Update on the Management of Irritable Bowel Syndrome
RELEASE DATE
December 1, 2022
EXPIRATION DATE
December 31, 2024
FACULTY
Brooke Barlow, PharmD
Neurocritical Care Clinical Pharmacy Specialist
Memorial Hermann The Woodlands Medical Center
The Woodlands, Texas
DISCLOSURE STATEMENTS
Dr. Barlow has no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT
 Pharmacy
Pharmacy
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-22-146-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients' conditions and possible contraindications or dangers in use, review of any applicable manufacturer's product information, and comparison with recommendations of other authorities.
GOAL
To educate pharmacists about the new FDA-approved medications and guideline recommendations for the management of irritable bowel syndrome (IBS).
OBJECTIVES
After completing this activity, the participant should be able to:
- Explain the epidemiology and pathophysiology of IBS.
- Identify the clinical presentation and diagnostic criteria for IBS.
- Describe guideline-based nonpharmacologic and pharmacologic treatments for IBS.
- Discuss the pharmacist's role in the care of patients with IBS.
ABSTRACT: Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder characterized by recurrent abdominal pain or discomfort and altered bowel habits. IBS is a broad diagnosis that requires characterization of stool frequency and consistency to identify the specific IBS subtype, which guides treatment selection. IBS has a significant disease burden, with symptoms severe enough to impact activities of daily living and overall quality of life. Efforts to identify targeted agents for IBS have led to recent advances in drug therapies. The American Gastroenterology Association has updated its guideline recommendations for the treatment of IBS with constipation and IBS with diarrhea. There are many ways in which pharmacists can help optimize care and improve outcomes in patients with IBS.
Irritable bowel syndrome (IBS) is a common, chronic, and often debilitating functional gastrointestinal (GI) disorder that occurs secondary to alterations in gut-brain interaction.1 IBS presents in distinct subtypes: IBS with constipation (IBS-C), IBS with diarrhea (IBS-D), IBS with mixed stool patterns (IBS-M), and IBS without abnormal stool patterns (i.e., unsubtyped; IBS-U).2 The global prevalence of IBS among adults is estimated to be 4.1% to 10%, with IBS-C accounting for more than one-third of cases.3 IBS is more common in women than in men, and it is more often diagnosed in persons aged 15 to 45 years.4 Although IBS is not life-threatening, the morbidity IBS patients experience can be severe, with worse reported health-related quality of life (QOL) than in patients with end-stage renal disease or diabetes.5 Approximately 40% to 60% of patients with IBS have a comorbid psychological disorder (e.g., depression or anxiety) or comorbid insomnia.6,7 IBS negatively impacts daily functioning, including increased absenteeism and inability to participate in social gatherings.8 IBS places a significant economic burden on the healthcare system, with high resource utilization resulting in direct medical costs of approximately $1.5 billion to $10 billion per year.9
PATHOPHYSIOLOGY
