Updated Guidance for Opioid Use in Pain Management
RELEASE DATE
March 1, 2024
EXPIRATION DATE
March 31, 2026
FACULTY
Jay P. Acharya, PharmD Candidate 2025
Jonathan Kao, PharmD Candidate 2024
Olga Hilas, PharmD, MPH, BCPS, BCGP, FASCP
Professor, Department of Pharmacy Practice
St. John’s University College of
Pharmacy and Health Sciences
Queens, New York
FACULTY DISCLOSURE STATEMENTS
Jay P. Acharya, Jonathan Kao, and Dr. Hilas have no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT
 Pharmacy
Pharmacy
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-24-029-H08-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients’ conditions and possible contraindications or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
GOAL
To provide pharmacists with updated knowledge to apply the latest CDC Clinical Practice Guideline for Prescribing Opioids in outpatient adults with acute, subacute, and chronic pain.
OBJECTIVES
After completing this activity, the participant should be able to:
- Explain key differences between the 2016 and 2022 CDC guidelines for prescribing opioids for pain.
- Develop individualized patient care plans for the treatment of pain based on the updated 2022 CDC Clinical Practice Guideline.
- Recognize the need for flexible patient-centered decision-making in the selection of treatment modalities for pain.
- Describe the value of collaboration among healthcare professionals to ensure a comprehensive approach to pain management.
ABSTRACT: The prevalence of pain in the United States remains a significant healthcare concern, affecting millions of people and impacting quality of life. Despite this widespread issue, inadequate pain management persists, and collaborative efforts among clinicians and patients are needed for personalized, multimodal approaches that are safe and effective. The use of medications is necessary in many cases; however, the opioid epidemic has posed a critical challenge as the number of related overdose deaths continues to rise along with hesitation to prescribe, dispense, or adhere to opioid regimens. The CDC has published two guidance documents in recent years to address these issues by encouraging the development of holistic patient care plans that include thorough patient assessment and follow-up and careful medication selection and risk evaluation.
Pain, defined by the International Association for the Study of Pain, is “an unpleasant sensory and emotional experience that is associated with actual or potential tissue damage.”1 It is one of the most common reasons adults seek medical care in the United States and is highly subjective in nature due to distressing symptoms, which result in poor quality of life (e.g., lack of or inadequate sleep, low mood, decreased appetite, change in behavior).2 The types of pain that individuals experience are commonly classified by the duration of pain: 1) acute pain refers to pain lasting less than 1 month, 2) subacute pain that lasts 1 to 3 months, and 3) chronic pain that lasts longer than 3 months.3 An estimated 50 million adults in the U.S. experienced chronic pain in 2016, resulting in substantial healthcare costs and lost productivity.4 In 2021, an estimated 20.9% of U.S. adults (nearly 52 million people) were reported to have chronic pain, which was similar to the 2016 reported estimate of 20.4%.4,5
Despite the millions of people affected by pain, many remain inadequately treated by the healthcare system.6 In 2019, the U.S. Department of Health and Human Services Pain Management Best Practices Inter-Agency Task Force published a report highlighting the importance of establishing an alliance between patients and clinicians and providing individualized, patient-centered care in the diagnosis and treatment of pain.7 This report also focused on the need for collaborative efforts among clinicians in the development of effective pain treatment plans aimed to improve their patients’ quality of life, functionality, and activities of daily living using multimodal approaches. These may include restorative therapies, behavioral interventions, complementary and integrative health practices, and medications.7
