding in the understanding of the asthma regimen is crucial for assisting the patient in maintaining proper adherence to a complex treatment regimen. Look-alike/sound-alike dosage forms of inhalers increase the risk of patient medication errors. Upon dispensing, community pharmacists should reinforce education and gauge where a patient’s understanding may be lacking. The teach-back method should be used to verify a patient’s understanding of correct technique and timing of inhalers. Adherence tools such as cell phone apps, reminders, or labels on inhalers should be encouraged in order to promote adherence and prevent medication mix-ups. During counseling, the pharmacist should ascertain that physical barriers to inhaler use (e.g., arthritis) are not present. General asthma education should cover the rationale or importance of treatment, prevention strategies, how to recognize worsening asthma, and when to seek medical attention.¹
In addition to treatment-related interventions, pharmacists should communicate nonpharmacologic strategies (TABLE 1). Smoking cessation improves asthma outcomes and reduces the risks of poor asthma control, hospitalization, and asthma-related death. Pharmacist monitoring of the patient’s smoking-cessation progress, combined with the provision of valuable resources, could make the difference between exacerbation-triggering deterioration and successful maintenance of control.1 Additional education could cover healthful dietary changes, exercise goals, and reminders about informing the HCP prior to starting any new medication. Patients should be made aware that certain medications, such as nonsteroidal anti-inflammatory drugs and cardioselective beta-blockers, can worsen asthma.
Patients treated with MABs should be counseled on storage and self-administration upon picking up new prescriptions. Because of the immense costs that these drugs may impose, various support programs are available to help cover patients’ copays and out-of-pocket costs. Pharmacists should keep abreast of existing program options to recommend to patients when dispensing specialty medications for asthma.
CONCLUSION
Asthma is an episodic respiratory disease characterized by variable signs and symptoms including, but not limited to, wheezing and shortness of breath. Asthma status can be assessed through a combination of symptom control, risk factors for exacerbations, and side effects from pharmacologic therapy, with asthma-control assessment tools including the ACQ and ACT. Nonpharmacologic interventions include vaccination, smoking cessation, weight reduction, and avoidance of triggers. The 2021 GINA guidelines recommend MART with ICS-formoterol for adults and adolescents. For uncontrolled asthma, MAB therapy is recommended. Pharmacists can play a crucial role in educating patients, assessing their symptom control, and counseling them on nonpharmacologic interventions.
REFERENCES
- Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention, 2021. https://ginasthma.org/wp-content/ uploads/2021/05/GINA-Main-Report-2021-V2-WMS.pdf. Accessed June 17, 2022.
- GINA. What’s new in GINA 2021? https://ginasthma.org/wp-content/ uploads/2021/05/Whats-new-in-GINA-2021_final_V2.pdf. Accessed June 17, 2022.
- Federman AD, O’Conor R, Mindlis I, et al. Effect of a self-management support intervention on asthma outcomes in older adults: the SAMBA study randomized clinical trial. JAMA Intern Med. 2019;179(8):1113-1121.
- CDC. People with certain medical conditions. www.cdc.gov/ coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html. Accessed June 17, 2022.
- Odactra. In: Lexi-Drugs. Hudson, OH: Lexicomp, Inc; 2022. https:// online.lexi.com. Accessed June 17, 2022.
- Odactra (house dust mite allergen extract) package insert. Hørsholm, Denmark: ALK-Abelló A/S; August 2019.
- Trelegy Ellipta (fluticasone, umeclidinium, and vilanterol) package insert. Research Triangle Park, NC: GlaxoSmithKline LLC; May 2022.
- Lee LA, Bailes Z, Barnes N, et al. Efficacy and safety of once-daily single inhaler triple therapy (FF/UMEC/VI) versus FF/VI in patients with inadequately controlled asthma (CAPTAIN): a double-blind, randomised, phase 3A trial. Lancet Respir Med. 2021;9(1):69-84.
- Hanania NA, Fortis S, Haselkorn T, et al. Omalizumab in asthma with fixed airway obstruction: post hoc analysis of EXTRA. J Allergy Clin Immunol Pract. 2022;10(1):222-228.
- Brusselle GG, Koppelman GH. Biologic therapies for severe asthma. N Engl J Med. 2022;386(2):157-171.
- Xolair (omalizumab) package insert. South San Francisco, CA: Genentech, Inc; July 2021.
- Fasenra (benralizumab) package insert. Wilmington, DE: AstraZeneca Pharmaceuticals LP; February 2021.
- Nucala (mepolizumab) package insert. Research Triangle Park, NC: GlaxoSmithKline; January 2022.
- Cinqair (reslizumab) package insert. West Chester, PA: Teva Respiratory, LLC; February 2020.
- Dupixent (dupilumab) package insert. Bridgewater, NJ: sanofi-aventis U.S. LLC, and Tarrytown, NY: Regeneron Pharmaceuticals, Inc; June 2022.
- Rabe KF, Nair P, Brusselle G, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. 2018;378(26):2475-2485.
- Hammad H, Lambrecht BN. The basic immunology of asthma. Cell. 2021;184(6):1469-1485.
- Busse WW, Maspero JF, Rabe KF, et al. Liberty Asthma QUEST: phase 3 randomized, double-blind, placebo-controlled, parallel-group study to evaluate dupilumab efficacy/safety in patients with uncontrolled, moderate-to-severe asthma. Adv Ther. 2018;35(5):737-748.
- Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486-2496.
- Tezspire (tezepelumab-ekko) package insert. Thousand Oaks, CA: AstraZeneca and Amgen Inc; December 2021.
- Kelsen SG, Agache IO, Soong W, et al. Astegolimab (anti-ST2) efficacy and safety in adults with severe asthma: a randomized clinical trial. J Allergy Clin Immunol. 2021;148(3):790-798.
- Wechsler ME, Ruddy MK, Pavord ID, et al. Efficacy and safety of itepekimab in patients with moderate-to-severe asthma. N Engl J Med. 2021;385(18):1656-1668.
- Gibson PG, Prazma CM, Chupp GL, et al. Mepolizumab improves clinical outcomes in patients with severe asthma and comorbid conditions. Respir Res. 2021;22(1):171.
- Máspero J. Reslizumab in the treatment of inadequately controlled asthma in adults and adolescents with elevated blood eosinophils: clinical trial evidence and future prospects. Ther Adv Respir Dis. 2017;11(8):311-325.
- Menzies-Gow A, Corren J, Bourdin A, et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med. 2021;384(19):1800-1809.
- Corren J, Parnes JR, Wang L, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med. 2017;377(10):936-946.