IBS is classified as a functional gut disorder with symptoms not otherwise explained by anatomical or biochemical abnormalities detectable by routine diagnostic testing.1,2 The precise mechanism underlying its pathogenesis is not fully understood, but IBS is proposed to be a multifactorial disorder of the gut-brain axis. The gut-brain axis is described as bidirectional communication between the enteric nervous system (ENS) and the central nervous system (CNS) through neurohormonal, immune, and neuroendocrine pathways. As with other functional disorders, psychosocial factors are believed to elicit abnormal sensorimotor responses of the GI tract that lead to dysmotility, visceral hypersensitivity, and abnormal secretory-function responses.1,10
Serotonin (5-HT) is well known for its role within the CNS for mood regulation; it also resides within the enterochromaffin cells of the GI tract, and in the ENS it is an integral neurotransmitter that mediates GI motility, secretion, and pain sensation.11 Serotonin dysregulation is thought to be a crucial mediator of IBS symptoms, with increased plasma concentrations seen in IBS-D and reduced concentrations seen in IBS-C.12 Stress has also been shown to be a critical mediator of IBS symptoms, highlighting the potential role of the autonomic nervous system (ANS) and the hypothalamic-pituitary-adrenal axis.1 In addition, alterations in gut microbiota, intestinal inflammation, genetic predisposition, food allergens, carbohydrate insensitivity, and environmental factors have been identified as key contributors to IBS development.10
CLINICAL PRESENTATION AND DIAGNOSIS
The predominant clinical features of IBS are a consequence of visceral hypersensitivity (abdominal discomfort or pain) and motility disturbances (diarrhea or constipation). Diagnosis of IBS is based on the presence of GI symptoms in the absence of structural or biochemical causes.2 In patients presenting with features of IBS, a careful clinical history should be taken and a physical examination focused on characterizing symptoms of abdominal pain, altered bowel habits, and duration of symptoms should be performed. Special attention should be paid to the presence of any potential alarming signs or symptoms—such as hematochezia, melena, severe pain, loss of appetite, or significant weight loss—that could indicate an alternative disease pathology.13 In IBS, changes in stool frequency and consistency are often chronic and recurring; therefore, its diagnosis requires the presence of symptoms for >6 months.14
Although in most cases the primary complaint is recurrent abdominal pain, the identification of stool patterns is critical for determining the correct IBS subtype and performing the appropriate diagnostic workup. Stool consistency should be characterized based on the Bristol Stool Form Scale (FIGURE 1), after which the IBS subtype should be classified according to the Rome IV criteria (TABLE 1).2,14 Given that IBS symptoms can mimic other GI disorders, serologic testing should be performed to rule out conditions such as celiac disease, inflammatory bowel disease, or enteric infections.14
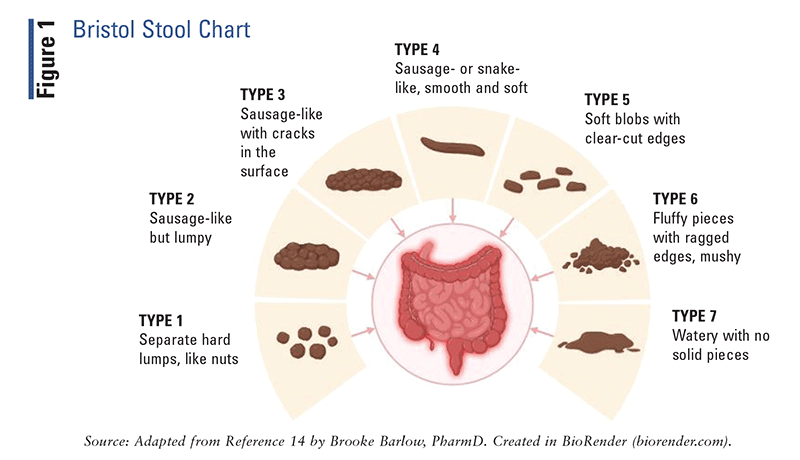 |
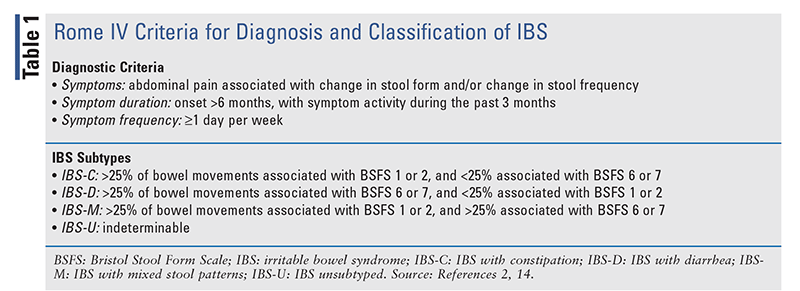 |
TREATMENT GOALS
The goal of IBS treatment is to provide symptom relief via a combination of nonpharmacologic and pharmacologic measures. Because each therapeutic option addresses a specific IBS subtype, using the wrong therapy for the particular subtype could result in worsened symptoms.14 This underscores the importance of accurately identifying the IBS subtype prior to treatment. The varied disease presentation and multiple underlying mechanisms of IBS have made it challenging to develop curative therapies, and treatment largely remains focused on improving QOL and lessening symptom frequency and severity.