With regard to the use of medications in pain management, clinicians should select agents based on an individual’s diagnosis, the mechanisms of pain, and related comorbidities. Additionally, the risks and benefits of the medication classes being considered should be carefully assessed to ensure that a safe and effective approach is taken.7,8 In 2016, the CDC published a guideline to assist in the decision-making for treatment of pain specifically with opioids, given the opioid epidemic in the U.S.5 Since 1999, more than 1 million individuals have died from a drug overdose in the U.S., and more than 70% of all drug overdose deaths over the past 5 years have involved opioids.9,10 In 2022, 109,680 overdose deaths were reported, which was an increase of 0.5% from the previous year and was likely driven by the continuous influx of synthetic nonpharmaceutical fentanyl, challenges posed by the COVID-19 pandemic, and increased combination-drug use.11,12 To provide additional guidance for the safer and appropriate use of opioids in pain management, the CDC updated its recommendations in 2022 (see TABLE 1).13

2016 CDC GUIDELINE FOR PRESCRIBING OPIOIDS FOR CHRONIC PAIN
The CDC released a national guideline in 2016 with recommendations for prescribing opioids in the management of chronic pain by primary care clinicians in outpatient settings (excluding patients with active cancer treatment, palliative care, and end-of-life care).5 The aim of this guidance was to improve appropriate opioid prescribing while minimizing opioid-related risks. However, certain recommendations within the 2016 CDC Guideline for Prescribing Opioids for Chronic Pain were misinterpreted, and an “anti-opioid” interpretation began to emerge, resulting in the reduction of opioid prescribing, dispensing, and overall use for chronic pain—even when clinically appropriate for patients.14 One example includes the implementation of some policies and practices that encouraged hard limits on quantities and dosages of opioid medications prescribed by providers, dispensed by pharmacists, and covered by insurance companies.14,15
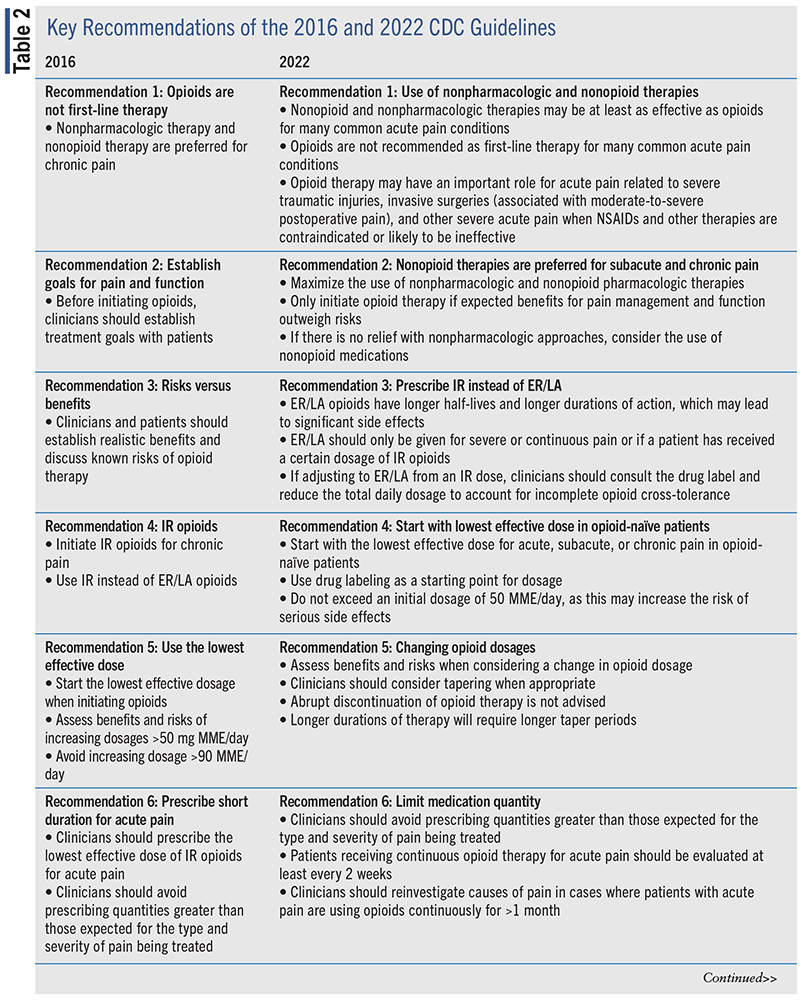
New evidence has emerged since 2016 on opioid use, including: 1) risks and benefits of use for acute and chronic pain; 2) dosing strategies; 3) dose-response relationships; 4) risk-mitigation strategies; 5) tapering and discontinuation; and 6) alternative and complementary nonopioid treatment modalities.13,14 This prompted the CDC to compose the 2022 CDC Clinical Practice Guideline for Prescribing Opioids for Pain.14 Released in November 2022, the guideline consists of five guiding principles (see TABLE 1) and 12 evidence-based recommendations (see TABLE 2) for clinicians who may prescribe opioid medications for acute, subacute, and chronic pain in outpatients aged 18 years or older (excluding persons with pain related to sickle cell disease, cancer-related pain, and those receiving palliative care or end-of-life care).13
2022 CLINICAL PRACTICE GUIDELINE FOR PRESCRIBING OPIOIDS FOR PAIN
The 2022 Clinical Practice Guideline for Prescribing Opioids for Pain is based on emerging evidence from observational studies or randomized clinical trials and is designed to be flexible for patient-centered decision-making and individualized care.12 Furthermore, this update aims to clarify recommendations that apply to patients who are being considered for initial treatment with prescription opioids and patients who have been receiving opioids chronically. It is important to note that this guidance should not replace clinical judgment of practitioners and an individualized, patient-centered care approach to the treatment of pain; nor is the guidance intended to set limits for policies or practices across populations by organizations, healthcare systems, or government entities as was seen after the misinterpretation and misapplication of the 2016 CDC Guideline for Prescribing Opioids for Chronic Pain.5
The 12 recommendations in the updated 2022 guideline document are grouped into four areas: 1) determining whether or not to initiate opioids for pain; 2) selecting opioids and determining dosages; 3) deciding the duration of initial opioid prescription and conducting follow-up; and 4) assessing risk and addressing potential harms of opioid use.6 A summary of each of the categories and their respective recommendations are provided on the following pages.