NONPHARMACOLOGIC TREATMENT
Dietary modifications, stress relief, routine exercise, and mind-body therapies have been demonstrated to provide significant symptom relief in IBS, regardless of the subtype. The elimination of dietary fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAP) has gained popularity for the management of IBS.15 FODMAPrich foods contain short-chain carbohydrates that are poorly absorbed by the intestine. These sugars undergo fermentation in the colon, leading to increased gas production and luminal distention, which trigger the bloating, flatulence, and abdominal discomfort associated with IBS. Several studies have shown that a low-FODMAP diet can alleviate meal-triggered IBS symptoms within 2 to 6 weeks of diet adherence without negatively impacting nutritional intake.16,17 Examples of low-FODMAP foods include cantaloupe, kiwi, grapes, and strawberries; eggplant, green beans, cucumber, and zucchini; dairy-free alternatives (almond milk, oat milk); unprocessed meats, eggs, and tofu; and wheat-free grains (oats, rice, sourdough bread).15,16 Fiber-containing foods and fiber-based supplements increase stool bulk and facilitate colonic transit, resulting in increased stool frequency. Dietary fiber may be helpful for IBS-C, and the consumption of foods containing soluble fiber (e.g., oat bran, barley, beans) and avoidance of insoluble fiber may also be beneficial in treating global IBS symptoms.14
Psychological interventions such as cognitive-behavioral therapy, hypnosis, and mindfulnessbased therapies may improve IBS-related symptoms, especially in patients who have comorbid psychiatric disorders.18 Routine physical activity ameliorates IBS-related symptoms; reduces fatigue, depression, and anxiety; and improves overall QOL.19 Yoga, acupuncture, and other relaxation techniques help reduce inappropriate activation of the ANS and have been demonstrated to lessen the IBS symptom burden.20 Patients should be educated about the importance of dietary and lifestyle modifications and encouraged to keep a daily food, exercise, and stress-level diary in order to help identify trends in symptom exacerbations
PHARMACOLOGIC TREATMENT
Historically, the treatment of IBS has taken a multimodal approach involving the use of agents that regulate bowel function (i.e., laxatives or antidiarrheals) in conjunction with drugs that relieve symptomatic pain of visceral hypersensitivity (i.e., antispasmodics, antidepressants). However, recent advances have expanded the therapeutic landscape for IBS to medications that target global symptom improvement. In 2022, the American Gastroenterology Association (AGA) updated its clinical practice guidelines on the pharmacologic treatment of IBS-C and IBS-D to incorporate some novel agents for management.21,22 To date, no approved therapies are available for IBS-M or IBS-U; therefore, the following discussion will be limited to guideline-based pharmacologic recommendations for IBS-C and IBS-D. A full listing of pharmacologic therapies used for IBS-C and IBS-D is provided in TABLE 2 and TABLE 3, respectively.
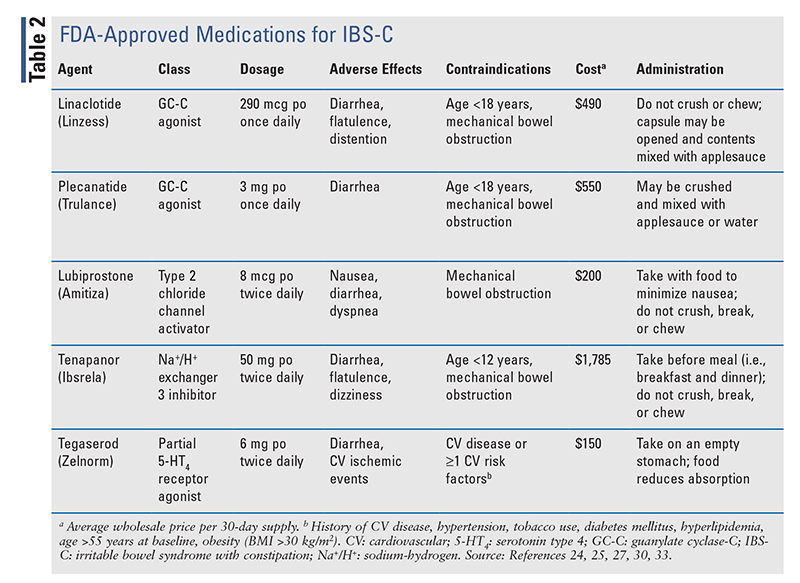 |
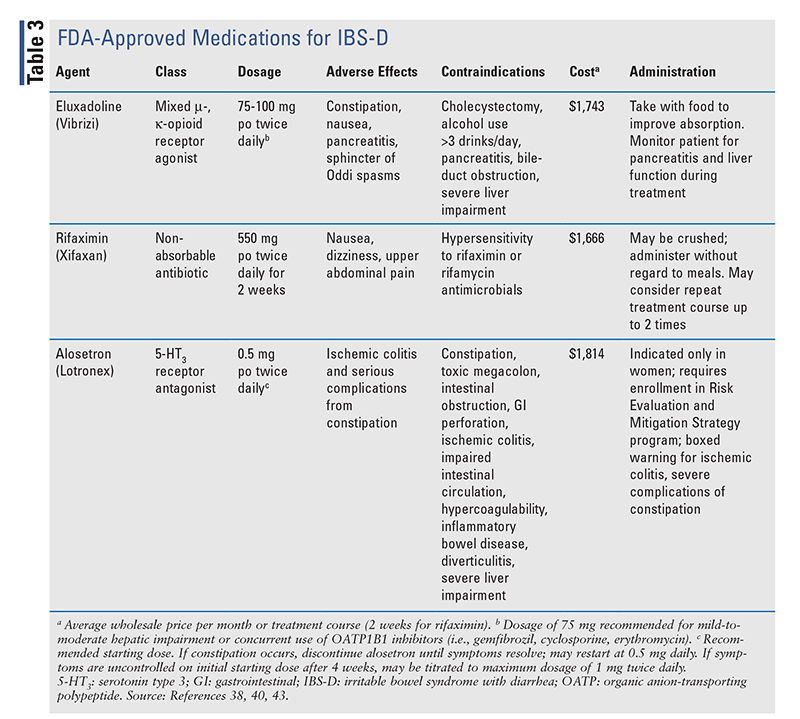 |
IBS WITH CONSTIPATION
To date, five secretagogues have been FDA approved for IBS-C: linaclotide, plecanatide, lubiprostone, tegaserod, and tenapanor. The AGA guideline strongly recommends the use of linaclotide and conditionally recommends the use of plecanatide, tegaserod, lubiprostone, and tenapanor.21 Adjunctive symptomatic therapy with antidepressants, laxatives, and antispasmodics may also be considered, although the evidence is of low certainty. The guideline emphasizes the importance of tailoring treatment to patient-specific factors such as cost, adherence, and potential for side effects.