Opioid Initiation
The first and second recommendations fall under the category of determining whether to initiate opioids for pain. The first recommendation highlights that nonopioid therapies are at least as effective as opioids for various types of acute pain (e.g., low back pain, neck pain, pain related to other musculoskeletal injuries involving sprains, strains, tendonitis, pain related to minor surgeries with minimal tissue injury, and mild postoperative pain such as dental pain, kidney stone pain, and migraines). Clinicians are advised to prioritize the use of nonpharmacologic interventions (e.g., ice, heat, elevation, immobilization, exercise, massage, or acupuncture) and nonopioid pharmacologic therapies (e.g., oral nonsteroidal anti-inflammatory drugs [NSAIDs] or acetaminophen) for specific conditions and to only consider the initiation of opioid therapy for acute pain if benefits outweigh risks. However, nonpharmacologic therapies may not always be accessible or affordable, as many are not covered by all insurances.
Topical NSAIDs (with or without menthol) were noted to have the highest benefit for musculoskeletal injuries other than low back pain, followed by oral NSAIDs or acetaminophen (with or without topical diclofenac). NSAIDs or skeletal muscle relaxants are preferred and recommended for low back pain. For acute dental pain management, NSAIDs are recommended as first-line treatment, and they are at least as effective as opioids for acute kidney stone pain. Lastly, NSAIDs, and combined triptans with NSAID therapy, antiemetics, dihydroergotamine, and acetaminophen are recommended as established treatments for acute migraines.
Opioids are also not recommended as first-line therapy for many common acute pain conditions such as low back pain, neck pain, musculoskeletal injuries, minor surgeries with minimal tissue injury, dental pain, kidney stone pain, and episodic migraine due to equivalent or lesser effectiveness for pain relief compared with NSAIDs and risk of long-term opioid use. However, opioid therapy may be appropriate for severe traumatic injuries, invasive surgeries associated with moderate-to-severe postoperative pain, and other severe acute pain when NSAIDs or alternative therapies are ineffective or contraindicated. If starting opioid therapy, pharmacists should ensure patient involvement in the treatment plan and a clear understanding of the benefits, risks, and other potential therapies to opioids (if applicable) before initiating or continuing opioid therapy. Patient education about the benefits and known risks of opioid therapy is important, and this includes discussing the potential for side effects, the risks of developing opioid use disorder (OUD), and the importance of proper storage conditions and disposal of unused opioids.
The second recommendation discusses use of non-opioid therapies as the preferred approach for subacute and chronic pain. Clinicians are advised to optimize the use of nonpharmacologic and nonopioid pharmacologic treatments based on the specific condition and patient, and opioid therapy should only be initiated if the expected benefits in terms of pain relief and function outweigh the risks to the patient. Nonpharmacologic treatment options include exercises for some conditions (e.g., back pain, fibromyalgia, osteoarthritis), weight loss for knee osteoarthritis, psychological therapy, yoga, acupuncture, and multidisciplinary rehabilitation. Low-cost exercise options like walking or utilizing public recreation facilities can be helpful for patients who have limited access to other approaches.