Linaclotide and Plecanatide: Linaclotide is a nonabsorbable 14-amino acid peptide that exhibits a dual mechanism of action. Stimulation of the guanylate cyclase C (GC-C) receptor on enterocytes initiates a cascade of intracellular events to draw fluid into the intestinal lumen, eliciting a laxative effect while also modulating colonic nociceptors to promote analgesia.1 Plecanatide is a synthetic analogue of uroguanylin, a 16-amino acid endogenous peptide with a mechanism similar to that of linaclotide. Compared with linaclotide, plecanatide has better pH stability, which confers higher affinity to the GC-C receptor in more acidic environments of the proximal duodenum.23 Both linaclotide and plencanatide are FDA approved for treatment of IBS-C and chronic idiopathic constipation.24,25 Linaclotide is recommended for IBS-C (strong recommendation, high quality of evidence) based on its proven ability to improve global IBS symptoms.21 In trials, plecanatide demonstrates similar efficacy in IBS-C, but no direct comparative trials of linaclotide and plecanatide have been performed.21,26 Diarrhea is the most common adverse effect of both agents, occurring in up to 16% of linaclotide-treated patients and 4% of plecanatide-treated patients in clinical trials.21 It has been proposed that the more selective effects of plecanatide in the proximal small bowel compared with the entire GI tract with linaclotide account for this difference.23 However, a recent meta-analysis that accounted for baseline differences in IBS symptoms found no difference in efficacy or rates of diarrhea between the agents.26
Lubiprostone: This chloride channel type 2 activator increases chloride influx into the gut lumen, resulting in accelerated intestinal transit.1,21 Lubiprostone is FDA approved for use in women with IBS-C.27 In a large meta-analysis of randomized, controlled trials, lubiprostone was found to be more effective than placebo in resolution of global symptoms including abdominal pain and discomfort, but this response may be delayed for up to 2 to 3 months.28,29 Notably, lubiprostone was not superior to placebo in achieving adequate spontaneous bowel movements.28 Nausea (19%) and diarrhea (16%) are the predominant adverse effects of lubiprostone, although discontinuation rates in clinical trials were comparable to rates for placebo.28 Nausea appears to be dose-dependent and may be mitigated by administration with meals.27 Dyspnea has been reported, with an acute onset of 30 to 60 minutes after administration, but it generally resolves within 3 hours.27
Tegaserod: Tegaserod is a partial agonist of the 5-HT4 receptor that stimulates gastric motility and increases fluid in the GI tract.30 This agent initially received FDA approval in 2002, but it was later withdrawn from the market based on reports of increased ischemic cardiovascular events (0.11%) versus placebo (0.01%).31 However, large epidemiologic analyses failed to confirm this association, and the drug was reapproved in 2019 with modified labeling.32 Tegaserod is approved for IBS-C in women aged <65 years without a history of myocardial infarction, stroke, transient ischemic attack, or angina, and the treatment course should be limited to a trial of 4 to 6 weeks.30 In terms of efficacy, tegaserod improves global IBS symptoms but shows no improvement in overall QOL. Given the potential safety concerns and minimal impact on patient-centered outcomes, tegaserod may be reserved for use after failure of alternative secretagogues.14,21
Tenapanor: The newest FDA-approved drug for IBS-C is a first-in-class small-molecule inhibitor of the sodium-hydrogen (Na+/H+) exchanger isoform 3.33 Na+/H+ exchanger is present on the apical surface of the small intestine and is responsible for sodium reabsorption. Inhibition by tenapanor decreases sodium reabsorption and increases water secretion into the intestinal lumen, resulting in a laxative effect.34 Tenapanor has also been shown to reduce visceral hypersensitivity through its antinociceptive effects.35 In the two phase III trials that led to FDA approval, a significantly greater proportion of patients receiving tenapanor reported symptom relief at 