Topical NSAIDs, duloxetine, or systemic NSAIDs can be considered in patients who do not experience relief with nonpharmacologic approaches if no contraindications exist. Various medications such as antidepressants and anticonvulsants can also be considered for neuropathic pain, with careful consideration in older adults due to potential adverse effects and risks. Specific medications such as duloxetine and pregabalin are FDA approved for diabetic peripheral neuropathy, while patients with fibromyalgia may benefit fromtricyclic or serotonin and norepinephrine reuptake inhibitor (SNRI) antidepressants, NSAIDs, and certain anticonvulsants.
In addition, opioids are not recommended as first-line or routine therapy for subacute or chronic pain; however, in certain clinical scenarios they may be appropriate. Before initiating opioid therapy for subacute or chronic pain, clinicians need to engage in discussions with patients regarding the benefits and risks associated with opioid therapy and collaborate with patients to set clear treatment goals for both pain management and improved functionality, along with creating a plan for discontinuation if the benefits do not outweigh associated risks.
Patients should always be well-informed about the potential alternatives to and expected benefits and risks of opioid therapy. There is insufficient evidence to establish the long-term benefits of opioid therapy for chronic pain, while increased risk for serious harms (likely dose-dependent) has been reported in some studies. Compared with patients receiving nonopioid therapy, those treated with opioid therapy face increased risks of OUD, overdose, all-cause mortality, fractures, falls, and myocardial infarction. Furthermore, opioids have been associated with nonadherence due to adverse gastrointestinal effects, somnolence, dizziness, and pruritus.
Selection and Dosage
The third, fourth, and fifth recommendations are categorized in the section of the guideline that discusses selection of opioids and determination of opioid dosages. The third recommendation advises that clinicians should prescribe immediate-release (IR) opioids (e.g., morphine, hydromorphone, oxymorphone, codeine, hydrocodone, and oxycodone) rather than extended-release (ER) and long-acting (LA) opioids when initiating opioid therapy for acute, subacute, or chronic pain. This is primarily due to ER/LA opioids having longer half-lives, longer durations of effects, and a higher risk for overdose compared with IR within the first 2 weeks of therapy. The FDA also notes that certain ER/LA opioids are only appropriate for opioid-tolerant patients.
ER/LA formulations are available for opioids, such as methadone, fentanyl, oxycodone, hydromorphone, hydrocodone, and morphine, and should be reserved only for patients who have severe and continuous pain or for patients who have received certain daily dosages of IR opioids (e.g., 60 mg of oral morphine, 30 mg of oral oxycodone, or equianalgesic dosages per day of other opioids) for at least 1 week.
The guideline also addresses certain considerations for specific opioid medications. For example, methadone is not preferred as an ER/LA opioid due to its potential to increase the risk for QT prolongation. ER/LA transdermal fentanyl is recommended to be prescribed only by clinicians who are familiar with its dosing and absorption properties along with comprehensive patient education regarding it proper use and administration. Caution should be taken when prescribing any opioid for patients with renal or hepatic dysfunction due to the possibility of reduced medication clearance and drug accumulation that may lead to prolonged effects and adverse events. Therefore, the risk for overdose should be discussed with all patients receiving IR and ER/LA opioids.
The fourth recommendation emphasizes the importance of prescribing opioids at the lowest effective dose upon initiation in opioid-naïve patients with acute, subacute, or chronic pain. Careful dosage increases are also important to mitigate the risk of misuse, overdose, and death. Pharmacists must be aware of these recommendations and ensure that product labeling is used for the dosage of opioids along with clinical factors and patient considerations. The lowest starting dose for opioid-naïve patients is approximately 5 to 10 morphine milligram equivalents (MME) or a daily dosage of 20 to 30 MME/day. Opioid dosages >50 MME/day should generally be avoided due to the potential for diminished benefits and increased risk for serious side effects. Any decision to increase opioid dosages should be carefully assessed based on individual needs, previous dosages/dosage increases, and benefit versus risk analyses.
The fifth recommendation discusses the importance of careful assessments of the benefits and risks of opioid use when considering dosage changes. Decisions should be based on benefits of continued opioid therapy outweighing the risks. In such cases, clinicians are encouraged to work with their patients to optimize nonopioid therapies while continuing opioid regimens. When benefits are not found to outweigh the risks of continued opioid therapy, clinicians should again work with their patients to optimize alternative therapies but discuss a plan to initiate a gradual taper to lower dosages for safe discontinuation of opioid treatment. Successful tapering plans usually require close collaborations between clinicians and patients with regular follow-up visits (at least once a month). Patients should be counseled to expect some worsening of pain that should diminish over time. Patients should also be warned about the increased risk of overdose with an abrupt return to a previously prescribed dosage of opioids after beginning a tapering plan because of dimished opioid tolerance.