12 weeks compared with placebo patients, with the greatest benefit seen in reduction of abdominal pain (relative risk, 0.81; CI, 0.73-0.88).36,37 Diarrhea was the most common adverse effect with tenapanor use (14.8% of patients), leading to discontinuation in 6.6% of recipients versus 1% of the placebo group.37 Although it has not been compared with other IBS-C treatments, tenapanor appears to cause modest improvements in IBS-C symptoms, with diarrhea limiting its use in some patients. Tenapanor is a substrate of organic anion-transporting polypeptide 2B1, and the potential exists for reduced exposure and efficacy of the concomitant substrates (e.g., enalapril).33
IBS With Diarrhea
Eluxadoline: This agent is a mixed mu- and kappa-opioid receptor agonist that reduces gastric peristalsis and delays transit time.38 Its limited systemic absorption restricts its effects selectively to the GI tract. FDA approval of eluxadoline for IBS-D was based on two large randomized, controlled trials in patients who failed loperamide treatment that demonstrated improved symptom relief, most notably abdominal pain and stool frequency, within weeks of eluxadoline initiation.39 Symptom improvement with eluxadoline use was correlated with an increase in patient-reported QOL compared with placebo. Despite the advantages, safety concerns have limited the routine use of this agent. Increased rates of pancreatitis and sphincter of Oddi spasms were observed in multiple studies, most notably in patients with a history of cholecystectomy.22,38 Given these risks, eluxadoline is contraindicated in patients with a history of pancreatitis, cholecystectomy, biliary-duct obstruction, excessive alcohol intake (>3 drinks/day), or severe hepatic impairment.38
Rifaximin: Rifaximin is a nonabsorbable oral antibiotic whose mechanism for IBS-D remains incompletely understood, but its benefit is thought to derive from modulation of the gut microbiome and reduction of intestinal inflammation.1,22 This agent is FDA approved for IBS-D as a short-term course (14 days) that may be repeated up to two times.40 In the trials that led to rifaximin's approval, a 2-week treatment course was shown to improve IBS-D-related symptoms with a durable response of up to 18 weeks.41 Repeat treatment was effective compared with placebo, and the risk of inducing antimicrobial resistance was minimal.42 Adverse events and discontinuation were comparable between rifaximin and placebo.22
Alosetron: This selective 5-HT3 antagonist modulates central and peripheral neurotransmission to block visceral pain sensations and reduce gastric motility.43 Alosetron was originally FDA approved in 2000; however, it was voluntarily withdrawn from the market based on reports of ischemic colitis and serious constipation requiring hospitalization or surgery.22 The drug was reintroduced in 2002 with a black box warning for ischemic colitis, and its use is limited to women with severe IBS-D after failure of conventional therapies.43 In order to mitigate the risk of adverse effects, enrollment in the alosetron Risk Evaluation and Mitigation Strategy program is required to ensure appropriate screening for contraindications, monitoring, and dose titration. Alosetron is contraindicated in patients with constipation, gastric perforation, stricture, toxic megacolon, impaired intestinal circulation, hypercoagulable states, inflammatory bowel disease, diverticulitis, severe hepatic impairment, or concurrent use of fluvoxamine.43
Adjunctive Therapies
Polyethylene Glycol (PEG): This long-chain polymer of ethylene oxide acts as an osmotic laxative. PEG, which is widely used to treat constipation, is available OTC. PEG is beneficial for relieving constipation of IBS-C, but it should not be used when pain is the predominant symptom, as it provides minimal benefit.44