Pharmacists can play a critical role in supporting clinicians and patients during this process via face-to-face or telehealth encounters. Tapering should be individualized based on a patient’s particular clinical situation and should be initiated slowly to minimize symptoms and signs of opioid withdrawal (e.g., anxiety, insomnia, abdominal pain, vomiting, diarrhea, diaphoresis, mydriasis, tremor, tachycardia, or piloerection). Rapid reductions of opioid dosages should be avoided, and clinicians are advised against abruptly discontinuing opioid therapy unless there are indications of life-threatening issues (e.g., warning signs of impending overdose such as confusion, sedation, or slurred speech). It is important to note that longer durations of opioid use may require a longer taper, especially in patients who have taken opioids for >1 year. These patients may require tapers of 10% per month, or slower, for greater tolerability.
Duration and Follow-Up
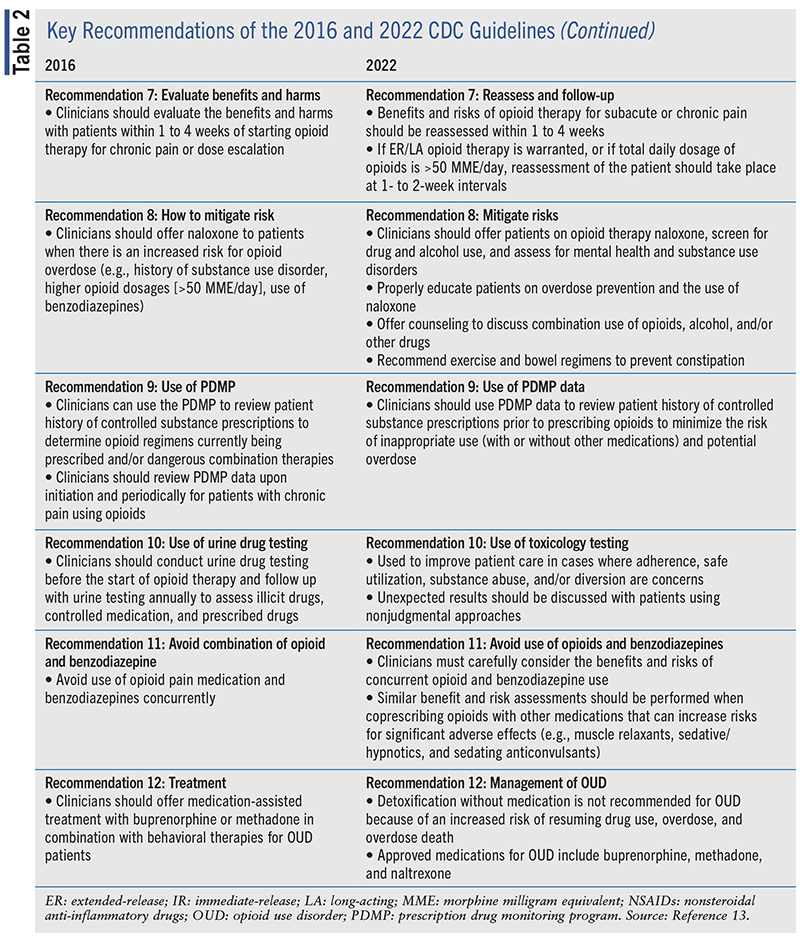
The sixth and seventh recommendations involve the duration of initial opioid therapy and follow-up assessments. The sixth recommendation states that when opioids are needed for acute pain, clinicians should prescribe no greater quantity than needed for the expected duration of pain severe enough to require opioids. Patients who experience nontraumatic or nonsurgical pain may be on opioid therapy for a few days or less, which is often sufficient and minimizes the need to taper opioids for the prevention of withdrawal symptoms. However, the duration of opioid therapy should be individualized based on personal clinical circumstances. Longer durations of opioid therapy (>7 days) may be required when the mechanism of injury is expected to result in prolonged severe pain (e.g., severe traumatic injuries). Patients should be evaluated at least every 2 weeks if opioids are continued for acute pain. Clinicians should reassess potential causes of pain in cases where opioids are needed and continued for >1 month to prevent the unintentional and inappropriate transition of opioid use from acute to chronic pain management.
The seventh recommendation highlights the importance of regularly evaluating the benefits and risks of starting opioid therapy for subacute or chronic pain or in cases of dosage escalation within 1 to 4 weeks. If ER/LA opioids are initiated or increased, a reassessment should be performed within 1 to 2 weeks due to the increased risk for overdose within the first 2 weeks of treatment or when the total daily dosage of opioids is >50 MME/day. If a patient is initiated on IR opioids at dosages ≤50 MME/day, an initial follow-up interval closer to 4 weeks may be considered by clinicians. Patients with subacute pain who are started on opioid therapy for 30 days should be reassessed to evaluate their pain, function, and treatment course to prevent the unintentional and inappropriate initiation of long-term opioid therapy. During each clinical reassessment, patient perspectives, preferences, and treatment goals should be discussed as well as the efficacy and safety of their current pain management regimen.
Risk and Potential Harms
The last five recommendations are placed under the category for assessing risk and addressing potential harms of opioid use. The eighth recommendation focuses on evaluating and discussing the risk for opioid-related harms with patients. Clinicians should work with patients to establish a strategy to mitigate risk such as offering naloxone and screening for alcohol and/or drug use, mental health disorders, and substance use disorders (SUDs; using validated tools and in collaboration with behavioral specialists). Joint decision-making between clinicians and patients should also include the establishment of overall health goals and the management of other comorbidities that may or may not be contributing to pain. Furthermore, clinicians should offer naloxone when prescribing opioids to all patients, particularly those at increased risk of overdose (e.g., patients with history of overdose, SUDs, sleep-disordered breathing, concurrent benzodiazepine use, higher dosage opioid regimens). Naloxone coprescribing can be facilitated through various clinics or practices through the development of collaborative practice models with pharmacists, statewide protocols, or standing orders at pharmacies across the nation. Educational efforts centered on opioid overdose prevention and naloxone use for patients and families/friends can help prevent overdose deaths.
The recommendation also cautions regarding the use of opioids in patients with hepatic or renal insufficiency and those who are aged 65 years and older, due to the potential for increased accumulation of the medications, increased risk for drug-drug interactions, and smaller therapeutic windows between safe dosages and dosages associated with adverse effects. In addition, exercise and bowel regimens to prevent constipation, risk assessment for falls, and patient monitoring for cognitive impairment are recommended, especially among older adults receiving opioid therapy. It is also important to counsel patients on the increased risk of overdose when opioids are combined with other drugs or alcohol. When considering opioid therapy for patients with SUDs, clinicians should discuss increased risks for overdose, carefully assess whether the benefits of opioid therapy outweigh the risks, and incorporate strategies to mitigate risks such as offering naloxone.
The ninth recommendation encourages clinicians to review patient histories of controlled substance prescriptions using state prescription drug monitoring program (PDMP) data before every opioid prescription is written/transmitted for the treatment of acute, subacute, or chronic pain. These reviews can assist clinicians in determining whether prescribing opioids is the most appropriate and safest option for their patients’ pain.
The tenth recommendation focuses on the benefits and risks of toxicology testing to assist clinicians in monitoring and managing patients who are prescribed opioids and other controlled substances. The main purpose of toxicology testing is to improve patient care by addressing issues that may arise with medication adherence, drug safety, substance misuse or abuse, and diversion of opioids. Clinicians should be mindful of bias and stigma that may be associated with toxicology testing and should not perform this testing based on assumptions about patients. Unexpected results from a toxicology test should be discussed with patients in a nonjudgmental, transparent manner, keeping in mind that false-negative or false-positive results are possible. Individual patient circumstances and additional clinical information should also be considered, along with results from toxicology testing to help guide subsequent directions in treatment.
The eleventh recommendation highlights the importance of exercising caution when prescribing opioids and benzodiazepines concurrently and considering whether the benefits of combination therapy with opioids and other agents (e.g., benzodiazepines, muscle relaxants, sedative-hypnotics, and sedating anticonvulsants) outweigh the risks—particularly the increased risk of respiratory depression. The decision to coprescribe opioids with the aforementioned medications should be based on individualized assessments of patient needs and goals.