Antidepressants: Antidepressants perform peripheral and central actions on neurotransmission to block pain-signal transmission, thereby reducing visceral hypersensitivity. These agents also help regulate bowel function by modulating 5-HT in the ENS.1 Tricyclic antidepressants (TCAs) and selective serotonin reuptake inhibitors (SSRIs) are the classes most widely studied for IBS management.45 TCAs modulate multiple neurotransmitters with activity including inhibition of 5-HT and noradrenergic reuptake and blockade of muscarinic, adrenergic, and histaminic receptors.1,21,22 Neuromodulation provides beneficial analgesic effects, but the anticholinergic properties of selected TCAs can worsen IBS-C. For patients who have predominant constipation, secondary amine TCAs such as nortriptyline or desipramine may be better tolerated than tertiary amines such as amitriptyline. Despite their widespread use, SSRIs are not recommended for IBS treatment given the inconsistent evidence regarding improvements in the global symptoms or abdominal pain of IBS; however, these agents may be beneficial for patients who have coexistent mood disorders.21,22
Antispasmodics: Antispasmodics (e.g., dicyclomine, hyoscyamine, peppermint oil) are thought to relieve IBS symptoms by reducing smooth-muscle contraction and possibly visceral hypersensitivity, especially when exacerbated by meals.14,21,22 These often-used agents have not been subjected to rigorous trials, but they have been found to improve abdominal pain compared with placebo in both IBS-C and IBS-D. Because these agents possess anticholinergic properties, caution is advised with their use in IBS-C given their potential to worsen constipation.
Loperamide: This synthetic selective opioid agonist reduces gut peristalsis and increases gut-transit time. Although it is an efficacious antidiarrheal agent, its effects on symptomatic improvement of IBS-D remain limited.22 Loperamide is readily available as an OTC agent, and it may be considered as an adjunct to alternative treatments in the setting of diarrhea-predominant features. Caution is advised in patients with prolonged QTc or cardiac risk factors for arrythmias because of the risk of torsades de pointes, especially at high doses.
THE PHARMACIST'S ROLE
Pharmacists can play an integral role in the management of IBS. Educating patients about the importance of lifestyle and dietary modifications is vital for reducing the symptom burden and, in some mild cases, mitigating the need for pharmacologic treatment. Pharmacists can encourage patients to maintain a food, activity, and symptom diary to help identify potential food-related allergens or other triggers of IBS symptoms. A thorough review of prescription and OTC therapies should be performed to identify any medications that could exacerbate constipation (i.e., anticholinergics, opioids) or diarrhea (i.e., magnesium supplements, metformin). Patients who are prescribed pharmacologic agents for IBS should be counseled on how to monitor for drugrelated adverse effects and should be advised to speak with their prescriber if extreme changes in bowel movements occur (e.g., severe constipation with IBS-D therapies or severe diarrhea with IBS-C therapies).
Ensuring patient adherence to treatment is critical for improving IBS symptoms and QOL, and pharmacists can assist in tailoring individual treatment based on cost, concerns about adherence, and treatment-related adverse effects. For example, for a patient with IBS-C in whom medication adherence is a concern, selection of a once-daily agent such as linaclotide or plecanatide may improve adherence compared with the alternative twice-daily secretagogues. Pharmacists can screen patients for contraindications or drug interactions related to IBS therapy (e.g., pancreatitis with eluxadoline, fluvoxamine, and alosetron). For patients who are prescribed alosetron, pharmacists are key educators regarding the risks of treatment and facilitators of appropriate monitoring and follow-up. Overall, pharmacists serve as essential healthcare providers for optimizing care and improving outcomes in patients with IBS.
REFERENCES
1. Enck P, Aziz Q, Barbara G, et al. Irritable bowel syndrome. NatRev Dis Primers. 2016;2:16014.
2. Lacy BE, Patel NK. Rome criteria and a diagnostic approach toirritable bowel syndrome. J Clin Med. 2017;6(11):99.
3. Sperber AD, Bangdiwala SI, Drossman DA, et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results ofRome Foundation global study. Gastroenterology. 2021;160(1):99-
114.e3.
4. Wilson S, Roberts L, Roalfe A, et al. Prevalence of irritable bowelsyndrome: a community survey. Br J Gen Pract. 2004;54(504):495-
502.
5. Singh P, Staller K, Barshop K, et al. Patients with irritable bowel syndrome-diarrhea have lower disease-specific quality of life than irritable bowel syndrome-constipation. World J Gastroenterol. 2015;21(26):8103-8109.
6. Hu Z, Li M, Yao L, et al. The level and prevalence of depression and anxiety among patients with different subtypes of irritable bowelsyndrome: a network meta-analysis. BMC Gastroenterol.2021;21(1):23.
7. Wang B, Duan R, Duan L. Prevalence of sleep disorder in irritable bowel syndrome: a systematic review with meta-analysis. Saudi J Gastroenterol. 2018;24(3):141-150.
8. Hungin APS, Whorwell PJ, Tack J, Mearin F. The prevalence, patterns and impact of irritable bowel syndrome: an international surveyof 40,000 subjects. Aliment Pharmacol Ther. 2003;17(5):643-650.
9. Canavan C, West J, Card T. Review article: the economic impact ofthe irritable bowel syndrome. Aliment Pharmacol Ther.
2014;40(9):1023-1034.
10. Saha L. Irritable bowel syndrome: pathogenesis, diagnosis, treatment, and evidence-based medicine. World J Gastroenterol. 2014;20(22):6759-6773.
11. Talley NJ. Serotoninergic neuroenteric modulators. Lancet. 2001;358(9298):2061-2068.
12. Dunlop SP, Coleman NS, Blackshaw E, et al. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. ClinGastroenterol Hepatol. 2005;3(4):349-357.
13. Brandler J, Chey WD. Fishing for irritable bowel syndrome: which alarm features weave the best net? Clin Gastroenterol Hepatol.2022;20(2):276-278.
14. Lacy BE, Pimentel M, Brenner DM, et al. ACG clinical guideline:management of irritable bowel syndrome. Am J Gastroenterol.
2021;116(1):17-44.
15. van Lanen AS, de Bree A, Greyling A. Efficacy of a low-FODMAP diet in adult irritable bowel syndrome: a systematic review and metaanalysis. Eur J Nutr. 2021;60(6):3505-3522.
16. Staudacher HM, Ralph FSE, Irving PM, et al. Nutrient intake, diet quality, and diet diversity in irritable bowel syndrome and theimpact of the low FODMAP diet. J Acad Nutr Diet. 2020;120(4):535-547.
17. Eswaran S, Dolan RD, Ball SC, et al. The impact of a 4-weeklow-FODMAP and mNICE diet on nutrient intake in a sample of US adults with irritable bowel syndrome with diarrhea. J Acad Nutr Diet.2020;120(4):641-649.
18. Ballou S, Keefer L. Psychological interventions for irritable bowel syndrome and inflammatory bowel diseases. Clin Transl Gastroenterol. 2017;8(1):e214.
19. Johannesson E, Simrén M, Strid H, et al. Physical activity improves symptoms in irritable bowel syndrome: a randomized controlled trial. Am J Gastroenterol. 2011;106(5):915-922.
20. Kavuri V, Raghuram N, Malamud A, Selvan SR. Irritable bowel syndrome: yoga as remedial therapy. Evid Based Complement Alternat Med. 2015;2015:398156.
21. Chang L, Sultan S, Lembo A, et al. AGA clinical practice guideline on the pharmacological management of irritable bowel syndromewith constipation. Gastroenterology. 2022;163(1):118-136.
22. Lembo A, Sultan S, Chang L, et al. AGA clinical practice guideline on the pharmacological management of irritable bowel syndrome with diarrhea. Gastroenterology. 2022;163(1):137-151.
23. Love BL. Plecanatide for treatment of chronic constipation and irritable bowel syndrome. Am J Med. 2019;132(5):572-575.