The last recommendation focuses on the management of patients with OUD using appropriate medications. Detoxification without medications for OUD is not recommended due to the increased risk of resuming drug use, overdose, and overdose death. Patients who meet the criteria for OUD should be offered treatment with an FDA-approved medication for OUD, which may include buprenorphine, methadone, or naltrexone. Treatment of OUD with indicated medications has been found to reduce the risk for overdose as well as overall mortality. In pregnant patients with OUD, buprenorphine or methadone is recommended and should be offered as early as possible during gestation to prevent harm to the patient and the fetus and allow clinicians to provide life-saving interventions.
CONCLUSION
The safe and effective treatment of pain continues to be a challenge for patients and clinicians. A multimodal and multidisciplinary approach to pain management is recommended by the updated CDC guideline to assess the physical health, behavioral health, and overall well-being of patients in a comprehensive and personalized manner. This approach explicitly recognizes the various roles of pharmacists on integrated healthcare teams (e.g., opioid tapering services, coprescribing and dispensing of naloxone, provision of patient education, monitoring PDMPs). The 2022 CDC guideline also brings much-needed attention to the misinterpretations and misapplications of the 2016 CDC Guideline for Prescribing Opioids for Chronic Pain by acknowledging the utility of opioids in the individualized treatment of certain types of pain. The recommendations presented should not be used as inflexible policies or practice, nor should they replace clinical judgement in patient care. Rather, clinicians are encouraged to engage in patient-centered decision-making processes that include discussions of nonopioid and nonpharmacologic interventions, careful evaluations of benefits and risks associated with opioid use, and risk mitigation strategies to best serve their patients.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
- International Association for the Study of Pain. Terminology. July 20, 2023. www.iasp-pain.org/resources/terminology/. Accessed January 31, 2024.
- Nahin RL, Feinberg T, Kapos FP, Terman GW. Estimated rates of incident and persistent chronic pain among US adults, 2019-2020. JAMA Netw Open. 2023;6(5):e2313563.
- CDC. Opioid therapy and different types of pain. November 3, 2022. www.cdc.gov/opioids/patients/therapy-expectations.html. Accessed January 31, 2024.
- Rikard SM, Strahan AE, Schmit KM, Guy GP Jr. Chronic pain among adults—United States, 2019-2021. MMWR Morb Mortal Wkly Rep.
- Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:1-49.
- Institute of Medicine Committee on Advancing Pain Research, Care, and Education. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington, D.C.: National Academies Press; 2011. www.uspainfoundation.org/wp-content/uploads/2016/01/IOM-Full-Report.pdf. Accessed January 31, 2024.
- U.S. Department of Health & Human Services. Pain Management Best Practices Inter-Agency Task Force Report: Updates, Gaps, Inconsistencies, and Recommendations. May 2019. www.hhs.gov/ash/advisory-committees/pain/reports/index.html. Accessed January 31, 2024.
- CDC. Safely and effectively managing pain without opioids. www.cdc.gov/drugoverdose/featured-topics/pain-management.html. Accessed January 31, 2024.
- CDC. Drug overdose deaths. August 22, 2023. www.cdc.gov/drugoverdose/deaths/index.html. Accessed January 31, 2024.
- Wilson N, Kariisa M, Seth P, et al. Drug and opioid-involved overdose deaths—United States, 2017–2018. MMWR. 2020;69:290-297.
- CDC. Provisional data shows U.S. drug overdose deaths top 100,000 in 2022. May 18, 2023. https://blogs.cdc.gov/nchs/2023/05/18/7365/. Accessed January 31, 2024.
- World Health Organization. Opioid overdose. August 29, 2023. www.who.int/news-room/fact-sheets/detail/opioid-overdose. Accessed January 31, 2024.
- Dowell D, Ragan KR, Jones CM, et al. CDC Clinical Practice Guideline for Prescribing Opioids for Pain—United States, 2022. MMWR Recomm Rep. 2022;71(3):1-95.
- MedCentral. CDC opioid prescribing guideline: clinical concerns lead to revision. February 16, 2022. www.medcentral.com/meds/opioids/cdc-opioid-prescribing-guideline-clinical-concerns-lead-revision. Accessed January 31, 2024.
- Bonner L. CDC releases new pain management guidelines, advocating tailored care for patients. Pharmacy Today. 2023;29(2):26-29.