24. Trulance (plecanatide) product information. Bridgewater, NJ: Salix Pharmaceuticals; April 2021.
25. Linzess (linaclotide) product information. Boston, MA: Ironwood Pharmaceuticals, Inc; August 2021.
26. Shah ED, Kim HM, Schoenfeld P. Efficacy and tolerability of guanylate cyclase-C agonists for irritable bowel syndrome with constipation and chronic idiopathic constipation: a systematic review and meta-analysis. Am J Gastroenterol. 2018;113(3):329-338.
27. Amitiza (lubiprostone) product information. Deerfield, IL: Takeda Pharmaceuticals America, Inc; November 2012.
28. Passos MDCF, Takemoto MLS, Corradino GC, Guedes LS. Systematic review with meta-analysis: lubiprostone efficacy on the treatment of patients with constipation. Arq Gastroenterol. 2020;57(4):498-506.
29. Drossman DA, Chey WD, Johanson JF, et al. Clinical trial: lubiprostone in patients with constipation-associated irritable bowel syndrome—results of two randomized, placebo-controlled studies. Aliment Pharmacol Ther. 2009;29(3):329-341.
30. Zelnorm (tegaserod) product information. Covington, LA: Alfa-sigma USA, Inc; July 2019.
31. Tack J, Camilleri M, Chang L, et al. Systematic review: cardiovascular safety profile of 5-HT4 agonists developed for gastrointestinal disorders. Aliment Pharmacol Ther. 2012;35(7):745-767.
32. Lacy BE, Brenner DM, Chey WD. Re-evaluation of the cardiovascular safety profile of tegaserod: a review of the clinical data. Clin Gastroenterol Hepatol. 2022;20(4):e682-e695.
33. Ibsrela (tenapanor) product information. Waltham, MA: Ardelyx, Inc; April 2022.
34. Spencer AG, Labonte ED, Rosenbaum DP, et al. Intestinal inhibition of the Na+/H+ exchanger 3 prevents cardiorenal damage in rats and inhibits Na+ uptake in humans. Sci Transl Med. 2014;6(227):227ra36.
35. Eutamene H, Charmot D, Navre M, Bueno L. Visceral antinociceptive effects of RDX5791, a first-in-class minimally systemic NHE3 inhibitor on stress-induced colorectal hypersensitivity to distension inrats. Gastroenterology. 2011;140(5 suppl 1):S57-S58.
36. Chey WD, Lembo AJ, Yang Y, Rosenbaum DP. Efficacy of tenapanor in treating patients with irritable bowel syndrome with constipation: a 26-week, placebo-controlled phase 3 trial (T3MPO-2). Am J Gastroenterol. 2021;116(6):1294-1303.
37. Chey WD, Lembo AJ, Rosenbaum DP. Efficacy of tenapanor in treating patients with irritable bowel syndrome with constipation: a12-week, placebo-controlled phase 3 trial (T3MPO-1). Am J Gastroenterol. 2020;115(2):281-293.
38. Viberzi (eluxadoline) product information. Madison, NJ: Allergan USA, Inc; June 2020.
39. Lacy BE, Chey WD, Cash BD, et al. Eluxadoline efficacy in IBS-D patients who report prior loperamide use. Am J Gastroenterol.2017;112(6):924-932.
40. Xifaxan (rifaximin) product information. Bridgewater, NJ: Salix Pharmaceuticals; October 2020.
41. Pimentel M, Lembo A, Chey WD, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl JMed. 2011;364(1):22-32.
42. DuPont HL, Wolf RA, Israel RJ, Pimentel M. Antimicrobial susceptibility of Staphylococcus isolates from the skin of patients with diarrhea-predominant irritable bowel syndrome treated with repeat courses of rifaximin. Antimicrob Agents Chemother.2016;61(1):e02165-16.
43. Lotronex (alosetron) product information. Roswell, GA: Sebela Pharmaceuticals, Inc; April 2019.
44. Chapman RW, Stanghellini V, Geraint M, Halphen M. Randomized clinical trial: macrogol/PEG 3350 plus electrolytes for treatment of patients with constipation associated with irritable bowel syndrome. Am J Gastroenterol. 2013;108(9):1508-1515.
45. Grover M, Drossman DA. Psychotropic agents in functional gastrointestinal disorders. Current Opin Pharmacol. 2008;8(6):715-723.